Simultaneous removal of copper and zinc ions by low cost natural snail shell/hydroxyapatite/chitosan composite
2021-08-26AbbasBambaeeroRezaBazarganLari
Abbas Bambaeero,Reza Bazargan-Lari
Department of Materials Science &Engineering,Marvdasht Branch,Islamic Azad University,Marvdasht,Iran
Keywords:Hydroxyapatite Chitosan Composite Adsorbent
ABSTRACT In this work,the snail shell/hydroxyapatite/chitosan composite was prepared as adsorbent.The adsorption potential of the composite was studied for simultaneous sorption behavior of Zn(II)and Cu(II)ions in a batch system.Chitosan and hydroxyapatite (HAP) were extracted from shrimp shell and bone ash,respectively,so this is a low cost natural composite.To prepare the composite,chitosan was dissolved in acetic acid,then HAP and snail shell powders were added to the chitosan solution.The morphology and characterization of the composite was studied by SEM and EDX analysis.Atomic adsorption was used to measure the amount of the ions.Experimental parameters were optimized with Design Expert Software and five parameters such as the concentration of ions,pH,adsorbent amount and contact time were studied at room temperature.Optimized value for the parameters of Zn (II) and Cu (II) concentrations,pH,adsorbent dose,and contact time were 3.01 mg∙L-1,5.5,0.02 g and 95 min,respectively.The adsorption isotherms for Zn (II) and Cu (II) showed Langmuir and Tempkin,respectively.Kinetic and equilibrium studies showed the experimental data of Zn (II) and Cu (II) ions were best described by the pseudo-second-order model.Studies on thermodynamic show the adsorption process were physical and spontaneous.
1.Introduction
In the past decade,the spreading of pollution resources such as heavy metals into the ecosystem has been significantly increased[1].Heavy metals are released into the environment on a large scale from different industries such as wastewaters,electroplating and electro winning factories,incinerators and casting industries[2–4].One of the most important issues in association with heavy metals is the lack of its metabolism in the human body.When heavy metals enter into the body they cannot excrete but accumulate in tissues such as fat,muscles,bones,and joints which can cause numerous diseases and complications in the body [5–7].Generally,neurological disorders (Parkinson’s,Alzheimer’s,depression,schizophrenia)[8],types of cancer[9],hormone imbalance,abortion,obesity,allergies and asthma,infertility,weakness of immune system,anorexia,hair loss and in acute cases death is a result of the effects of heavy metals in the human body[10–13].Copper and zinc are some of the most consumed metals in different industries such as electroplating [14,15],castings and synthetic dyes [16].The excessive amounts of them in the biological system of the human body cause various diseases and toxicity.In this way,different approaches and methods such as chemical removal,ion exchange,adsorption,reverse-osmosis,electrochemical oxidation and reduction,electrodialysis and solvent extraction have been reported in the literature to remove metals from wastewater and drinking water[17–20].The absorption process is a low cost,high removal efficiency,and simple procedure to reduce the percentage of hazardous metals in wastewaters.
Recently,the production of biopolymers is considered as a powerful tool for the removal of pollutants and metals due to their lack of toxicity in the environment and their availability.Chitosan is widely used as a sorbent for the removal of heavy metals [21–24].Chitosan is a hydrophilic and cationic polymer that is derived from the removal of acetyl chitin moiety in basic media.This adsorbent has an excellent performance in removing cations from neutral environments due to the presence of hydroxyl and amine groups in its structure[25–29].It is easily extractable from various animal and plant sources including crab and snail shell and the cell wall of some fungi and algae.Furthermore,HAP with general formula Ca10(PO4)6(OH)2is an availabile and low-cost adsorbent to remove metals with the high-efficiency removal [30–33].HAP has been used as an adsorbent in the removal of Sr,Zn,Co,Cd ions in various environments,including electrolyte solutions [34–37].Recent studies show that natural and low-cost material such as rice straw,palm tree leaves and shrimp shell are efficient adsorbents for the removal of heavy metal ions from aqueous solution[31,38,39].In this manuscript,the composite was developed as an adsorbent for the simultaneous removal of copper and zinc ions.For this purpose,the effective parameters such as temperature,pH were investigated and optimum conditions were obtained for thermodynamic and kinetic experiments.After the characterization of the surface by SEM analysis,EDX analysis,Adsorption isotherms,the kinetic and thermodynamic investigation was performed for removal of metal ions(Zn and Cu)by the adsorbent.Notable characteristics of adsorbents,were used,they were all waste,low-cost and natural.
2.Materials and Methods
2.1.Adsorbent preparation
2.1.1.Preparing of the snail shell
The shell of snails was washed several times with water to remove dirt and any other impurities.After that,they were dried at 105°C in the oven for 24 h.The dried snail shells were calcined for 4 h in a furnace at 1000 °C then used in the adsorption composite.
2.1.2.Preparing of HAP
HAP powder was extracted from the bovine cortical bone.In following,removing the spongy bones,de-fleshing the cortical bone,and cleaning the bone marrow and all pieces of meat and fat were performed in this approach.A gas torch was applied in order to burn the organic components of the bone by a direct flame.This thermal process generated some chars as a result of burning the organic components to remove the remaining chars,the black powder was placed in a furnace at 800 °C for 3 h.in following,the black bone ash changed to a white granular powder.In this method,270 g of HAP was extracted from 500 g bovine cortical bone [40,41].The Ca/P ratio of this HAP various between 1.46 and 2.01.
2.1.3.Preparing of the chitosan
Initially,the shell of shrimp was prepared and then was placed in the sun for 2–3 days for drying.It was then placed in 7% HCl solution for 24 h and immerse in 10% NaOH for 24 h solution to remove the minerals and proteins,respectively.In the following,the process of deacetylation of chitosan was carried out in a 40%solution of NaOH for 5 h at 100–120 °C.The resulting chitosan was separated from the sodium hydroxide solution by filtration then washed with distilled water until attaining a neutral pH,dried at room temperature [41,42].
2.1.4.Synthesis of the composite
After the preparation of chitosan,1 g of chitosan is dissolved in 100 ml 2% acetic acid and sonicated for one hour to remove the bubbles.HAP (1 g HAP suspended in 25 ml of distilled water)was added to Chitosan at a ratio of(1:1)and a ratio of(1:0.5)snail shell powders added to chitosan and was stirred for 48 h at room temperature and finally filtered and dried at 80 °C.
2.2.Apparatus
The pH measurements were carried out using pH meter(Metrohm,model 827,Switzerland,Swiss).An ultrasonic bath with the heating system (model LBS2) at 40 KHz of frequency and 285 W of power was used for the ultrasound-assisted adsorption procedure.Atomic absorption spectroscopy was measured using a Perkin-Elmer AA800 using a hollow cathode ion-cathode lamp at the desired wavelength in the acetylene-air flame.All of the chemical reagents used were of analytical grade and were obtained from Merck.The concentration of the samples was determined with the drawing of the standard diagram.
2.3.Optimization of experimental parameters on the removal of copper and zinc ions
The design of the experiment consists of a test or a series of tests that intentionally cause a change in the process of input variables to observe and identify the amount of variation in the output response of the process [43].This process can be considered as a combination of devices and methods that convert inputs (variables) to output product.This output has one or more quality features with visible responses.Some process variables are controllable and others are uncontrollable(although they can also be controllable under test conditions).In order to minimize the impact of uncontrolled factors on the results of the experiments to the minimum in the design of the experiment,three principles should be taken into consideration so that the effect of effective parameters with uncontrollable and unpredictable parameters are not mistaken [44].In other words,the effect of the effective parameters on the desired response is not affected by the uncontrollable parameters.The above principles are repetition,randomization and categorization.
We used the experiment design method for the following reasons:
1.Reducing the number of tests,costs,time and performed work comparing to the one at a time method.
2.Ability to simultaneously investigate and measure the sensitivity of several factors.
Statistical methods were used to analyze the results of experimental design.In this work,we investigated five factors of copper metal concentration,zinc metal concentration,pH,adsorbent amount and contact time using the experimental design method.The equation that is known and conventional in the surface response methods is a polynomial containing three different sentence types or terms and a constant value[45].These polynomials include the main factors as linearly(first and second-order model),and the interaction between the two parameters with each other plus a constant value.Polynomials are written as the below equation:

In this equation,Y is the desired response,b0constant coefficient,biithe coefficient of the second order and bijis the coefficient of interaction.Xiand Xjare coded values.The above equation is processed for design and investigation and the effective and ineffective factors are usually performed using software developed for the design of the experiment.Statistical tests are used to determine meaningful and non-meaningful sentences.To evaluate the reliability of the correlation coefficient (R2),P value (95% probability level),the Fisher test (F-test) is used [46].Tables 1 and 2 show the analysis obtained from the experimental design for copper and zinc metals,respectively,Coefficients with a small p value are significantly greater than zero.Here p <0.05 means significant at α=0.05 level and corresponds to 95% confidence level.If the p-value for Lack-of-Fit exceeds the value chosen to be significant with the specified confidence level,it indicates that the model is desirable.Therefore,if the p value for Lack-of-Fit is greater than this,this model is appropriate.

Table 1Experimental factors and levels in the central composite design

Table 2Experimental conditions and values obtained through the CCD

Table 3Analysis of variance (ANOVA) for CCD.
For optimization of the process,Cu concentration,Zn concentration,time,adsorbent amount and pH were used as independent and input variables of RSM which values are presented in Table 1.The range of changes in these parameters were as follows:copper concentration:1–9 mg∙L-1,zinc concentration:1–9 mg∙L-1,solution pH:4–6(because of the copper ions precipitation,the pH values above 6 were not studied.),adsorbent used:0.01–0.03 g,test time:20–120 min.
The percentage of simultaneous removal of Cu and Zn ions by the composite adsorbents depends on the changes in the studied parameter,so this quantity is called the dependent variable,which is given to the software as a response,to analyze,model and optimize.Based on the number of factors,26 experiments were designed according to the range of variations of each parameter.Table 2 shows the experiments for the simultaneous removal of Cu and Zn ions by the composite adsorbents and proposed by the software in accordance with the range of parameter changes.It should be noted that in order to increase the software repeatability,it has proposed a number of iterative tests.
3.Results and Discussion
3.1.Central composite design
By using central composite design(CCD),the effects of variables such as pH,adsorbent dosage,Cu(II)concentration,Zn(II)concentration and contact time on binary ions removal were examined and optimized values were found to be 5.5,0.02 g,3.01 mg∙L-1,3.01 mg∙L-1and 95 min,respectively.The design model shows the amount of copper adsorbent is greater than zinc.
On the other,Table 3 can show the analysis of variance of the data obtained.According to previous researchers,if the probe value>F was less than 0.05 for the input parameters,their interaction and prediction model,subsequently,model and parameters are in good agreement.However,when this value was less than 0.05 for the non-fitting parameter,then for the latter parameter,Probe >F should be greater or better than 0.05.The point to notable is if the value of Probe >F is in the range of 0.05–0.1,the expression under consideration is significant.The results in Table 3 can show the implementation of this software model is desirable due to the smaller p-value of 0.05 and reports significant information for presentation.Also,the lack of fit factor can be justified and meaningful since it is greater than 0.05.The p-value for Lack-of-fit in this design is for Cu (II) and Zn (II) indicating that the model is suitable.Table 3.Make it possible to obtain the following equation for Cu (II) ions and Zn (II) ions,respectively.
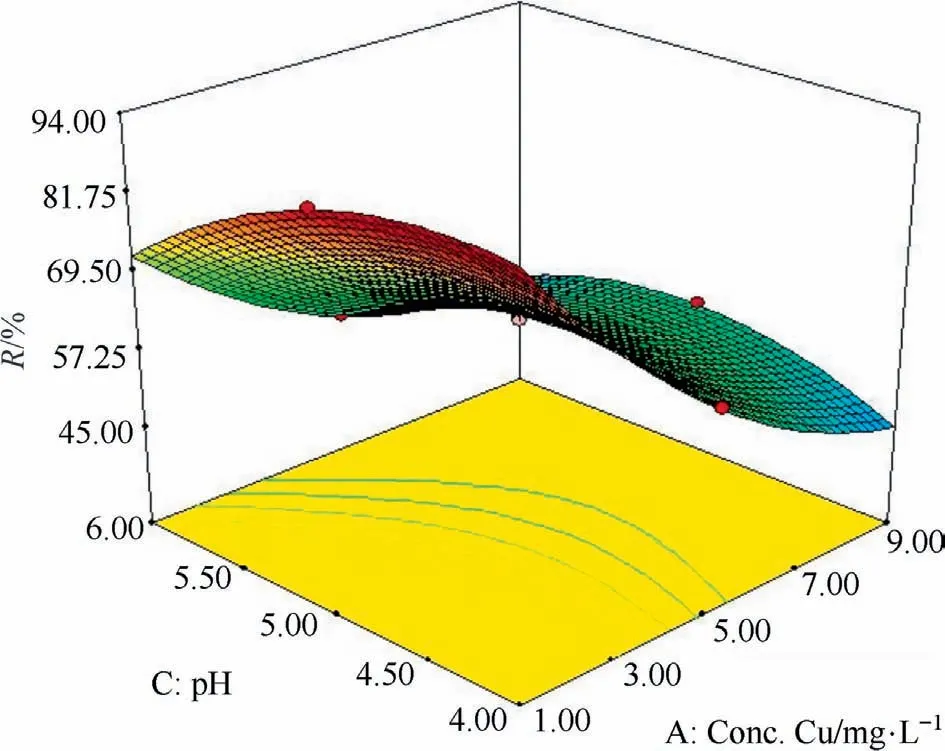
Fig.1.The response surface plots of adsorbent for the Cu(II) removal (%) for Cu concentration and pH.

Fig.2.The response surface plots of adsorbent for the Cu(II) removal (%) for Zn(II)concentration and pH.

Fig.3.The response surface plots of adsorbent for the Cu(II)removal(%)for amount of adsorbent and pH.
The results were analyzed by regression analysis of centeral composite design in Table 3,And make it possible to obtain the followin equation for Cu and Zn ions:

Table 4Isotherm constant parameters and correlation coefficients calculated for the 0.02 g adsorption Cu2+ and Zn2+on the composite adsorbent.(0.02 g of adsorbent,1–10 mg∙L-1 for Cu2+ to Zn2+ concentration ratio at pH=5.5 and Time=95 min and V=50 ml)
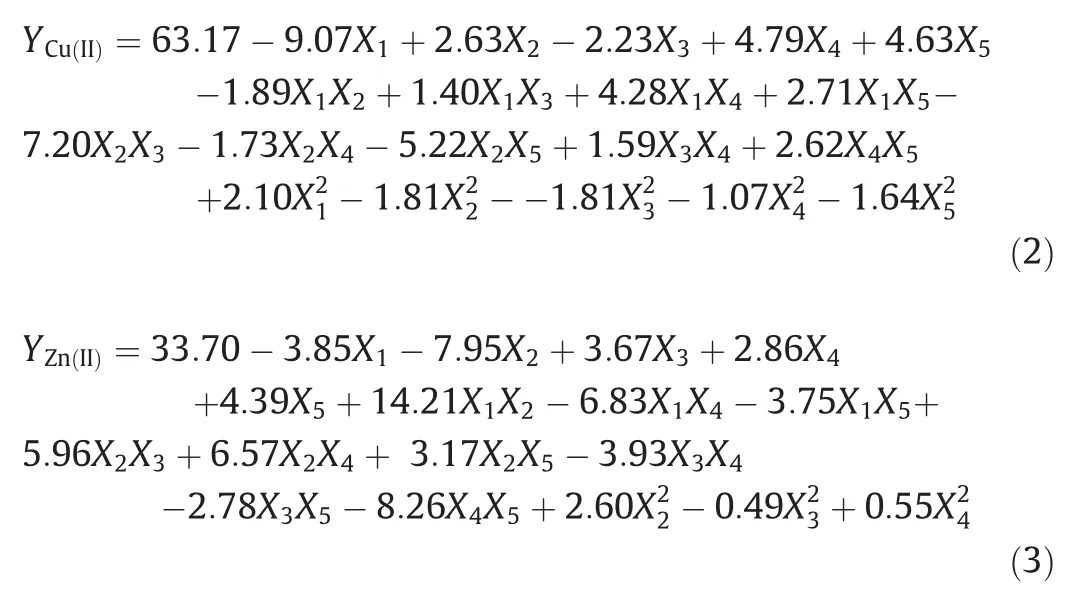
The successful solution of above equations according to the desirability function (DF) makes it possible to achieve an experimental equation that is able to interpret the behavior of the proposed method for simultaneous removal of Cu and Zn ions in aqueous sample with least experiments and high accuracy andrepeatability.
In the next section,the simultaneous effect of parameters on the rate of simultaneous sorption of Cu and Zn ions was investigated by the composite(Figs.1 and 2).The following figure shows the concomitant effect of copper concentrations and soluble pH on the percentage of copper removal by the adsorbent.The results indicate that low concentrations of copper and an increase in solution pH will increase the amount of copper ion removal and adsorption.The higher the concentration of copper ion presented in the solution,the lower the percentage removal under the same conditions and at a constant time.

Fig.4.Three types of adsorption isotherms for Zn2+ and Cu2+ on the composite.
The simultaneous effect of the pH of the solution and the presence of zinc ions on the rate of removal of copper ions by the adsorbents were investigated.The results show that increasing the concentration of zinc ion in solution will slightly decrease the percentage of copper ion removal.As the adsorption capacity is occupied by zinc ions,the observed trend is predictable.
To determine the effect of adsorbent on the removal rate of the graph below,the software was extracted.As can be seen (Fig.3),increasing the amount of adsorbent will increase the removal efficiency of the copper ion in the solution.The maximum amount of Cu ion removal was obtained at 0.030 g of chitosan adsorbent at pH 5.
3.2.Adsorption isotherms
The relationship between ions concentration in the desired media and the number of adsorbed metals (Cu2+and Zn2+) are determinate with adsorption isotherms.Based on the extracted information from the surface features and adsorption mechanism,some adsorption isotherm such as Langmuir Freundlich,and Tempkin isotherm are available to the investigation of characteristics of the adsorption process [47–52].The isotherm equation of these mechanisms is expressed in Table 4.The Langmuir parameters are the energy of adsorption [Ka(L∙mg-1)] and the maximum metal adsorption[Qm(mg∙g-1)].For Freundlich isotherm,the most important parameters are adsorption intensity (n) and adsorption capacity [KF(L∙g-1)],also,B1and KT(L∙mg-1) are Tempkin parameters and related to the heat of adsorption and the equilibrium binding,respectively.These parameters for adsorption of Cu2+and Zn2+are reported in Table 4.

Table 5Kinetic parameters for the adsorption of Cu2+ and Zn2+ on the composite.Kinetic parameters for the adsorption of Cu2+and Zn2+on the composite.(0.02 g of adsorbent,3 and 3 mg∙L-1 for Cu2+ to Zn2+ concentration ratio at pH=5.5 and 5–220 min and V=50 ml)

Fig.5.The SEM images of (a),(b) the composite adsorbents and (c),(d) adsorbent after adsorption.

Fig.6.The EDX analysis of the adsorbent system.
To calculate and obtain the adsorption parameters,the graphs of these isotherms were plotted and the parameters were calculated by using the slope and intercepts.Based on correlation coefficients(R2),the adsorption of Cu2+and Zn2+on the composite surface follow according to this order:Langmuir

Fig.7.Quantitative results of the adsorbent.

Fig.8.The EDX analysis of the adsorbent after the adsorption.
3.3.Characterization of the sorbents
Scanning electronic microscopy (SEM) and energy dispersive analysis of X-ray (EDX) were used to investigate about the morphology of the composite surface during simultaneousremoval of Zn(II) and Cu(II) (Fig.5).The SEM results indicate that the composite surface has many pores which significate change proportion to the composite component,So the composite surface has a high adsorption potential for removal of these ions.
Furthermore,the major constituents in the composite surface are determine by EDX analysis (Figure 6,7,8 and 9).The results indicate that the major element is Ca (21.04%),C (19.22%),O(36.09%)P(8.70%),Zn(0.48%)and Cu(0.63%)in the adsorbent system.The remarkable point is the adsorption of the desired metals(Zn and Cu) on the composite surface which indicates the good performance of the adsorbent.

Fig.9.Quantitative results of the adsorbent after the adsorption.

Fig.10.Kinetic adsorption results of Zn2+ and Cu2+ on the composite surface.

Fig.11.Effect of time on the performance of Zn2+ and Cu2+ adsorption on the composite surface.
3.4.Adsorption kinetics
Based on literature,the study of sorption kinetics can provide important information about the adsorption mechanism of heavy metals on natural adsorbents [53–57].In this work,four models such as pseudo-first-order,second-order,Elovich and Intraparticle diffusion were examined for the adsorption of Zn2+and Cu2+on the composite surface in room temperature.The result in Table 5 indicates that the correlation coefficients(R2) base on pseudo-secondorder kinetic is near 1 for both Zn2+and Cu2+.Therefore,the adsorption mechanism follows the pseudo-second-order model.The details of these investigations are 0.02 g of adsorbent,3 and 3 mg∙L-1for Cu2+to Zn2+concentration ratio at pH=5.5 and 5–220 min and V=50 ml.

Table 6Thermodynamic parameters for adsorption of Cu2+and Zn2+.(0.02 g of adsorbent,3 and 3 mg∙L-1 for Cu2+to Zn2+concentration ratio at pH=5.5 and Time=95 min and V=50 ml)
The plots of kinetic models for Cu2+to Zn2+is shown in Fig.10.The best conditions are obtained for higher correlation coefficients.The maximum values of R2are obtained for the pseudo-secondorder model and the results are 0.999 and 0.995 for Zn2+and Cu2+,respectively.

Fig.12.Thermodynamic evaluation of Zn2+ and Cu2+ adsorption process on the composite surface.
3.5.Effect of contact time
The removal percentages of Cu2+to Zn2+on the composite surface were explored as a function of contact time in at initial concentrations of 3 mg∙L-1,adsorbent of 0.02 g∙L-1,pH of 5.5,and temperature of 25 °C is illustrated in Fig.11.As has been shown in the early metal ion adsorption process,the adsorption percentage increased dramatically and reached equilibrium after a certain time.The equilibrium time for adsorption of Zn2+is lower than Cu2+and the removal percentages for Zn2+and Cu2+on the adsorbent composite surface are 90% and 60%,respectively.
3.6.Thermodynamic treatment of the sorption process
Thermodynamic parameters are one of the most important parameters for predicting and comparing the facilitation of the adsorption process of metal ions on the desired surface (Fig.12).The intercept and slope of the diagram of lg(qe/Ce) versus 1/T indicated the Gibbs free energy G°,enthalpy H°and the entropy of the adsorption S°.The more negative values of ΔG°confirmed the feasibility and spontaneous process of the metal sorption by the synthesized composite (Table 6).The result of thermodynamic investigation indicated that the negative values of Gibb’s free energy change(ΔG°<0)of the process was spontaneous.The negative value of enthalpy change(ΔH°<0)indicated that the process was exothermic.
4.Conclusions
Heavy metals such as zinc and copper pollute wastewater but they could be potentially purified by biosorption.In this work,chitosan can be easily modified by physical and chemical reactions by using HAP and snail shell,this is done for the purpose of fabricate desirable composite with good sorption capacity for removal of metal ions.This composite can be used in wastewater filtration.Several isotherm models were investigated to explain the experimental data and their parameters,and also correlation coefficients were determined.Langmuir and Tempkin model show the proper agreement with the experimental data of Zn(II)and Cu(II),respectively.Conventional kinetic models were utilized,and it seems that pseudo-second order equation is proper for fitting the experimental data.It is shown that the pseudo-second-order model has proper fitting with the adsorption data for both ions.Based on our investigation the negative values of enthalpy change (ΔH°)and negative values of Gibbs free energy change (ΔG°) are indicated that the natures of metal ions sorption into adsorbent are feasible,endothermic and spontaneous.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Comparison of catalyst-coated membranes and catalyst-coated substrate for PEMFC membrane electrode assembly:A review
- Experimental investigation of different brines imbibition influences on co-and counter-current oil flows in carbonate reservoirs
- Experimental investigation on the effect of surface characterization of electrodes on the gas bubble dynamics in electrolyte flow and performance of FLA batteries by using PIV
- Mechanically activated starch magnetic microspheres for Cd(II)adsorption from aqueous solution
- Robust,fluorine-free and superhydrophobic composite melamine sponge modified with dual silanized SiO2 microspheres for oil–water separation
- Efficient adsorption to hexavalent chromium by iron oxalate modified D301:Characterization,performance and mechanisms
