复合纳米材料修饰丝网印刷电极检测土壤中铅和镉
2021-09-16王旭明
刘 宁,赵 国,王旭明,刘 刚
•农业生物环境与能源工程•
复合纳米材料修饰丝网印刷电极检测土壤中铅和镉
刘 宁1,2,赵 国3,王旭明2,刘 刚1,2※
(1. 中国农业大学现代精细农业系统集成研究教育部重点实验室,北京 100083;2. 中国农业大学农业农村部农业信息获取技术重点实验室,北京 100083;3. 南京农业大学人工智能学院,南京 210031)
为了快速、准确、低成本检测土壤中痕量Pb(Ⅱ)和Cd(Ⅱ),该研究应用电化学还原和滴涂方法制备了一种铋膜/Nafion/还原氧化石墨烯/离子液体复合纳米材料修饰的丝网印刷电极(Bi/Nafion/rGO/IL/SPE)。通过扫描电子显微镜、循环伏安法和能量色散谱手段表征了修饰电极的电化学分析性能,结果显示修饰材料增大了电极的比表面积、增强了电极的电子传输能力并提高了电极对目标重金属的沉积量。利用标准溶液优化了试验参数,最优参数下的修饰电极检测性能为:在1~80g/L范围内,Pb(Ⅱ)和Cd(Ⅱ)的峰值电流与其浓度的校正模型决定系数分别为0.993和0.985,Pb(Ⅱ)和Cd(Ⅱ)的理论检测限分别为0.124和0.232g/L(S/N=3)。应用实际土壤样品验证了Bi/Nafion/rGO/IL/SPE的实用性,结果显示:Pb(Ⅱ)和Cd(Ⅱ)的平均加标回收率分别为98.71%和98.93%,表明该修饰电极可以用于土壤中痕量Pb(Ⅱ)和Cd(Ⅱ)的检测。
重金属;土壤;离子液体;氧化石墨烯;铋膜;Nafion;丝网印刷电极
0 引 言
重金属Pb(Ⅱ)和Cd(Ⅱ)的毒性高且难以被生物降解,即使在痕量水平也会对大脑、肾脏、血液、神经等器官造成严重损害[1-2]。由于污水灌溉、化肥农药滥用以及工业废物、废液、废气过量排放等不当的人类活动,导致Pb(Ⅱ)和Cd(Ⅱ)在土壤中大量沉积[3]。Pb(Ⅱ)和Cd(Ⅱ)会被农作物根系吸收并在动物体内积累,可在食物链的生物放大作用下,成千百倍地富集进入人体,最终造成经济损失和危害人类健康[4-5]。因此,提出一种快速、准确、可靠且低成本的土壤重金属检测方法,对土壤重金属污染的监控和治理具有重要意义。
传统的光谱法检测精度高,但由于光谱仪器体积庞大,检测周期长、成本高,需要专业人员操作,无法用于土壤重金属的现场快速检测[6-7]。方波阳极溶出伏安法(Square Wave Anodic Stripping Voltammetry,SWASV),作为一种被广泛报道的电化学检测技术,具有速度快、灵敏度高、选择性强和成本低等优点,且检测设备小型化、可便携,适合用于痕量重金属的现场快速分析[8-9]。近年来,丝网印刷电极(Screen Printed Electrode,SPE)由于易制备、可抛弃、成本低等优点被广泛用于电化学分析[10-12]。相对于由玻碳工作电极、铂丝对电极和Ag/AgCl参比电极构成的传统三电极体系,SPE的体积更小[13],更适合作为小型电化学检测设备的敏感器件[14],可用于流通池、微流控等多种场景[15]。但是,SPE的电化学分析性能较差,需要进一步修饰以提高其检测能力。
为了有效增大电极的比表面积、增加电极表面的活性位点,众多研究者利用还原氧化石墨烯(reduced Graphene Oxide,rGO)材料修饰电极[16-19]。如李杜娟等[18]原位修饰金纳米颗粒/石墨烯修饰玻碳电极检测Pb(Ⅱ),检测范围为1~90g/L,检测限为0.27g/L。Zhao等[19]提出一步电沉积金离子和氧化石墨烯修饰玻碳电极实现了土壤中痕量Pb(Ⅱ)和Cd(Ⅱ)的准确检测。上述研究表明,电还原氧化石墨烯是一种可控、稳定性高的电极修饰方法。但在上述研究中,rGO均用于修饰玻碳电极,在丝网印刷电极修饰方面的研究报道较少。而且与玻碳电极相比,SPE的电子传输能力和电催化性能较差[20],所以本研究在应用rGO修饰SPE的基础上,仍需进一步修饰以提高其对重金属离子的检测能力。
为了进一步改善SPE的电分析性能,本研究又应用离子液体(Ionic Liquid,IL)、Nafion和铋膜,与rGO一同制备一种复合材料对SPE进行修饰。IL的高电导率可以提高SPE的电子传输能力[21]。Nafion表面丰富的磺酸基团,使其作为一种阳离子选择性透过膜,可以有效抑制土壤样本中活性物质的干扰[22]。铋膜(Bismuth film,Bi)可以与重金属离子形成合金,有效降低重金属电还原时所需的活化能,大大提高重金属离子的溶出伏安响应[23-24]。综上,本研究制备了一种铋膜/Nafion/还原氧化石墨烯/离子液体复合材料修饰的丝网印刷电极(Bi/Nafion/rGO/IL/SPE),以期实现土壤中痕量Pb(Ⅱ)和Cd(Ⅱ)的准确检测。
1 试验部分
1.1 试剂与仪器
硝酸铅(Pb(NO3)2)、硝酸镉(Cd(NO3)2)和硝酸铋(Bi(NO3)3)原液(1mg/mL)购买于国家标准物质中心并按需要稀释到指定浓度。氧化石墨烯(Graphene Oxide,GO)水溶液(1mg/mL)购买于先锋纳米有限公司(南京)。Nafion D-520分散液(Nafion)购买自美国Sigma-Aldrich公司。离子液体(1-丁基-3-甲基咪唑双氰胺盐,([bmin+][N(CN)]2-))购买自上海成捷化学有限公司,1-丁基-3-甲基咪唑双氰胺盐具有高电导率和良好的粘性度,常用于修饰丝网印刷电极[25]。乙酸/乙酸钠缓冲液购买自奥博来公司(北京)作为电解质以电化学分析Pb(Ⅱ)和Cd(Ⅱ)。NaOH、KCl、K3[Fe(CN)6]购于国药基团化学试剂有限公司(上海),所有试剂均为分析纯。电阻率为18.2 MΩ的超纯水用于稀释试剂和清洗容器。
使用型号为蔡司Supra 55的场发射扫描电镜(光学仪器,上海)进行扫描电子显微镜(Scanning Electron Microscope,SEM)和能量色散谱的分析(Energy Dispersive Spectroscopy,EDS)。用上海辰华公司型号为CHI660D电化学工作站执行循环伏安法和方波溶出伏安法测量分析。裸丝网印刷电极(Screen Printed Electrode,SPE)购买自长三角系统生物科学研究院公司(上海分公司)。裸SPE的参比电极材料为Ag/AgCl、对电极和工作电极(=3 mm)材料均为碳糊。电化学测量均在30 mL电解池中进行,其中电沉积过程中,用磁力搅拌器和搅拌子对待测溶液进行搅拌。
1.2 复合纳米材料修饰丝网印刷电极的制备
修饰之前,先将SPE浸入pH值9.0的磷酸盐缓冲液(Phosphate Buffer Solution,PBS)中执行3 min循环伏安,清除工作电极上的杂质,用超纯水冲洗后,用氮气吹干备用。IL和Nafion均用无水乙醇稀释,得到1%质量分数的IL和0.1%质量分数的Nafion。将1 mg/mL的氧化石墨烯悬浊液超声10 min使之均匀分散,再用pH值为 4.5的PBS溶液稀释,得到0.1 mg/mL的氧化石墨烯-磷酸盐缓冲液(GO-PBS)混合液,PBS在此充当电解质以电还原氧化石墨烯。
取5L离子液体滴涂到SPE的工作电极表面,放入温度为60 ℃的干燥箱中固化,得到离子液体修饰的SPE(IL/SPE)。将IL/SPE浸入到GO-PBS混合液中从−1.4至0.6 V循环扫描10圈,将氧化石墨烯电还原至工作电极表面,同时伴随着300 r/min速度的搅拌,进一步得到还原氧化石墨烯修饰的IL/SPE(rGO/IL/SPE)。之后,用超纯水清洗rGO/IL/SPE并用氮气吹干,取5L的Nafion溶液滴涂到rGO/IL/SPE电极表面,干燥后得到Nafion/rGO/IL复合材料修饰的丝网印刷电极(Nafion/rGO/IL/SPE)。在重金属沉积阶段,向待测液中加入一定量的铋离子,铋离子与目标重金属离子被同步电沉积到工作电极表面,实现原位电镀铋膜修饰Nafion/rGO/IL/SPE,最后得到Bi/Nafion/rGO/IL/SPE。
1.3 电化学测量
用乙酸-乙酸钠缓冲液(0.2 mol/L,pH值5.0)配置20 mL标准浓度的Pb(Ⅱ)和Cd(Ⅱ)待测液置于电解池中,并加入一定浓度的铋离子,将修饰过的丝网印刷电极与电化学工作站连接执行方波阳极溶出伏安(SWASV)测量。主要步骤如下:向工作电极施加−1.2 V的电压同步电沉积铋离子和目标重金属离子,同时以300 r/min的速度搅拌待测溶液,电沉积一段时间后停止搅拌,静置10 s,执行SWASV测量并记录结果。方波溶出伏安法的参数如下:溶出电压区间为−1~ −0.4 V;方波频率为25 Hz;方波振幅为25 mV;电压增量为5 mV。每次SWASV测量后,应用计时电流技术在0.4 V的恒电压下,清洗掉电极表面残留的重金属和铋膜。
1.4 土壤样本制备
本研究按照Tessier等[26]提出的土壤样本顺序浸提方法,用弱酸制备实际土壤待测液样本,以测试本研究制备的修饰电极检测性能。本研究共准备3个土壤样本,取自本实验室先前采集的全国34个地区土样[27]。土壤样本的处理过程如下:首先对土壤样品进行研磨、并使用200m的筛子过筛,取2 g过筛后的土壤样品转移至浸提池中,加入40 mL 0.2 mol/L的乙酸-乙酸钠缓冲液,将浸提液在室温下用混匀仪进行24 h振荡,然后用转速为2 000 r/min的离心机对土壤浸提液进行2 min离心处理,取出上清液。之后使用孔隙为0.2m的滤纸对上清液进行过滤,去除不溶杂质,再用 NaOH将土壤待测液的pH值调整到5.0,得到土壤待测液。最后,取20 mL土壤待测液置入电解池中,供方波溶出伏安检测。
2 结果与分析
2.1 不同修饰电极性能表征
图1a为IL/SPE在磷酸盐缓冲液-氧化石墨烯混合液中的10圈循环伏安扫描图。可以看出图中有一个阳极峰(a)和两个阴极峰(b和c)。随着循环伏安圈数的增加,电流峰值先逐渐增大,表明氧化石墨烯被成功电还原到工作电极表面构成了石墨烯层,提高了工作电极的导电能力;之后趋于稳定,是由于当还原氧化石墨达到一定厚度后,电子的传输路径达到饱和,导致电流没有进一步提高。阳极峰a和阴极峰b的存在,分别是由于石墨烯氧化物表面一些含氧基团被氧化和还原所致[18];阴极峰c主要由于氧化石墨烯中不可逆氧化基团被还原所致[19]。
本研究使用[Fe(CN)6]3-/4-作为氧化还原探针,研究了裸SPE和各修饰SPE的电化学属性。图1b所示为裸SPE、IL/SPE、rGO/IL/SPE和Nafion/rGO/IL/SPE 4种电极在5.0 mmol/L的[Fe(CN)6]3-/4-和0.1 mol/L的KCl混合溶液中的循环伏安扫描结果。对比图中4条循环伏安曲线可知修饰IL和rGO后,电极的氧化/还原峰电流逐步增大且氧化/还原峰电压差略有减小,原因是修饰离子液体能增大SPE的电导率,氧化石墨烯被电还原到IL/SPE上后,电极的电子传输速率得到进一步增大,而且增大幅度高于离子液体。而修饰Nafion后,电极的氧化/还原峰比裸SPE还小且氧化/还原峰电压差比裸SPE还大,这是由于Nafion膜是阳离子交换膜对氧化还原探针[Fe(CN)6]3-/4-阴离子不导电所致。但是Nafion仍会提高SPE对重金属的分析能力;因为Nafion的负电荷架构可以促进重金属离子在电极表面的吸附,进而提高重金属的检测灵敏度;并且,Nafion的高粘度特性可以防止修饰材料的脱落,大大提高了修饰电极的稳定性[28]。
本研究应用扫面电子显微镜(Scanning Electron Microscope,SEM)表征了不同修饰电极的表面形貌,结果如图2所示。图2a显示出裸SPE表面分布杂乱且形态不一,部分为糊状、部分为片状。图2b显示IL/SPE表面材质平整且分布均匀,这是由于离子液体的填充在裸SPE的表面形成一层均匀分布的薄膜,这有助于氧化石墨烯被均匀地电还原至电极表面。从图2c中可以清楚地看到rGO/IL/SPE表面上石墨烯薄膜的褶皱纹理,这说明rGO被成功修饰至电极表面,rGO的褶皱分布可提高修饰电极的比表面积,为电沉积重金属提供更多的活性位点。

注:试验条件:图a为在0.1 mg∙mL−1的氧化石墨烯和pH值为4.5的磷酸盐混合液中以0.1 V∙s−1的速度进行CV扫描;图b为在5.0 mmol∙L−1的[Fe(CN)6]3-/4-和0.1 mol∙L−1的KCl混合溶液中以20 mV∙s−1的扫描速度进行CV扫描。SPE为丝网印刷电极;IL为离子液体;rGO为还原氧化石墨烯;下同。
2.2 电极修饰条件优化
图3a显示了使用循环伏安法电还原氧化石墨烯修饰IL/SPE时,扫描圈数对20g/L Pb(Ⅱ)和Cd(Ⅱ)溶出信号的影响。当扫描圈数自0增加到10时,Pb(Ⅱ)和Cd(Ⅱ)的峰值电流逐步增大,并在10圈时取得最大值。扫描10圈之后,峰值电流随着圈数的增加逐渐减小。该现象可解释如下:当循环伏安扫描圈数小于10时,工作电极表面没有被rGO完全覆盖,所以在0~10圈内,随着圈数的增加,工作电极的电子传输速率和活性位点数量逐渐增大,进而可以提高Pb(Ⅱ)和Cd(Ⅱ)的沉积量、增大溶出伏安信号;当扫描圈数超过10之后,过多的石墨烯层在电极表面堆积,影响了电极的电子转移速率,从而降低重金属的溶出伏安响应[18-19]。该结果与图1a一致,均说明过多的rGO会对电极的检测性能产生负面影响。因此,选择10圈作为rGO修饰电极的最佳电还原圈数。
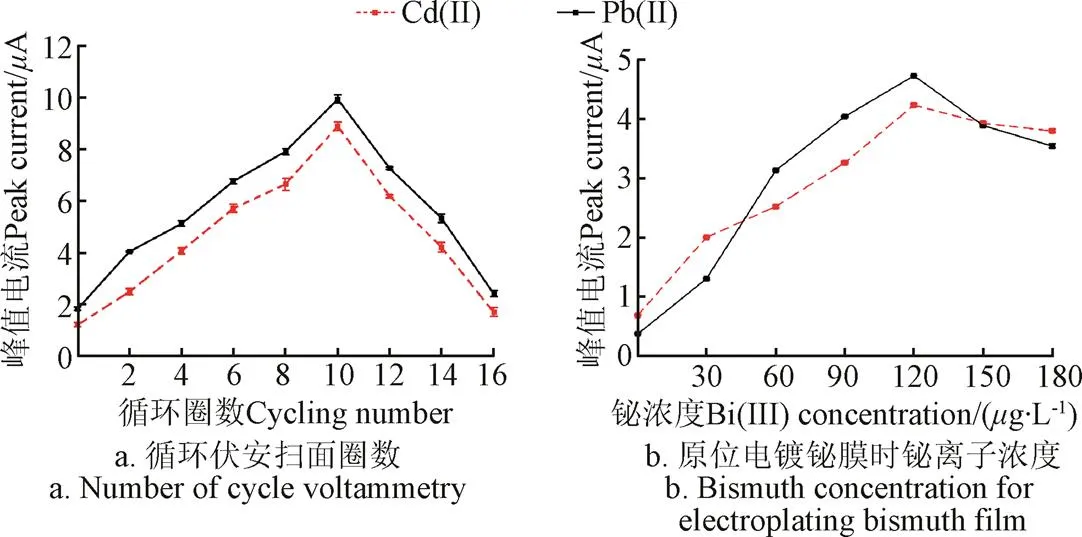
注:图a试验条件:CV扫描速度为0.1 V∙s−1,氧化石墨烯浓度为0.1 mg∙mL−1,缓冲液为磷酸盐(pH值为4.5),铅和镉的浓度为20 μg∙L−1;图b试验条件:沉积电压为−1.2 V,缓冲液为乙酸缓冲液(pH值为5.0),铅和镉的浓度为10 μg∙L−1。
为了进一步提高电极的分析性能,用Nafion/rGO/IL复合材料修饰SPE之后,又向待测溶液中加入铋离子,利用原位电镀的方式对工作电极修饰铋膜,得到Bi/Nafion/rGO/IL/SPE电极。图3b显示了铋离子浓度对电极检测10g/L Pb(Ⅱ)和Cd(Ⅱ)性能的影响。当铋离子浓度在0~120g/L范围内增加时,Pb(Ⅱ)和Cd(Ⅱ)的峰值电流随之逐渐增大,铋离子高于120g/L之后峰值电流逐渐减小。上述现象主要因为:当铋离子浓度较低时,不能与重金属离子充分形成合金,无法有效降低Pb(Ⅱ)和Cd(Ⅱ)还原所需的活化能[29];但是,铋离子浓度过高时,会占据工作电极表面的大量活性位点,降低重金属的沉积量,进而减小Pb(Ⅱ)和Cd(Ⅱ)的溶出伏安电流[30]。因此,选择120g/L为最佳铋离子浓度。
2.3 电化学测量条件优化
图4a显示了乙酸缓冲液的pH值对10g/L Pb(Ⅱ)和Cd(Ⅱ)溶出峰值电流的影响。pH值自3.5升至5.0时,峰值电流随之增大,当pH值大于5.0之后,峰值电流随之减小。其现象可解释为:当pH值太低时,大量的H+离子会占据工作电极表面,由于静电力作用和“析氢”作用降低Pb2+和Cd2+的电沉积量[31];当pH过高时,缓冲液中存在相当量的OH−,会与Pb(Ⅱ)和Cd(Ⅱ)形成不溶性的氢氧根配合物,也会降低Pb(Ⅱ)和Cd(Ⅱ)的溶出伏安信号[32]。因此,选择pH值为5.0的乙酸缓冲液作为溶出伏安测量的支持电解质。
图4b显示了在−1.4~−0.8V范围内,沉积电压对10g/L Pb(Ⅱ)和Cd(Ⅱ)溶出伏安响应的影响。沉积电压自−1.4 V升高至−1.2 V时,Pb(Ⅱ)和Cd(Ⅱ)的峰值电流随之增大,在−1.2 V之后,随着沉积电压的升高峰值电流快速下降,而且Cd(Ⅱ)的下降程度大于Pb(Ⅱ)。此现象可解释为:过负的电压会导致电极表面发生“析氢”现象,影响重金属的电沉积;但是过正的电压无法提供充足的电化学能以电还原重金属离子[33]。在过正的电压下,Cd(Ⅱ)的峰值电流下降更快的原因是:金属镉的活泼性高于铅,需要更高的还原电压才能将其还原。
图4c显示了沉积时间对10g/L Pb(Ⅱ)和Cd(Ⅱ)溶出伏安检测结果的影响。在210 s之前随着沉积时间的增长,Pb(Ⅱ)和Cd(Ⅱ)的峰值电流逐渐增大,表明越来越多的重金属离子被沉积到了电极表面。但是210 s之后,由于工作电极表面的活性位点有限,峰值电流的增速减缓,该现象与文献[28, 34]报道一致。综合考虑检测效率和灵敏度,本研究最终选择150 s作为Pb(Ⅱ)和Cd(Ⅱ)的沉积时间。
2.4 电极检测性能分析
图5显示了不同修饰电极对25g/L的Pb(Ⅱ)和Cd(Ⅱ)的溶出伏安响应。从图中可以看出,Pb(Ⅱ)和Cd(Ⅱ)在裸SPE(曲线a)上的电流信号非常微弱,在Bi/SPE上峰值电流增大(曲线b),这是因为铋与铅和镉形成合金,降低了目标重金属的电还原活化能,提高了重金属的电沉积量。在Bi/IL/SPE上(曲线c)电流响应再次增强,是由于离子液体提高了电极的电子传输能力。相对于Bi/IL/SPE,铅离子和镉离子在Bi/rGO/IL/SPE(曲线d)上的峰值电流提升幅度较大,这是因为rGO增大了工作电极的比表面积和活性位点数量,较大幅度地提高了重金属的电沉积量,进而较大程度地增强了溶出伏安响应。由于Nafion的负电荷架构对重金属离子具有吸附作用,会进一步提高铅离子和镉离子的沉积量,最终使Pb(Ⅱ)和Cd(Ⅱ)在Bi/Nafion/rGO/IL/SPE(曲线e)上取得最大的溶出伏安响应。
为了探究修饰材料对电极检测性能的改善机理,本研究应用能量色散谱(Energy Dispersive Spectroscopy,EDS)表征了不同修饰电极表面Pb(0)和Cd(0)的沉积量,结果如图6所示。图6a显示裸SPE表面铅和镉的沉积量最少、且镉的沉积量低于铅,原因是裸SPE比表面积小、活性位点少且导电性差。图6b显示Bi/SPE电极表面上铅和镉的沉积量相对于裸SPE明显增多,证明了铋膜可以增大Pb(Ⅱ)和Cd(Ⅱ)的沉积量。图6c显示rGO和IL的修饰进一步提高了铅和镉的沉积量,结合图1b和图2c可分析其原因为:IL和rGO提高了电极的导电性且rGO增大了电极的比表面积进而增多了供重金属沉积的活性位点。图6d显示Nafion材料再次增多目标重金属的沉积量,证实了Nafion的负电荷骨架可以促进电极表面对重金属阳离子的吸附、提高重金属离子的沉积量。
在最佳试验条件下,应用Bi/Nafion/rGO/IL/SPE对浓度范围为1~95g/L的Pb(Ⅱ)和Cd(Ⅱ)标准溶液进行溶出伏安检测,伏安图谱如图7a所示。在1~80g/L内Pb(Ⅱ)和Cd(Ⅱ)的峰值电流随浓度增高逐步上升,但当重金属浓度超过80g/L时,峰值电流趋于稳定。该现象主要是因为工作电极的活性位点有限,即使浓度继续增高,工作电极上也无法沉积更多的重金属所致。如图7b和图7c所示,在1~80g/L内Pb(Ⅱ)和Cd(Ⅱ)浓度和其峰值电流表现出良好的线性相关关系,Pb(Ⅱ)和Cd(Ⅱ)的检测校准模型分别为=0.544−2.962(2=0.993)和=0.427−2.155(2=0.985)。在线性范围内,电极对Pb(Ⅱ)和Cd(Ⅱ)的检测灵敏度分别为0.544和0.427A/(g/L)。在信噪比为3:1条件下(S/N=3),Pb(Ⅱ)和Cd(Ⅱ)的理论检测下限分别为0.124和0.232g/L。
表1对比了Bi/Nafion/rGO/IL/SPE与其他修饰电极对Pb(Ⅱ)和Cd(Ⅱ)的检测性能,结果如下:与2D Biexf/SPCE 相比Bi/Nafion/rGO/IL/SPE的检测限较高但是具有更宽的检测范围;与其他修饰电极相比Bi/Nafion/rGO/IL/SPE有相当的检测范围且具有更低的检测限。

表1 不同修饰电极对Pb(Ⅱ)和Cd(Ⅱ)的检测性能对比
Note: GR/PANI/PS/SPCE: Graphene/polyaniline/polystyrene fibers modified screen-printed carbon electrode; Bi/RGO-MWCNT-AuNP/SPE: Bismuth film/reduced grophene oxide-multiwalled carbon nanotubes-gold nanoparticle modified SPE; Poly-dendrimer/carbon black/SPE: Poly-dendrimer/carbon black modified screen-printed electrode; Bi/glassy carbon microparticle/SPE: Bismuth film/glassy carbon microparticle modified screen-printed electrode; 2D Biexf/SPCE: two-dimensional exfoliated layered bismuth modified screen-printed carbon electrode.
2.5 电极稳定性和抗干扰性分析
2.5.1 稳定性分析
为了验证Bi/Nafion/rGO/IL/SPE的稳定性和重复性,应用该电极在最佳试验条件下对20g/L的Pb(Ⅱ)和15g/L的Cd(Ⅱ)进行6次重复测试,结果如图8所示。Pb(Ⅱ)和Cd(Ⅱ)6次溶出伏安测量结果的相对标准偏差(Relative Standard Deviation,RSD)分别为1.57%和2.32%,说明Bi/Nafion/rGO/IL/SPE具有较高的稳定性和可重复性。
2.5.2 Pb(Ⅱ)和Cd(Ⅱ)的相互干扰分析
当同时检测多个重金属离子时,必须确保两种目标重金属之间不存在严重的相互干扰,否则会大大降低校准模型的检测精度[35]。因此,本研究评估了Bi/Nafion/rGO/IL/SPE同时检测Pb(Ⅱ)和Cd(Ⅱ)时的相互干扰程度。如图9a所示,当Cd(Ⅱ)浓度在10~80g/L范围变化时,20g/L Pb(Ⅱ)溶出伏安响应的相对标准偏差(RSD)为2.97%。如图9b所示,当Pb(Ⅱ)浓度在10~80g/L变化时,20g/L Cd(Ⅱ)溶出伏安信号的相对标准偏差(RSD)为2.52%。综上分析表明,Pb(Ⅱ)和Cd(Ⅱ)的溶出伏安响应之间无显著的相互干扰,同时说明本研究制备的Bi/Nafion/rGO/IL/SPE具有同时检测Pb(Ⅱ)和Cd(Ⅱ)的能力。
此外,本研究还考察了当Pb(Ⅱ)或Cd(Ⅱ)在某一固定浓度存在时,Bi/Nafion/rGO/IL/SPE对铅和镉的单独检测性能。如图9c所示,当存在20g/L的Pb(Ⅱ)时,Cd(Ⅱ)在10~80g/L内的校准模型为=0.416−4.116(2=0.983),灵敏度为0.416A/(g/L)。如图9d所示,当存在20g/L的Cd(Ⅱ)时,Pb(Ⅱ)的校准模型为=0.507−2.274(2=0.999),灵敏度为0.507A/(g/L)。与在1~80g/L范围内同时检测Pb(Ⅱ)和Cd(Ⅱ)的校准模型相比(如图7所示),检测灵敏度分别降低了6.86%和2.37%,误差在10%以内、差异较小[36],进一步说明了Bi/Nafion/rGO/IL/SPE可以同时检测Pb(Ⅱ)和Cd(Ⅱ)。
2.5.3 其他非目标重金属的干扰分析
本实验室前期研究结果表明,Na+、K+和Ca2+等非重金属离子对Pb(Ⅱ)和Cd(Ⅱ)溶出伏安响应的干扰非常小,可以不做考虑[37]。在最佳试验条件下,本研究分析了Zn2+、Hg2+、Cr2+、As2+、Cu2+对Pb(Ⅱ)和Cd(Ⅱ)溶出伏安响应的干扰程度。设置干扰离子与目标检测离子的浓度比为10:1。结果如图10所示,除了Cu2+之外,其他重金属离子均没有对Pb(Ⅱ)和Cd(Ⅱ)伏安响应产生严重干扰(峰值电流的变化小于5%)。Cu(Ⅱ)作为最大的干扰离子,对Pb(Ⅱ)和Cd(Ⅱ)峰值电流的抑制程度分别是40.02%和62.85%,干扰原因可能是:铜与铅和镉会形成Cu-Pb-Cd金属合金,影响了Pb(Ⅱ)和Cd(Ⅱ)的电沉积[38]。据文献报道,铁氰化物(ferricyanide,FCN)可以与Cu2+发生配位反应形成不溶性络合物[39-40]。因此,为了抑制Cu2+的干扰,向待测液中加入适量的铁氰化钾溶液。并对铁氰化钾的浓度进行优化,结果如图10所示,铁氰化钾的最佳浓度为0.1 mmol/L。进一步分析可知,加入铁氰化物较大程度上削弱了Cu2+对Pb(Ⅱ)的干扰,而对Cd(Ⅱ)的干扰仍然较为严重。
2.6 实际土壤样本的检测分析
为了测试Bi/Nafion/rGO/IL/SPE的实用性,应用该电极检测3个土壤样本中Pb(Ⅱ)和Cd(Ⅱ)的含量。采用标准添加法分析土样中的重金属浓度,并考察了Pb(Ⅱ)和Cd(Ⅱ)的加标回收率。标准添加法是向待测实际样本中滴定目标检测离子,以测定样本中目标离子的含量。由于滴定前后样本基质一致,所以该方法可以消除实际样本存在的基质效应[41]。首先,按照1.4节准备土壤待测液样本,然后向待测液中加入0.1 mmol/L的亚铁氰化钾,以抑制土壤中Cu2+的干扰。图11显示了3个土壤样本及加标后的溶出伏安检测结果。由于不同土壤有不同的理化性质(即样本基质不同),所以即使加标浓度相同,样本间溶出电流的变化量也不一致,如图11a和11b所示。因此,对于特定的样本需要建立各自的检测校准模型,再采用标准添加法计算实际样本加标前后的Pb(Ⅱ)和Cd(Ⅱ)浓度,进一步计算样本的加标回收率。为了验证修饰电极与火焰原子吸收光谱(Flame Atomic Absorption Spectroscopy,FAAS)在检测结果上无显著差异,在95%置信水平下进行双样本-检验,结果如表2所示。Pb(Ⅱ)和Cd(Ⅱ)的值均小于临界值(2.776,4个自由度),说明修饰电极的检测结果与FAAS的检测结果没有显著性差异。此外表2显示了3个土壤样本的Pb(Ⅱ)和Cd(Ⅱ)检测结果和其加标回收率结果,Pb(Ⅱ)的回收率在96.99%~101.85%之间,平均回收率为98.71%,Cd(Ⅱ)的回收率在97.34%~101.73%之间,平均回收率为98.93%。试验结果表明,本研究制备的Bi/Nafion/rGO/IL/SPE电极可以用于实际土壤样本中痕量Pb(Ⅱ)和Cd(Ⅱ)的检测。
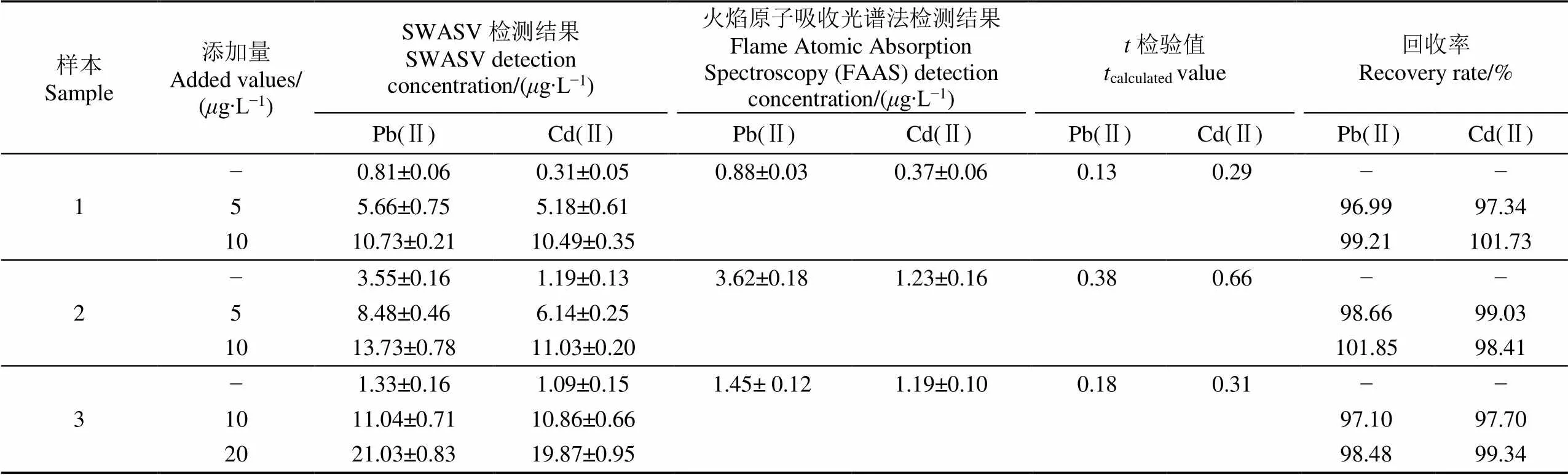
表2 Bi/Nafion/rGO/IL/SPE电极对实际土壤样本中Pb(Ⅱ)和Cd(Ⅱ)的检测结果
3 结 论
本研究制备了一种铋膜/Nafion/还原氧化石墨烯/离子液体复合材料修饰的丝网印刷电极(Bi/Nafion/rGO/IL/SPE)用于检测土壤中Pb(Ⅱ)和Cd(Ⅱ)含量。在最佳检测条件下,Bi/Nafion/rGO/IL/SPE对Pb(Ⅱ)和Cd(Ⅱ)的检测线性范围均为1~80g/L、校准模型的2分别为0.993和0.985、检测限分别为0.124和0.232g/L。应用修饰电极进行6次溶出伏安测量,Pb(Ⅱ)和Cd(Ⅱ)峰值电流的相对标准偏差分别为1.57%和2.32%,表明修饰电极具有较高的稳定性和可重复性。考察了Pb(Ⅱ)和Cd(Ⅱ)的相互影响,当Cd(Ⅱ)浓度在10~80g/L范围变化时,20g/L Pb(Ⅱ)峰值电流的相对标准偏差为2.97%,当Pb(Ⅱ)浓度在10~80g/L变化时,20g/L Cd(Ⅱ)峰值电流的相对标准偏差为2.52%,表明Pb(Ⅱ)和Cd(Ⅱ)对双方的溶出伏安响应之间无显著相互干扰,同时表明Bi/Nafion/rGO/IL/SPE具有同时检测Pb(Ⅱ)和Cd(Ⅱ)的能力。在干扰离子与目标离子的浓度比为10:1时,Zn2+、Hg2+、Cr2+、As2+、Cu2+对Pb(Ⅱ)和Cd(Ⅱ)峰值电流的干扰程度均小于5%,但Cu2+对Pb(Ⅱ)和Cd(Ⅱ)峰值电流的抑制程度分别是40.02%和62.85%。向待测溶液中加入0.1 mmol/L的铁氰化钾溶液,能够基本上消除Cu2+的对Pb(Ⅱ)干扰,但是Cu2+对Cd(Ⅱ)的干扰仍然较为严重。应用标准添加法对实际土壤样本进行检测,结果显示Pb(Ⅱ)和Cd(Ⅱ)的平均加标回收率分别为98.71%和98.93%。表明本研究制备的Bi/Nafion/rGO/IL/SPE电极可以用于土壤中痕量铅和镉的检测。
研究结果显示:加入适量的铁氰化钾可以一定程度上抑制Cu2+对Pb(Ⅱ)和Cd(Ⅱ)溶出信号的干扰,但并不能消除其干扰。虽然Cu2+会严重干扰Pb(Ⅱ)和Cd(Ⅱ)的溶出信号,但是仍可检测到Pb(Ⅱ)和Cd(Ⅱ)的溶出峰值电流。因此,在未来的研究中可以分析不同浓度Cu2+对Pb(Ⅱ)和Cd(Ⅱ)峰值电流的干扰规律,通过构建化学计量学检测模型消除Cu2+的干扰。
[1] Rehman A U, Nazir S, Irshad R, et al. Toxicity of heavy metals in plants and animals and their uptake by magnetic iron oxide nanoparticles[J]. Journal of Molecular Liquids, 2021, 321: 114455.
[2] Wang L, Cui X F, Cheng H G, et al. A review of soil cadmium contamination in China including a health risk assessment[J]. Environment Science and Pollution Research, 2015, 22: 16441-16452.
[3] 张秋霞,张合兵,刘文锴,等. 高标准基本农田建设区域土壤重金属含量的高光谱反演[J]. 农业工程学报,2017,33(12):230-239.Zhang Qiuxia, Zhang Hebing, Liu Wenkai, et al. Inversion of heavy metals content with hyperspectral reflectance in soil of well-facilitied capital farmland construction areas[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2017, 33(12): 230-239. (in Chinese with English abstract)
[4] 黄迪,黄志红,孔辉,等. 重金属污染农田土壤的稳定化修复技术及其修复实践研究[J]. 中国农学通报,2021,37(8):72-78.
Huang Di, Huang Zhihong, Kong Hui, et al. Stabilization remediation technology and remediation practice of heavy metal contaminated farmland soil[J]. Chinese Agricultural Science Bulletin, 2021, 37(8): 72-78. (in Chinese with English abstract)
[5] 李新民,刘桀佳. 农田土壤重金属污染快速检测及修复方法研究[J]. 环境科学与管理,2021,46(2):128-133.
Li Xinmin, Liu Jiejia. Rapid detection and remediation of heavy metal pollution in farmland soil[J]. Environmental Science and Management, 2021, 46(2): 128-133. (in Chinese with English abstract)
[6] Liu N, Zhao G, Liu G. Accurate SWASV detection of Cd(II) under the interference of Pb(II) by coupling support vector regression and feature stripping currents[J]. Journal of Electroanalytical Chemistry, 2021, 889: 115227.
[7] Wang L W, Li X R, Tsang D C W, et al. Green remediation of Cd and Hg contaminated soil using humic acid modified montmorillonite: Immobilization performance under accelerated ageing conditions[J]. Journal of Hazardous Materials, 2020, 387: 122005.
[8] Ye J J, Lin C H, Huang X J, et al. Analyzing the anodic stripping square wave voltammetry of heavy metal ions via machine learning: Information beyond a single voltammetric peak[J]. Journal of Electroanalytical Chemistry, 2020, 872: 113934.
[9] Zhang T, Jin H N, Fang Y N, et al. Detection of trace Cd2+, Pb2+and Cu2+ions via porous activated carbon supported palladium nanoparticles modified electrodes using SWASV[J]. Materials Chemistry and Physics, 2019, 225: 433-442.
[10] Promphet N, Rattanarat P, Rangkupan R, et al. An electrochemical sensor based on graphene/polyaniline/ polystyrene nano-porous fibers modified electrode for simultaneous determination of lead and cadmium[J]. Sensors and Actuators B-Chemistry, 2015, 207: 526-534.
[11] Wang H, Yin Y, Zhao G, et al. Graphene oxide/multi-walled carbon nanotubes/gold nanoparticle hybrid functionalized disposable screen-printed carbon electrode to determine Cd(II) and Pb(II) in soil[J]. International Journal of Agricultural and Biological Engineering, 2019; 12(3): 194-200.
[12] Mazzaracchio V, Tshwenya L, Moscone D, et al. A poly (propylene imine) dendrimer and carbon black modified flexible screen printed electrochemical sensor for lead and cadmium co-detection[J]. Electroanalysis, 2020, 32: 3009-3016.
[13] Kava A, Beardsley C, Hofstetter J, et al. Disposable glassy carbon stencil printed electrodes for trace detection of cadmium and lead[J]. Analytica Chimica Acta, 2020, 1103: 58-66.
[14] Tapia M A, C Pérez-Ràfols, Rui G, et al. Enhanced voltammetric determination of metal ions by using a bismuthene-modified screen-printed electrode[J]. Electrochimica Acta, 2020, 362: 137144.
[15] Alejandro G M F, Samuel J R N, Craig E B. Screen-printed electrodes: Transitioning the laboratory in-to-the field[J]. Talanta Open, 2021, 3: 100032.
[16] Bao Q W, Li G, Yang Z C, et al. Electrochemical performance of a three-layer electrode based on Bi nanoparticles, multi-walled carbon nanotube composites for simultaneous Hg(II) and Cu(II) detection[J]. Chinese Chemical Letters, 2020, 31(10): 2752-2756.
[17] Kanjana K, Phuktra C, Eda M, et al. A highly sensitive fenobucarb electrochemical sensor based on graphene nanoribbons-ionic liquid-cobalt phthalocyanine composites modified on screen-printed carbon electrode coupled with a flow injection analysis[J]. Journal of Electroanalytical Chemistry, 2019, 855: 113630.
[18] 李杜娟,徐枫,樊凯,等. 原位合成纳米金/石墨烯修饰玻碳电极检测水和土壤中痕量铅[J]. 农业工程学报,2018,34(11):203-208.
Li Dujuan, Xu Feng, Fan Kai, et al. In-suit synthesis of graphene/gold nanoparticles modified glassy carbon electrode for detection of lead in water and soil[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2018, 34(11): 203-208. (in Chinese with English abstract)
[19] Zhao G, Wang H, Liu G, et al. Simultaneous determination of trace Cd(II) and Pb(II) based on Bi/Nafion/reduced graphene oxide-gold nanoparticle nanocomposite film-modified glassy carbon electrode by one-step electrodeposition[J]. Ionics, 2017, 23(3): 767-777.
[20] Wang Z Q, Wang H, Zhang Z H, et al. Electrochemical determination of lead and cadmium in rice by a disposable bismuth/electrochemically reduced graphene/ ionic liquid composite modified screen-printed electrode[J]. Sensors and Actuators B: Chemical, 2014, 199: 7-14.
[21] Chaiyo S, Mehmeti E, Žagar K, et al. Electrochemical sensors for the simultaneous determination of zinc, cadmium and lead using a Nafion/ionic liquid/graphene composite modified screen-printed carbon electrode[J]. Analytica Chimica Acta, 2016, 918: 26-34.
[22] Rehacek V, Hotovy I, Vojs M, et al. Nafion-coated bismuth film electrodes on pyrolyzed photoresist/alumina supports for analysis of trace heavy metals[J]. Electrochimica Acta, 2012, 63: 192-196.
[23] Lee S, Park S K, Choi E, et al. Voltammetric determination of trace heavy metals using an electrochemically deposited graphene/bismuth nanocomposite film-modified glassy carbon electrode[J]. Journal of Electroanalytical Chemistry, 2016, 766: 120-127.
[24] Carlos R R, Margarita E A, Verónica A. Determination of molybdenum (VI) via adsorptive stripping voltammetry using an ex‒situ bismuth screen-printed carbon electrode[J]. Microchemical Journal, 2020, 154: 104589.
[25] Abo-Hamad A, AlSaadi M A, Hayyan M, et al. Ionic liquid-carbon nanomaterial hybrids for electrochemical sensor applications: A review[J]. Electrochimica Acta, 2016, 193: 321-343.
[26] TessierA, CampbellPG, BissonM. Sequential extraction procedure for the speciation of particulate trace metals[J]. Analytical Chemistry, 1979, 51(7): 844-851.
[27] 王圣伟. 农田环境土壤重金属评测技术与时空信息发布系统研究[D]. 北京:中国农业大学,2013.
Wang Shengwei. Soil Heavy Metals Evaluation Technology Based on Farmland Environment with Spatial-temporal Information Publish Research[D]. Beijing: China Agriculture University, 2013. (in Chinese with English abstract)
[28] Liu N, Zhao G, Liu, G. Sensitive stripping voltammetric determination of Pb(II) in soil using a Bi/single-walled carbon nanotubes-Nafion/ionic liquid nanocomposite modified screen-printed electrode[J]. International Journal of Electrochemical Science, 2020, 15(8): 7868-7882.
[29] Domańska K, Tyszczuk-Rotko K. Integrated three-electrode screen-printed sensor modified with bismuth film for voltammetric determination of thallium(I) at the ultratrace level[J]. Analytica Chimica Acta, 2018, 1036: 16-25.
[30] MarianM, PavolM, MiroslavB, et al. Bismuth modified boron doped diamond electrode for simultaneous determination of Zn, Cd and Pb ions by square wave anodic stripping voltammetry: Influence of boron concentration and surface morphology[J]. Vacuum, 2019, 167: 182-188.
[31] Deng X J, Lyu L L, Li H W, et al. The adsorption properties of Pb(II) and Cd(II) on functionalized graphene prepared by electrolysis method[J]. Journal of Hazardous Materials, 2010, 183(1/2/3): 923-930.
[32] Xu R X, Yu X Y, Gao C, et al. Non-conductive nanomaterial enhanced electrochemical response in stripping voltammetry: The use of nanostructured magnesium silicate hollow spheres for heavy metal ions detection[J]. Analytica Chimica Acta, 2013, 790: 31-38.
[33] Jayadevimanoranjitham J, Sriman N S. A mercury free electrode based on poly O-cresophthalein complexone film matrixed MWCNTs modified electrode for simultaneous detection of Pb(II) and Cd(II)[J]. Microchemical Journal, 2019, 148: 92-101.
[34] Liu, N, Zhao G, Lyu G. Coupling square wave anodic stripping voltammetry with support vector regression to detect the concentration of lead in soil under the interference of copper accurately[J].2020,: 6792.
[35] Zhao G, Liu G. Interference effects of Cu(II) and Pb(II) on the stripping voltammetric detection of Cd(II): Improvement in the detection precision and interference correction[J]. Journal of Electrochemical Society, 2018, 165: H488-H495.
[36] Kitte A S, Li S P, Nsabimana A, et al. Stainless steel electrode for simultaneous stripping analysis of Cd(II), Pb(II), Cu(II) and Hg(II)[J]. Talanta, 2019, 191: 485-490.
[37] Xiao L L, Xu H B, Zhou S H, et al. Simultaneous detection of Cd(II) and Pb(II) by differential pulse anodic stripping voltammetry at a nitrogen-doped microporous carbon/Nafion/bismuth-film electrode[J]. Electrochimica Acta, 2014, 143: 143-151.
[38] Zhang X, Zhang Y, Ding D, et al. On-site determination of Pb2+and Cd2+in seawater by double stripping voltammetry with bismuth-modified working electrodes[J]. Microchemical Journal, 2016, 126: 280-286.
[39] Rashid O K, Ibtisam E T. Development of disposable bulk-modified screen-printed electrode based on bismuth oxide for stripping chronopotentiometric analysis of lead (II) and cadmium (II) in soil and water samples[J]. Analytica chimica acta, 2008, 623(1): 76-81.
[40] Christos K, Anastasios E, Ioannis R. Lithographically fabricated disposable bismuth-film electrodes for the trace determination of Pb (II) and Cd (II) by anodic stripping voltammetry[J]. Electrochimica Acta, 2008, 53(16): 5294-5299.
[41] Zhao G, Liu G. A portable electrochemical system for the on-site detection of heavy metals in farmland soil based on electrochemical sensors[J]. IEEE Sensors Journal, 2018, 18(14): 5645-5655.
Detection of lead and cadmium in soil using composite nanomaterials modified screen-printed electrode
Liu Ning1,2, Zhao Guo3, Wang Xuming2, Liu Gang1,2※
(1. Key Laboratory of Modern Precision Agriculture System Integration Research, Ministry of Education, China Agricultural University, Beijing 100083, China; 2. Key Laboratory of Agricultural Information Acquisition Technology, Ministry of Agriculture and Rural Affairs, China Agricultural University, Beijing 100083, China; 3. College of Artificial Intelligence, Nanjing Agricultural University, Nanjing 210031, China)
Lead Pb (Ⅱ) and cadmium Cd (Ⅱ) are toxic heavy metals, particularly difficult to be biodegraded in soil. Furthermore, lead and cadmium at trace levels can cause serious damage to brains, kidneys, blood, nerves, and other organs. A large amount of Pb (Ⅱ) and Cd (Ⅱ) can also be deposited in the soil environment after anthropogenic improper activities, such as sewage irrigation, the abuse of chemical fertilizers and pesticides, as well as the excessive discharge of industrial wastes. Pb (Ⅱ) and Cd (Ⅱ) can be absorbed by crops, and then accumulated in animals, finally enriched thousands of times into the human body under the biomagnification of the food chain, thereby causing economic losses and a great threat to human health. Therefore, it is highly urgent to accurately detect the accumulation of Pb (Ⅱ) and Cd (Ⅱ) in soil. An electrochemical Square Wave Anodic Stripping Voltammetry (SWASV) is widely utilized to combine with chemically modified working electrodes for the detection of heavy metal ions. Screen-Printed Electrodes (SPEs) have also been commonly used in recent years, due to easy preparation, disposable capability, and low cost. Particularly, SPEs with a small size are quite qualified as the sensing device ofminiature electrochemical detection equipment, suitable for many detection scenarios, such as flow cells and microfluidics. In this study, electrochemical reduction and coating were applied to fabricate a modified SPE (Bi/Nafion/rGO/IL/SPE) with the bismuth film/Nafion/reduced graphene oxide/ionic liquid composite nanomaterials, to accurately, fast, and reliably detect trace Pb (Ⅱ) and Cd (Ⅱ) in soil with a low-cost. Cyclic Voltammetry (CV) was also selected to characterize the electron transport capability of the modified electrodes. It was found that the composite nanomaterials greatly improved the electron transport capability of SPE and the stripping voltammetry responses for Pb (Ⅱ) and Cd (Ⅱ) on the SPE. Moreover, Scanning Electron Microscopy (SEM) was utilized to characterize the morphology of the modified electrodes. It was found that the rGO greatly enhanced the specific surface area of bare SPEs, thereby obtaining much more active sites for the electro-deposition of heavy metal ions. Energy Dispersive Spectroscopy (EDS) was used to identify the deposition amount of heavy metal ions on the surface of different modified electrodes. The results demonstrated that the modification with the bismuth film, rGO, and Nafion gradually increased the deposition amount of Pb (Ⅱ) and Cd(Ⅱ) on the modified SPE surface. Additionally, the Pb(Ⅱ) and Cd(Ⅱ) standard solutions were selected to optimize the experimental parameters, including the pH value of support electrolyte, bismuth ions concentration, deposition potential, and potential time. There were linear responses of Bi/Nafion/rGO/IL/SPE to Pb(Ⅱ) and Cd(Ⅱ) in the concentration from 1 to 80g/L, with the critical values of 0.124g/L for Pb(Ⅱ) and 0.232g/L for Cd(Ⅱ) (S/N=3), under an optimal experimental condition. The determination coefficients (2) of linear correction models were 0.993 and 0.985 for Pb(Ⅱ) and Cd(Ⅱ), respectively. In six SWASV measurements, the relative standard deviation (RSD) of Pb(Ⅱ) and Cd(Ⅱ) peak currents were 1.57% and 2.32%, respectively, indicating high stability and repeatability of Bi/Nafion/rGO/IL/SPE. Other heavy metal ions were also added to investigate the anti-interference performance of modified electrodes. It was found that there was no serious interference of other heavy metal ions (except for Cu(Ⅱ)) on the voltammetry responses of Pb(Ⅱ) and Cd(Ⅱ), where the changes of peak currents were all less than 5%. Since Cu(Ⅱ) inhibited the Pb(Ⅱ) and Cd(Ⅱ) peak currents by 40.02% and 62.85%, respectively, the Cu2+interference could be alleviated by adding ferricyanide. Finally, actual soil samples under the standard addition were used to verify the practicability of modified SPEs. Results demonstrated that the average recovery rates of Pb(Ⅱ) and Cd(Ⅱ) were 98.71% and 98.93%, respectively, indicating that the Bi/Nafion/rGO/IL/SPE can be applied to detect the trace lead and cadmium in soil.
heavy metals; soils; ionic liquid; graphene oxide; bismuth film; Nafion; screen-printed electrode
刘宁,赵国,王旭明,等. 复合纳米材料修饰丝网印刷电极检测土壤中铅和镉[J]. 农业工程学报,2021,37(13):180-189.
10.11975/j.issn.1002-6819.2021.13.021 http://www.tcsae.org
Liu Ning, Zhao Guo, Wang Xuming, et al. Detection of lead and cadmium in soil using composite nanomaterials modified screen-printed electrode[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2021, 37(13): 180-189. (in Chinese with English abstract) doi:10.11975/j.issn.1002-6819.2021.13.021 http://www.tcsae.org
2021-04-06
2021-05-29
国家自然科学基金资助项目(32071898,32001411);中央高校基本科研业务费专项资金资助项目(2021TC111)
刘宁,博士生,研究方向为电化学分析和光谱分析。Email:ningliu@cau.edu.cn
刘刚,博士,教授,博士生导师,研究方向为电子信息技术在农业中应用。Email:pac@cau.edu.cn
10.11975/j.issn.1002-6819.2021.13.021
S24; O657.1
A
1002-6819(2021)-13-0180-10
