Phosphorene-Based Heterostructured Photocatalysts
2021-12-25YunZhengYilinChenBifenGoBizhouLinXinchenWng
Yun Zheng, Yilin Chen, Bifen Go, Bizhou Lin, Xinchen Wng*
a Xiamen Key Laboratory of Optoelectronic Materials and Advanced Manufacturing, College of Materials Science and Engineering, Huaqiao University, Xiamen 361021, China
b State Key Laboratory of Photocatalysis on Energy and Environment, College of Chemistry, Fuzhou University, Fuzhou 350116, China
ABSTRACT
Keywords:Phosphorene Heterostructure Photocatalysis Water splitting Pollutant degradation Semiconductor photocatalysis is a potential pathway to solve the problems of global energy shortage and environmental pollution.Black phosphorus(BP)has been widely used in the field of photocatalysis owing to its features of high hole mobility,adjustable bandgap,and wide optical absorption range.Nevertheless,pristine BP still exhibits unsatisfactory photocatalytic activity due to the low separation efficiency of photoinduced charge carriers. In recent years, the construction of heterostructured photocatalysts based on BP has become a research hotspot in photocatalysis with the remarkable improvement of photoexcited charge-separation efficiency. Herein, progress on the design, synthesis, properties, and applications of BP and its corresponding heterostructured photocatalysts is summarized. Furthermore, the photocatalytic applications of BP-based heterostructured photocatalysts in water splitting,pollutant degradation,carbon dioxide reduction, nitrogen fixation, bacterial disinfection, and organic synthesis are reviewed.Opportunities and challenges for the exploration of advanced BP-based heterostructured photocatalysts are presented.This review will promote the development and applications of BP-based heterostructured photocatalysts in energy conversion and environmental remediation.
1. Introduction
Photocatalysis is a renewable and economic technology aimed at energy and environmental applications[1,2].Research on semiconducting photocatalysis was stimulated in 1972 by the pioneering investigation of Fujishima and Honda[3]on TiO2electrodes for photoelectrochemical water splitting. To date, plenty of photocatalysts have been designed and developed, most of which are transition-metal-based semiconductors. Based on chemical composition, they can be classified as oxides (e.g., TiO2) [4], sulfides(e.g., SnS2) [5], phosphides (e.g., CoP) [6], nitrides (e.g., Ta3N5) [7],oxynitrides (e.g., BaNbO2N) [8], and more. Since the discovery of the activity of melon-based carbon nitride (denoted as CN) polymer in photocatalytic water splitting[9],non-metal photocatalysts have started to receive more attention in photocatalysis[10].Subsequent emergences of non-metal photocatalysts include selenium[11], sulfur [12], boron [13], doped graphene [14], and red phosphorus(RP)[15].The development of a photocatalyst that can efficiently utilize visible and near-infrared (NIR) light and have high photoexcited charge separation/transfer efficiency is highly desirable in order to pursue the maximum utilization efficiency of solar energy [16,17].
Recently,black phosphorus(BP),also known as phosphorene,as an emerging single-element two-dimensional (2D) material, has received great attention in photocatalysis due to its fascinating features, which include a layer-dependent direct bandgap (0.3–2.1 eV), strong visible and NIR light absorption ability, high carrier mobility (~1000 cm2∙V-1∙s-1), in-plane structural anisotropy, low toxicity, and biocompatibility [18–20]. These properties enable BP to serve as a potential catalyst for water splitting and other photoredox reactions [21,22]. However, a pristine BP photocatalyst also suffers from shortcomings, which include the rapid recombination of photoexcited electron holes,and therefore exhibits moderate catalytic activity in many photoredox reactions [23].Accordingly, the construction of BP-based heterostructures can suppress the recombination of photogenerated charge and carriers,thus remarkably enhancing photocatalytic activity [24].

Fig. 1. Schematic illustration of the categories and photocatalytic applications of BP-based heterostructures.
2. BP-based photocatalysts for water splitting
2.1. The mechanism of BP nanosheets for water splitting
The band structure of 2D BP semiconductors has been investigated by density functional theory (DFT) calculation. BP has a bandgap of 1.79 eV under 7% tensile strain along the a axis and a bandgap of 1.82 eV under 5% tensile strain along the b axis at pH 8.0(Fig.2)[25].The conduction band minimum(CBM)of BP engineered with strain is more negative than the H+/H2potential,while the valence band maximum (VBM) is more positive than the O2/H2O potential[25]. Therefore,BP could serve as a potential photocatalyst for visible-light-driven water splitting due to the suitable bandgaps and band edge alignments [25].
2.2. Design and synthesis of BP nanosheets for water splitting
In the past decades, a great deal of research effort has been spent on the preparation of monolayer or few-layer BP (FP). The synthetic methods of 2D BP can be divided into top-down methods(e.g., liquid exfoliation, microwave exfoliation, and ball milling)and bottom-up methods (e.g., hydrothermal and solvothermal methods)[26].Zhu et al.[27]showed that BP nanosheets prepared by a ball-milling method (BP-BM) exhibited higher photocatalytic activity in H2evolution than bulk BP, without using a cocatalyst(Fig.3).The formation of BP nanosheets leads to an enlarged bandgap,improved electron-reduction ability,accelerated electron-hole separation rate, and enlarged specific surface area, resulting in enhanced photocatalytic activity in H2evolution [27]. In addition,BP nanosheets prepared by a solvothermal method and loaded with platinum (Pt) can generate H2from pure water, and possess a 24-fold higher H2evolution rate than CN nanosheets [28]. The partial oxidation of the surface of BP leads to the increase of both the photocatalytic activity and stability of BP nanosheets [28].The synthesis of FP nanosheets with a top-down or bottom-up approach brings us a step closer to real-world applications of BPbased photocatalysts for solar energy conversion.
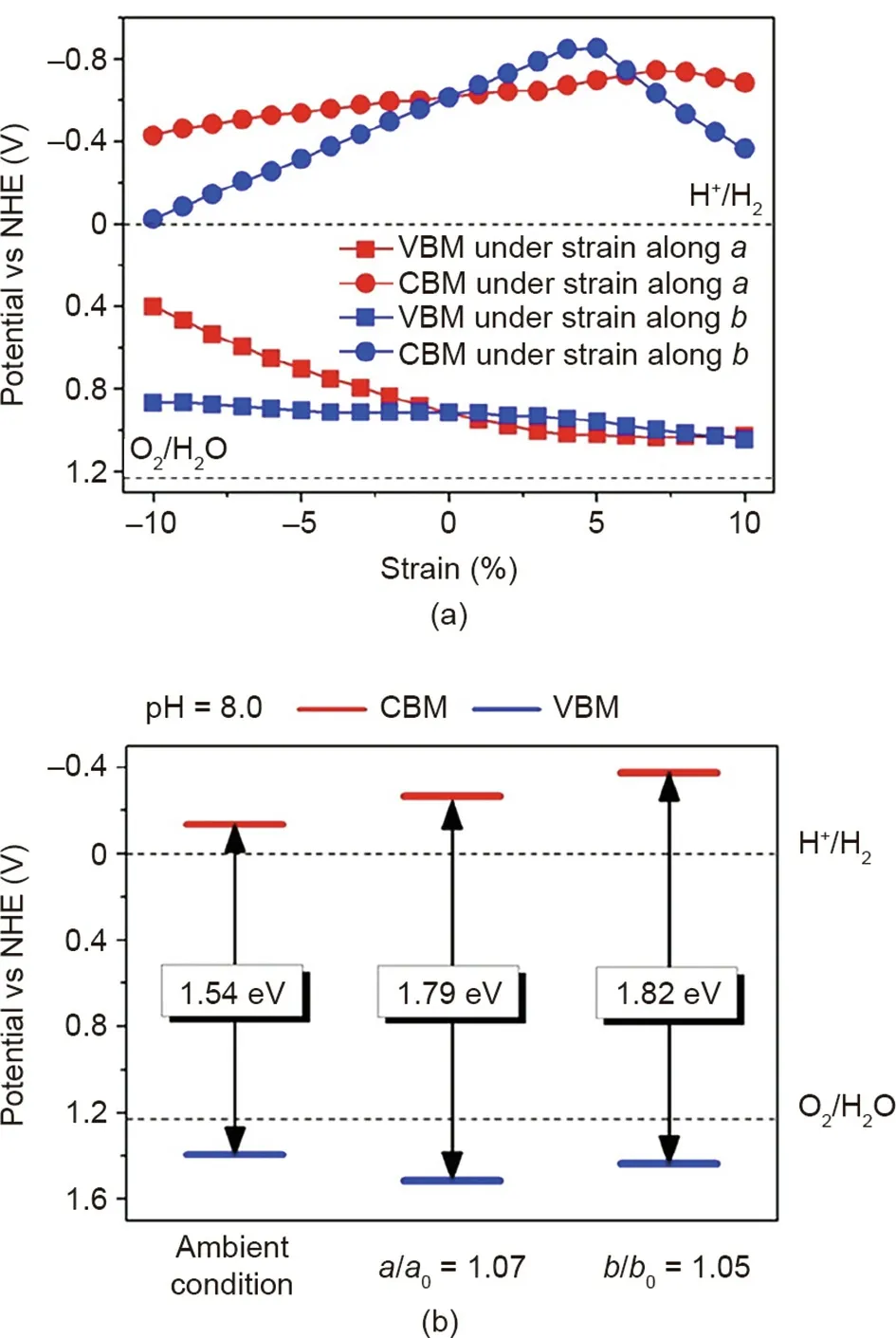
Fig. 2. (a) Formation of the conduction band minimum (CBM) and valence band maximum (VBM) of BP by introducing uniaxial strain using Heyd–Scuseria–Ernzerhof (HSE06) functional; (b) band edge alignments of BP under ambient condition or engineered with uniaxial strain. NHE: normal hydrogen electrode.Reproduced from Ref. [25] with permission of American Chemical Society, ©2014.
In addition,the hydrogen evolution rate(HER)of BP nanosheets in photocatalytic water splitting can be improved by surface modification and dye sensitization [16]. Functionalized BP with indium(III) chloride, tris(pentafluorophenyl)borane, and a benzyl group showed stability under ambient conditions and exhibited higher HER than pristine BP [16]. The HER activity of BP was also significantly improved when Eosin Y was used as the photosensitizer[16].
2.3. Recent advances of BP-based heterostructured photocatalysts in water splitting
Although pristine 2D BP has gained great attention as a potential candidate for photocatalytic water decomposition, the high charge recombination of charge carriers and the poor stability of BP nanosheets restricts their practical application.The heterostructure construction can promote the spatial separation of photoinduced electrons and holes and can endow photocatalysts with optimized photocatalytic performance [29,30]. This section discusses the development of metal/BP, semiconductor/BP, carbon materials/BP, and BP-based ternary heterostructures in photocatalytic water splitting. The photocatalytic activities of BP-based photocatalysts in H2evolution are summarized in Table 1 [23,26–29,31–53].

Fig.3. (a)The synthetic process of BP-BM;(b)photocatalytic water-splitting activities of BP-BM photocatalysts under visible-light irradiation(λ>420 nm).Reproduced from Ref. [27] with permission of Wiley-VCH Verlag GmbH & Co. KGaA, ©2017.
Construction of a metal/BP heterostructure is an important method to enhance the photocatalytic activities in hydrogen evolution.With Pt loaded as a cocatalyst,BP nanosheets prepared using the hydrothermal method exhibited superior photocatalytic H2evolution activity, which was attributed to the layered polycrystalline structure and stronger light absorption ability of BP [29].
In addition, BP has been coupled with various semiconductors(e.g., metal oxide, metal sulfide, metal phosphide, metal vanadate,and CN) to constitute highly efficient photocatalytic watersplitting systems [31,32]. In these hybrid systems, BP serves as a non-metal cocatalyst to raise the photocatalytic activities of the semiconductors in water decomposition, due to the following advantages: ①a large surface area and abundant active sites;②a tuneable band gap that can be used to form a suitable band structure with other semiconductors; and ③high mobility for shuttling photogenerated charges [33]. For example, a BP nanosheet/TiO2mesocrystal hybrid was utilized as a photocatalyst for H2evolution under visible and NIR light when depositing with Pt as a cocatalyst,because the hybrid could harvest ultraviolet(UV)to NIR light and exhibited high charge-separation efficiency [34].Furthermore, the hybridization of FP with CdS contributed to a remarkably enhanced photocatalytic H2evolution rate [35]. The beneficial band structure, remarkable charge mobility, and abundant active sites of BP,as well as the intimate electronic interaction between BP and CdS,are the major reasons for the promoted photocatalytic activity of BP/CdS toward H2evolution (Fig. 4) [35].Apart from that, the 2D/2D heterostructure of WS2/BP [36] and MoS2/BP [37] photocatalysts can effectively accelerate the separation of charge carriers, and increase the H2evolution rate under visible light and NIR light illumination. Moreover, an apparent quantum efficiency (AQE) of 42.55% at 430 nm for H2evolution and solar-to-hydrogen conversion efficiency of 5.4% was obtained when using CoP/BP nanosheets as a photocatalyst in pure water without hole scavengers and bias [38]. The efficient utilization of solar light by BP nanosheets and the high efficiency of chargecarrier separation by amorphous CoP contributed to the enhanced photocatalytic activity of CoP/BP in water splitting [39].
Metal-free heterostructured photocatalysts composed of BP and CN were designed and prepared for water splitting under visible and NIR light irradiation [40]. The strong interfacial contact and efficient charge transfer between BP and CN via the formation of N–P bonds contributed to the improved photocatalytic activity of the BP/CN hybrids for H2evolution(Fig.5)[40].Moreover,the intimate electronic coupling and accelerated charge separation and migration in the 2D/2D van der Waals heterostructure resulted in more superior photocatalytic activity and stability of the BP/CN heterostructure in H2evolution [41]. In addition, phosphorene quantum dots (QDs) were coupled with layered CN to form a zero-dimensional(0D)/2D heterojunction in order to facilitate free carriers’separation and improve photocatalytic activities for water splitting [42–44]. The enhanced photocatalytic activity of BP QDs/CN is ascribed to the type II band alignment, the formation of P–C bonds, and the efficient interfacial charge separation between BP QDs and CN.
总体而言,现实的情况是为最大限度的优化住院医师培训,陪练式现场督导教育实践模式在短期内可能仍然需要结合传统带教模式,由带教老师对其进行儿童保健相关专业知识总培训,然后再进行陪练式现场督导教育实践,增加练兵的机会。
To improve the photocatalytic activity of BP, the hybrid nanostructure that is formed by the combination of BP and carbon materials has attracted extensive attention. Benefiting from the efficient charge transfer between Pt/reduced graphene oxide(RGO) and excited BP nanoflakes, the BP/Pt/RGO hybrid can serve as a photocatalyst for water splitting under visible and NIR light illumination [45]. When Pt nanoparticles, BP, and RGO coexisted,the H2generation amounts reached about 5.13 and 1.26μmol under >420 and >780 nm irradiation for 4 h, respectively [45].
Aside from the H2evolution half-reaction, BP-based materials have also been applied to the water oxidation half-reaction. The BP-Ni(OH)2hybrid exhibited an O2production rate of 15.7μmol per gram catalyst per hour or 224.3μmol per gram BP per hour under simulated sunlight illumination [54]. Furthermore, overall water splitting was realized over a Z-scheme heterostructure artificial photosynthetic system composed of 2D BP/BiVO4.In this photosystem, the O2generation reaction was driven by the photogenerated holes in the valence band (VB) of BiVO4, while the H2evolution reaction was initiated by the photogenerated electrons in the conduction band(CB)of BP[47].These discoveries demonstrate the promising prospect of semiconductor/BP heterostructured photocatalysts in water splitting.
Extensive effort has been focused on the design of BP-based binary nanocomposites for photocatalytic water splitting. To further improve the physicochemical properties and photocatalytic activities, BP-based ternary composites have been developed. The construction of a ternary heterostructure by combining widebandgap semiconductors, narrow-bandgap semiconductors, and metal takes full advantage of the respective superiorities of each component,hence providing an effective approach to realize better photocatalytic performance. BP–Au/La2Ti2O7can be utilized as a broadband solar-response photocatalyst for H2production, because plasmonic gold (Au) and BP can harvest broadband light, and electrons can efficiently migrate from BP and Au to La2Ti2O7(Fig. 6)[48]. Furthermore, BP QDs–Au–CdS [49], BP QDs–CdS–La2Ti2O7[50] and CdS/BP–MoS2[51] heterostructures can efficiently photocatalytically generate H2for a wide solar spectrum,and show higher H2production rates than those of binary hybrids.Moreover,2D BP-supported Ni2P can act as an earth-abundant cocatalyst to couple with a 2D porous CN nanosheet to further enhance visible-light photocatalytic H2production activity[52].These findings demonstrate the superiority of BP-based heterostructured photocatalysts in water splitting.

Table 1 Summary of the photocatalytic activities of BP-based photocatalysts in H2 evolution.

Fig.4. Schematic illustration of the photocatalytic mechanism of the CdS/BP hybrid for water splitting. SHE: standard hydrogen electrode; CB: conduction band; VB:valence band; Eg: band gap energy. Reproduced from Ref. [35] with permission of Wiley-VCH Verlag GmbH & Co. KGaA, ©2017.
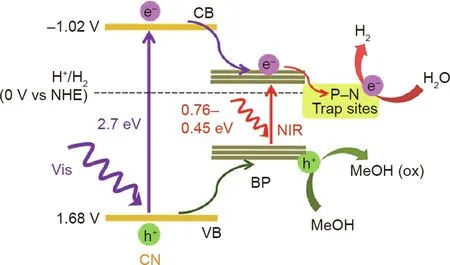
Fig. 5. Schematic diagram for the photocatalytic water-splitting mechanism over BP/CN. Ox: oxidation. Reproduced from Ref. [40] with permission of American Chemical Society, ©2017.
3. BP-based photocatalysts for pollutant treatment and bacterial disinfection
BP-based photocatalysts have stimulated continuous research interest due to their promising photocatalytic application in pollutant treatment. With the advantages of their unique electronic structure and physicochemical properties, BP and its hybrids have been used for the photocatalytic degradation of various pollutants,such as rhodamine B (RhB),reactive black 5 (RB 5), methyl orange(MO), methylene blue (MB), 2-chlorophenol (2-CP), bisphenol A(BPA), 1,3-diphenylisobenzofuran (DPBF), dibutyl phthalate(DBP), Cr(VI), and NO. This section discusses the application of BP nanosheets,BP QDs,and BP-based heterostructured photocatalysts in pollutant treatment.

Fig. 6. (a) Photocatalytic H2 evolution over BP–Au/La2Ti2O7 (LTO) under Vis–NIR(>420 nm) and NIR (>780 nm) light irradiation; (b) effect of the Au ratio on photocatalytic H2 evolution rate for BP–Au/La2Ti2O7. Reproduced from Ref. [48]with permission of Wiley-VCH Verlag GmbH & Co. KGaA, ©2017.
3.1. BP nanosheets and BP QDs for pollutants treatment
The molecular oxygen activation and pollutant degradation ability of 2D BP nanosheets has aroused great research interest.Ultrathin 2D BP nanosheets were prepared by Wang et al. [55]via a sonication-mediated liquid exfoliation method in degassed water (Fig. 7). The BP nanosheets served as a photosensitizer for singlet oxygen (1O2) production with a high quantum yield of about 0.91[55].Furthermore,ultrathin BP nanosheets also present an exotic,excitation-energy-related,optical-switching effect in the production of reactive oxygen species(ROS)[56].The primary ROS products are hydroxyl radicals(·OH)and1O2under UV and visiblelight irradiation, respectively. BP nanosheets exhibited excellent activity in the photocatalytic degradation of MO under different illumination conditions [56]. In another case,1O2and O2·-were generated by energy/charge transfer from the excited P* to the ground state of O2when 2D BP nanosheets, oxygen, water, and light coexisted [57]. The photodegradation efficiency of DBP for 2D BP nanosheets was greatly improved via the1O2oxidation reaction, whereas the effects of O2·-with lower oxidative reactivity were negligible. These findings indicate a promising technique for the removal of water-soluble organic pollutants by 2D BPbased photocatalysts [57].

Fig. 7. (a) Synthetic process of ultrathin BP nanosheets; (b) transmission electron microscope (TEM) image; (c) atomic force microscope image; (d) corresponding height image of ultrathin BP nanosheets. Reproduced from Ref. [55] with permission of American Chemical Society, ©2015.
In addition, bandgap-tunable BP QDs were prepared by Yuan et al. [58] via a liquid exfoliation approach in a mixed solvent of oleic acid and N-methyl pyrrolidone.The as-prepared BP QDs with a bandgap of 2.82 eV showed high photocatalytic activity for RhB degradation [58]. The major ROS products,·OH and O2·-, account for the superior photocatalytic activities of BP QDs for RhB degradation. Furthermore, a heterostructure of BP QDs and attapulgite(BP QDs/ATP) demonstrated higher photocatalytic activity toward the decomposition of BPA due to the sensitization of BP QDs and the facilitation of charge separation [59]. The development of BPbased photocatalysts will open up new opportunities for environmental remediation [59].
3.2.BP-based heterostructured photocatalysts for pollutant treatment and bacterial disinfection
Diverse categories of BP-based heterostructured photocatalysts have been developed for pollutant degradation,including semiconductor/BP, carbon materials/BP, metal/BP, and BP-based ternary composites [60]. This section discusses the progress that has been made on the application of these heterostructures for pollutant degradation. The photocatalytic activities of BP-based photocatalysts in pollutant degradation are summarized in Table 2[46,53,55–59,61–68,70–75].
The loading of metal nanoparticles on BP to construct a metal/BP heterostructure is a key method for enhancing photocatalytic activity. Plasmonic Ag/BP nanohybrids synthesized via a chemical reduction approach were applied to photocatalytic pollutant degradation (Fig. 8) [61]. When decreasing the layer thickness of BP from multi-layers to few-layers,or increasing the size of the silver (Ag) nanoparticles, Ag/BP plasmonic nanohybrids exhibited higher photocatalytic activity than pristine BP nanosheets in RhB degradation. This research opens a new path for the application of plasmonic metal/BP nanohybrids in photocatalytic pollutant degradation [61].
Various semiconductors, such as TiO2, CeO2, MoS2, RP, and CN,have been coupled with BP to form heterostructures with improved photocatalytic activity for pollutant degradation [62].Lee et al. [63] were the first to apply FP–titanium dioxide(BP@TiO2) hybrids to pollutant degradation and bacterial disinfection.In comparison with BP,TiO2,and P25,BP@TiO2hybrid showed a higher rate constant in the decomposition of anionic (RB 5) and cationic (RhB) dyes, and exhibited higher photocatalytic activity in bacterial disinfection with visible-light illumination [63]. The heterojunction between FP and TiO2promotes photoinduced charge-carrier separation with the injection of electrons from the CB of TiO2to FP, while the holes trapped from the VB in the FP and/or TiO2will have longer lifetimes [63]. In addition, CeO2/BP hybrids with oxygen vacancies were prepared by a hydrothermal and deposition approach[64].The photocatalytic degradation rate of BPA for the CeO2/BP composite reached 82.3% within 180 min,which was higher than that of the individual counterparts [64].The Z-scheme heterostructure was responsible for the optimized photocatalytic degradation activity, because of the accelerated charge-carrier separation rate and the high redox potential of the photogenerated charge carriers [64]. Furthermore, a 0D/2D nanohybrid of BP QDs/MoS2was formed by anchoring BP QDs onto MoS2nanosheets via a grinding and sonicating approach [65]. The enhanced photocatalytic activity of the BP QDs/MoS2in MO decomposition originates from its type II band alignment,improved light-harvesting ability, and accelerated spatial charge separation [65]. A hybrid nanocomposite of a zeolitic imidazolate framework (ZIF-8) and BP (ZIF-8/BP) was formed by functionalizing BP with polyvinylpyrrolidone [66]. Since 2D BP functions as an effective electron acceptor and promotes the charge separation and transfer in ZIF-8, the ZIF-8/BP composites exhibited higher photocatalytic reactivity toward the degradation of MB [66]. In addition to the photodecomposition of pollutants in the liquid phase, BP-based heterostructured photocatalysts have been designed and applied to the removal of NO gas. A Z-scheme 2D/2D heterojunction of monolayered Bi2WO6/BP (MBWO/BP)showed improved photocatalytic performance in NO removal to purify air [46]. O2·-,·OH, NO2, and NO3-species were detected in the reaction (Fig. 9) [46].
In addition to the construction of metal-containing semiconductor and BP hybrids, the design of metal-free heterostructured photocatalysts has stimulated great research interest. An RP/BP heterostructure, which was synthesized via a high-energy ballmilling technique,showed remarkably higher photocatalytic activity in the degradation of RhB than those of pristine RP and CdS[67].Aside from the ball-milling method, RP/BP heterostructure prepared using a microwave-assisted liquid-phase synthetic method showed superior photocatalytic ability in MB decomposition with visible-light irradiation [68].
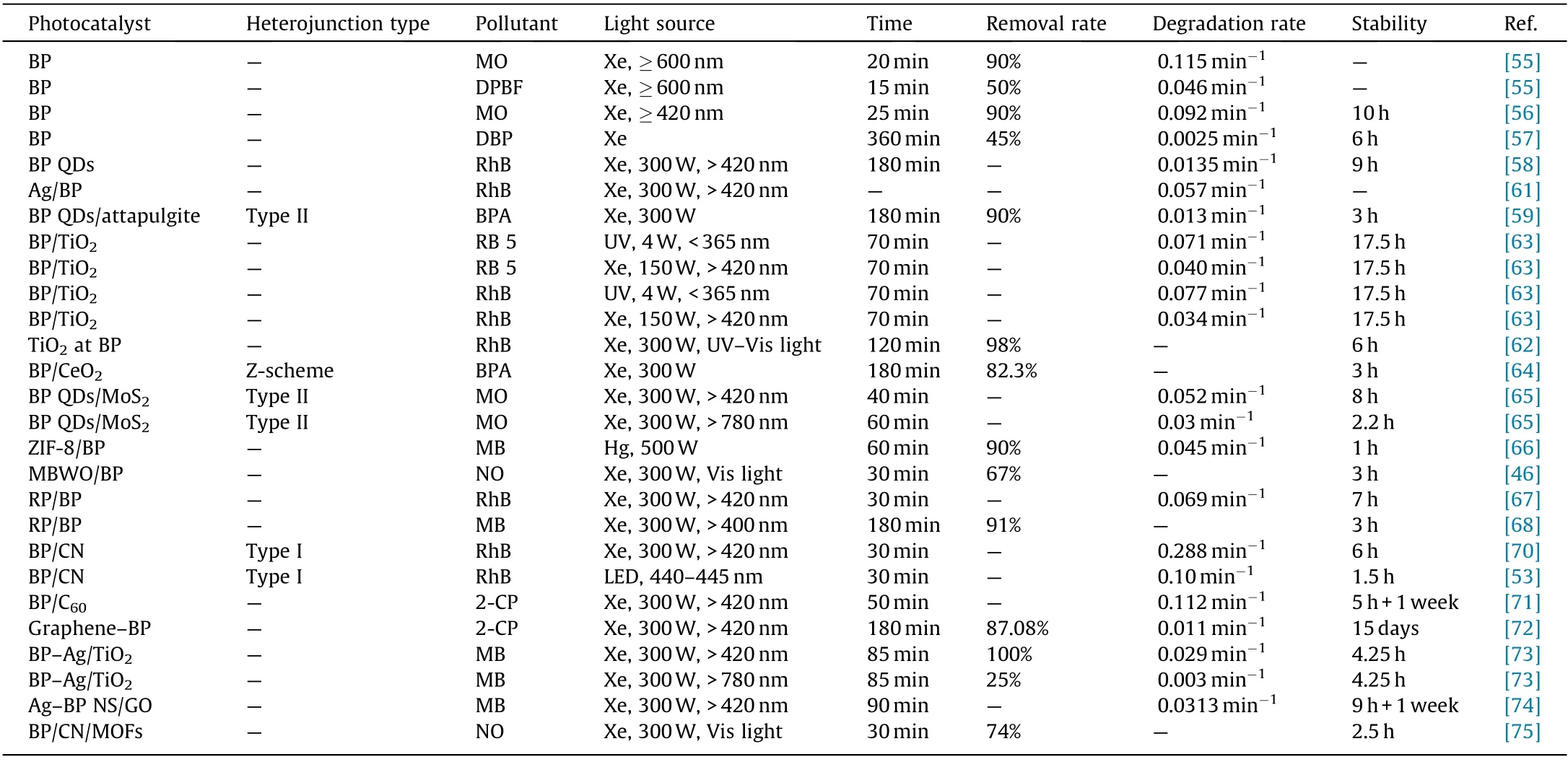
Table 2 Summary of the photocatalytic activities of BP-based photocatalysts in pollutant degradation.

Fig.8. (a)Photocatalytic activities of Ag/multi-layered BP(m-BP)and Ag/few-layered BP(f-BP)hybrids for RhB degradation(insets show the TEM images of Ag/m-BP and Ag/f-BP); (b) energy bands of f-BP, Ag, and m-BP. Ef,m: Fermi energy level. Reproduced from Ref. [61] with permission of American Chemical Society, ©2016.

Fig.9. Synthetic process of the MBWO/BP heterojunction.NMP:N-methyl-pyrrolidone.Reproduced from Ref.[46]with permission of Wiley-VCH Verlag GmbH&Co.KGaA,©2019.
Furthermore, a metal-free heterostructure composed of BP/CN was designed and applied to the photocatalytic removal of RhB and Cr(VI) [69]. BP/CN hybrids were synthesized using a one-step liquid exfoliation strategy in N-methyl-pyrrolidone (NMP) [70].The BP/CN hybrids showed enhanced photocatalytic activity in the degradation of RhB,which was mainly ascribed to the stronger visible-light harvesting capability, accelerated charge-separation rate, and enhanced ability in ROS generation (Fig. 10) [70]. To lower the preparation cost,a BP/CN heterostructured photocatalyst was synthesized from urea and RP via a ball-milling technique[53]. The BP/CN heterostructure also exhibited superior photocatalytic activity in the degradation of RhB, which was attributed to accelerated charge separation and migration, and the formation of P–N bonds [53].
Carbon materials/BP heterostructures with enhanced stability and photocatalytic activity have shown great potential in pollutant degradation.The edge-selective functionalization of BP nanosheets by the covalent bonding of C60molecules via covalent P–C bonds was accomplished via a ball-milling method[71].The participation of C60results in photoinduced electron transfer from BP to C60,and hinders the recombination of charge carriers,thus greatly increasing the stability and photocatalytic activity of the BP–C60hybrid in RhB degradation [71]. In another case, graphene–BP hybrids were synthesized using a chemical vapor transport approach under mild growth conditions [72]. The graphene–BP hybrid displayed higher photocatalytic performance in the removal of 2-CP than BP under visible-light irradiation, owing to the promoted transfer of photoexcited charge carriers [72]. This breakthrough of hybridizing BP nanosheets with carbon materials opens up a new path toward environmental manipulation.
BP-based ternary composites have been designed and developed to further promote their photocatalytic activity in pollutant degradation and air purification. Benefiting from a better harvest of solar light and improved charge-separation efficiency, a Ag/TiO2nanohybrid sensitized by BP nanosheets(BP–Ag/TiO2)showed outstanding photocatalytic performance in MB degradation under visible and NIR light illumination[73]. Ag–BP/graphene oxide also exhibited promoted photocatalytic activity in the decomposition of MB, which was attributed to the broad-spectrum response of the BP nanosheets and the effective trapping of electrons by the Ag nanoparticles[74].In addition,a ternary heterojunction composed of BP,porous CN,and metal–organic framework(BP/CN/MOF)was utilized as a macroscopic membrane material for the photocatalytic removal of NO[75].The nanocomposite showed higher photocatalytic performance than CN-related heterojunctions in NO removal under visible-light illumination [75]. These ternary nanocomposites will become effective photofunctional materials in environmental remediation.
The studies described above hold great promise for the preparation of highly efficient BP-based heterostructured photocatalysts toward environmental remediation.
4. BP-based photocatalysts for N2 fixation, CO2 reduction, and selective organic photosynthesis

Fig. 10. (a) Schematic illustration of the synthetic process of the BP/CN heterostructure; (b) photocatalytic activities of BP/CN hybrids in RhB degradation (C0 is the initial concentration of RhB,and C is the concentration of RhB after photocatalytic reaction);(c)electron paramagnetic resonance spectra of 10%BP/CN hybrid for the detection of O2·- radicals (B is magnetic field, 1 G=10-4 T). Reproduced from Ref. [70] with permission of Wiley-VCH Verlag GmbH & Co. KGaA, ©2018.
In addition to applications in water splitting and pollutant degradation,BP-based heterostructures have been used in N2fixation, CO2reduction, and selective organic photosynthesis. The composite of BP and CN nanosheets(BPCNS)exhibits high activity in photocatalytic N2fixation (Fig. 11) [69]. The photocatalytic N2fixation rate of 0.05BPCNS (0.05 means the mass ratio of BP to CN nanosheets is 0.05) is 347.5μmol∙L-1∙h-1, which is more than eight times higher than that of CNS (40.5μmol∙L-1∙h-1) [69]. The electrons in 0.05BPCNS can be excited more easily due to the change of the π-conjugated CN system and the formation of C–P bonds [69]. Recombination of photogenerated charge carriers is suppressed due to the efficient transfer of photogenerated electrons via the formation of C–P bonds [69]. Some of the lone pairs on phosphorus atoms are occupied, owing to the formation of C–P bonds in 0.05BPCNS, and the oxidation of BP is inhibited by reducing the number of oxygen reactive sites. In addition, BP QDs-modified CN composites can act as a candidate to initiate the photocatalytic reaction of CO2reduction [76]. In comparison with pristine CN (2.65μmol∙h-1∙g-1), BP/CN composites exhibited enhanced carrier-separation efficiency and higher activities for photocatalytic CO2reduction to CO (6.54μmol∙h-1∙g-1) [76].
2D BP has also been used for a solar-to-chemical energy conversion reaction, in which triethylamine (TEA) was oxidized to TEA+,and chloro(triphenylphosphine)gold(I) (AuITPP) was converted to AuIBP and reduced to Au nanoparticles [77]. Furthermore, a Pt/BP heterostructure with strong Pt–P interactions and good stability has been demonstrated to show superior performance in the hydrogenation of styrene and benzaldehyde, as well as the oxidation of benzyl alcohol under simulated solar light irradiation(Fig. 12) [78].

Fig. 11. The mechanism of photocatalytic nitrogen fixation over 0.05BPCNS catalyst. CNS: carbon nitride nanosheets. Reproduced from Ref. [69] with permission of Elsevier B.V., ©2018.
5. Conclusions
This review presents an overview of the synthesis,modification,and photocatalytic applications of BP-based photocatalysts. Various BP-based heterostructured photocatalysts have been designed and prepared, including metal oxide/BP, metal sulfide/BP, metal phosphide/BP, metal vanadate/BP, metal tungstate/BP, MOFs/BP,RP/BP,CN/BP,metal/BP,carbon materials/BP,and BP-based ternary composites. These BP-based heterostructured photocatalysts are promising candidates toward water splitting, pollutant degradation,disinfection,CO2reduction,N2fixation,and organic synthesis.
Despite essential advances in the development of BP-based photocatalysts,the performance of BP-based photocatalysts cannot yet satisfy the demands of practical applications.Future efforts are highly desired in the following aspects. First, the exploration of effective methods to optimize the photocatalytic properties of BP-based materials is still required.Rationally tuning the bandgap configuration and surface properties of BP is required for the design of highly efficient photocatalysts.Second,more comprehensive investigation of the reaction mechanism via theoretical calculations and in situ characterization techniques is necessary to clarify the thermodynamics and kinetics of the catalytic process as well as the structure–activity relationship of photocatalysts[19,20]. Third, the exploitation of suitable approaches to enhance the long-time stability of BP-based materials in photocatalytic systems is highly necessary. The degradation of BP arises from the reaction between O2and the lone pair electrons perpendicular to the BP surfaces, which may induce the generation of POxspecies[79]. In general, the stability of BP can be enhanced by the formation of a heterostructure. Strategies including passivation, the covalent surface modification of BP, and encapsulating BP with protective layers could also be developed and utilized to prevent the oxidation of BP from the air[80].Finally,an environmental risk assessment of these nanostructured photocatalysts should be adequately conducted before practical application. The toxicity of intermediate products should be evaluated, since some pollutants cannot be mineralized during the photocatalytic degradation process [81]. The real commercial and industrial applications of BPbased catalysts via the photocatalytic route require more extensive investigation, and we expect their possible realization in the future, with continued efforts in this direction.
Acknowledgements

Fig. 12. (a) Conversion rates of styrene to ethylbenzene under simulated solar light illumination for Pt/BP catalysts; (b) turnover frequency (TOF) in the hydrogenation of styrene for Pt/BP. R.t.: room temperature, 28°C. Reproduced from Ref. [78] with permission of Wiley-VCH Verlag GmbH & Co. KGaA, ©2018.
This work was financially supported by the National Natural Science Foundation of China (21902051, 21861130353,U1905214, 21961142019, 22032002, 21761132002, and 21425309),the Fundamental Research Funds for the Central Universities(ZQN-807),the Natural Science Foundation of Fujian Province(2019J05090 and 2017J01014),the Graphene Powder and Composite Research Center of Fujian Province (2017H2001), the Scientific Research Funds of Huaqiao University(20171XD033),the Open Project Program of the State Key Laboratory of Photocatalysis on Energy and Environment of Fuzhou University (SKLPEE-KF201803), the NationalKeyTechnologiesR&DProgramofChina(2018YFA0209301), the National Basic Research Program of China(2013CB632405), the Chang Jiang Scholars Program of China(T2016147),and the 111 Project(D16008).
Compliance with ethics guidelines
Yun Zheng,Yilin Chen,Bifen Gao,Bizhou Lin,and Xinchen Wang declare that they have no conflict of interest or financial conflicts to disclose.
猜你喜欢
杂志排行
Engineering的其它文章
- COVID-19 Vaccine Allocation: Modeling Health Outcomes and Equity Implications of Alternative Strategies
- Fast Marching Method for Microseismic Source Location in Cavern-Containing Rockmass: Performance Analysis and Engineering Application
- Non-Communicable Diseases During the COVID-19 Pandemic and Beyond
- Next Steps for Efficacy Evaluation in Clinical Trials of COVID-19 Vaccines
- Facilities for Centralized Isolation and Quarantine for the Observation and Treatment of Patients with COVID-19
- Temporal Profiles of Antibody Responses, Cytokines, and Survival of COVID-19 Patients: A Retrospective Cohort
