Ionic liquid catalyzed solvent-free synthesis of chalcone and its derivatives under mild conditions
2021-08-26QiuZhaoGangWangFuxiaLiaoYifanShaFeiXuChunshanLiZengxiLiYijunCao
Qiu Zhao,Gang Wang,Fuxia Liao,Yifan Sha,Fei Xu,Chunshan Li,Zengxi Li,Yijun Cao,*
1 School of Chemical Engineering and Energy,Zhengzhou University,Zhengzhou 450001,China
2 Key Laboratory of Green Process and Engineering,State Key Laboratory of Multiphase Complex Systems,Beijing Key Laboratory of Ionic Liquids Clean Process,Institute of Process Engineering,Chinese Academy of Sciences,Beijing 100190,China
3 School of Chemical Sciences,University of Chinese Academy of Sciences,Beijing 100049,China
Keywords:Ionic liquid Chalcone Aldol reaction Kinetic model
ABSTRACT An ionic liquid(IL)catalyzed solvent-free process was developed for the direct synthesis of chalcone and its derivatives by using substituted acetophenones and benzaldehydes via aldol reaction under mild conditions.A series of acidic and basic ILs were selected and screened.The influences of cations and reaction conditions on product yield and selectivity were systematically investigated.The [Bmim]OH was identified as the optimal IL,with the highest yield and selectivity reaching up to 96.7%and 100%,respectively.A reaction mechanism-based kinetic model was established and regressed with experimental data,revealing the β-Hydroxylketone dehydrolysis with activation barrier of 37.8 kJ∙mol-1 was observed as the ratecontrolling step.
1.Introduction
Chalcone and its derivatives with relatively high drug activities are common natural products that can be directly extracted from ashitaba,tea,and ginkgo [1–3].These compounds are very important for human health due to their antitumor,anti-oxidation,oxygen radical elimination,antibiosis,and phosphodiesterase inhibition properties.However,natural plants contain relatively low amounts of these substances,resulting in the requirement for a large amount of organic solvent for plant extraction.Therefore,developing a simple,economic,and environmentally friendly synthetic method for these chemicals from available stocks is necessary [4–11].
Chalcones are traditionally synthesized from benzaldehyde and acetophenone via aldol condensation catalyzed by inorganic base of sodium hydroxide,potassium hydroxide or alkali alcoholate[12];dry HCl [13];Lewis acids [14,15];and p-toluenesulfonic acid[16].With the increasing public concern on environmental protection and future resources,developing new synthetic route for chalcone and its derivatives is of great significance.The earliest method is the use of benzaldehyde and acetophenone as raw materials in alkali solution.The process is simple,but the yield is as low as 10%–70%[17].The major disadvantage of this traditional method is the long reaction time.Sebti proceeded the reaction of benzaldehyde and acetophenone with forged sodium nitrate or lithium nitrate as catalyst in methanol solution at room temperature,and the yield could reach 70%–98% after 16–48 hours reaction [18].Kazemi designed a series of transition metal catalysts for the synthesis of chalcones at 120 °C and the yield could reach up to 90%–97%[19].Yao used 4% NaOH solution as the catalyst for chalcone synthesis under mild conditions,but the reaction time was long,and the yield was very low[20].When the methanol and 10%NaOH solution were used as solvent and catalyst,respectively,only 65% yield was achieved after 4 h.Dang prepared chalcone and derivatives with the catalysis of NaOH solution at room temperature,the yield was onlyapproximately 50%[21].Park proposed alcohol-ketone condensation for the production of chalcone and its derivatives[22].However,noble Pd catalyst should be used with strong base under oxygen atmosphere and high temperature.Although the alkalicatalyzed route is simple,some problems are still encountered,including high equipment corrosion,product separation and catalyst recovery.Some other acid catalysts,such as H3BO4and H2SO4,can also be used for chalcone synthesis.Although H3BO4can be employed as a catalyst for the aldol condensation between 4-hydroxyacetophenone(or 2,4-dihydroxyacetophenone)and substituted benzaldehyde in the solvent of ethylene glycol,only 30%–54%yield can be achieved at 110–120°C after 8 h.By using acid catalysts,the reaction time is generally long,but the yield is relatively low,and the product is difficult to be separated.
Ionic liquids (ILs) have attracted great attention due to their special physicochemical properties,such as good stability,negligible vapor pressure,and unique dissolving abilities.As ecofriendly alternatives,ILs have been widely used as solvents and catalysts for chemical reactions.Green chemistry concept also provides a good and environmentally friendly method for organic synthesis under solvent-free conditions.And the application of functionalized ILs as catalysts in organic synthesis has recently become a popular issue [23–28].Acidic ILs,such as [BPy]HSO4and [Bmim]HSO4,are used as catalysts for chalcone synthesis because of their benign nature as solvents and ability to dissolve a wide range of compounds.However,the high cost,viscosity and problems related to the product separation,especially when the product is solid or has high boiling point,are some of the disadvantages[29–34].
In this study,chalcone and its derivatives were synthesized from substituted benzaldehydes and acetophenones by using IL as catalyst.The effects of cations and reaction conditions on the product yield and selectivity were investigated,and the reaction mechanism-based kinetic model was established.
2.Experimental
2.1.General information
Benzaldehyde (99.0%),acetophenone (99.0%),and octane(99.0%) were purchased from Alfa Aesar Chemical Co.,Ltd.1-Butyl-3-methylimidazolium bromide ([Bmim]Br,99.0%),potassium hydroxide (95.0%),potassium bromide (99.0%) were purchased from Shanghai Aladdin Biological Technology Co.,Ltd.Ionic liquids including 1-(3-aminopropyl)-3-methylimidazolium tetrafluoroborate ([H2N-C3mim]BF4),1-butyl-3-methylimidazolium bis((trifluoromethyl)sulfonyl)imide ([Bmim]TFSI),1-butyl-3-methylimidazolium tetrachloroferrate ([Bmim]FeCl4),1-butyl-3-methylimidazolium tetrachloroferrate ([Bmim]FeCl4),1-propyl-3-methylimidazolium hexafluorophosphate([Pmim]PF6),1-(3-aminopropyl)-3-methylimidazolium bis(trifluor omethyl-sulfonyl)imide ([H2N-C3mim]NTf2,1-butyl-3-methylimidazolium tetrafluoroborate ([Bmim]BF4),1-Butyl-3-methylimidazolium hydrogen sulfate ([Bmim]HSO4),1-butyl-3-methylimidazolium hexafluorophosphate ([Bmim]PF6),1-hexyl-3-methylimidazolium p-toluenesulfonate ([Hmim]TsO),1-hexyl-3-methylimidazolium hydrosulfate ([Hmim]HSO4),1-Butyl-3-methylimidazolium Dihydrogen phosphate ([Bmim]H2PO4),1-butyl-3-methylimidazolium tosylate ([Bmim]Ts),[18]crown-6 potassium bromide ([18-C-6K]Br) and [15]crown-5 sodium bromide ([15-C-5Na]Br) were purchased from Linzhou Ke Neng Co.Ltd.and Shanghai Cheng Jie Chemical Co.Ltd.All other chemicals and solvents such as methylene chloride (99.8%) were purchased from Xilong Chemical Co.,Ltd.All chemical compounds were used directly without further purification.
Product samples were quantitatively analyzed using GC–MS(QP-2020,SHIMADZU (HONG KONG LIMITED) equipped with Rtx-5MS (30 m,0.25 mm ID,0.25 μm) by comparing them with standard samples and MS information.The test conditions were as follows:carrier gas,He;temperature,40–250 °C with a heating rate of 20 °C∙min-1;detector temperature,250 °C;and injector temperature,200 °C.The concentration of each component was determined using octane as internal standard.
2.2.Preparation of [Cation]OH ILs
[Cation]OH ILs were prepared as previously reported with some modifications [35]. The preparation of 1-butyl-3-methylimidazolium hydroxide ([Bmim]OH) was presented as an example to illustrate the process.Solid potassium hydroxide(4.20 g,75 mmol) was added to a solution of [Bmim]Br (14.33 g,75 ml) in dry methylene chloride (50 ml),then the mixture was stirred vigorously at room temperature for 12 h.After that,the precipitated KBr was filtered off and the filtrate was evaporated to yield crude[Bmim]OH,a viscous liquid.The[Bmim]OH at approximately 85.6% yield was then washed with ether (3 × 20 ml) and dried at 80°C for 12 h in high vacuum to obtain the pure IL for later use.The basic IL was kept in an alcohol solution due to the chemical instability.While the crown ether complex cation ionic liquids of[18-C-6K][OH]and[15-C-5Na][OH]were prepared according to the method reported in literature [36].
2.3.Typical procedure and apparatus for synthesis reaction
All reactions for the synthesis of chalcone and derivatives were conducted in a round-bottomed flask equipped with magnetic stirrer.Reagents and catalyst were added into the flask,and the reaction mixture was kept at 20–40 °C in a temperature-controlled oil bath,as shown in Fig.1.Typically,benzaldehyde (2.60 g,2.5 ml),acetophenone (1.03 g,1 ml),and IL (0.25 g) were added into the flask,and the mixture was kept at 30 °C for 4 h.After the reaction was completed,the product samples were analyzed using GC–MS and GC with octane as an internal standard.
3.Results and Discussion
3.1.Screening of ILs
The synthesis of chalcone from benzaldehyde and acetophenone was selected as the model reaction for screening IL catalysts(Fig.2).
The use of an appropriate catalyst is an alternative and simple approach to obtain high yield and selectivity of chalcone.A series of ILs based on [imidazolium] and [phosphonium] cations countered with OH-,TFSI-,and TSO-anions were used as catalysts for chalcone synthesis under identical reaction condition,and the results are shown in Table 1.[Bmim]PF6and [Bmim]BF4,which are neutral ILs,show lower catalytic activity compared with the basic ILs (Table 1,entries 1,2,and 4).[Bmim]HSO4and[Bmim]H2PO4,with relatively strong acidity,are also inactive for the reaction (Table 1,entries 7 and 8).While basic IL tetrabutylphosphonium hydroxide (TBPOH)exhibits the highest catalytic activity with the yield of 80.2% and selectivity of 95.2% even at 30 °C,which is considerably higher than previously reported temperatures (>100 °C) [19–22].

Table 2The obtained parameters at different temperatures

Fig.1.Experimental apparatus for synthesis reactions.

Fig.2.Reaction pathway for the synthesis of chalcone.
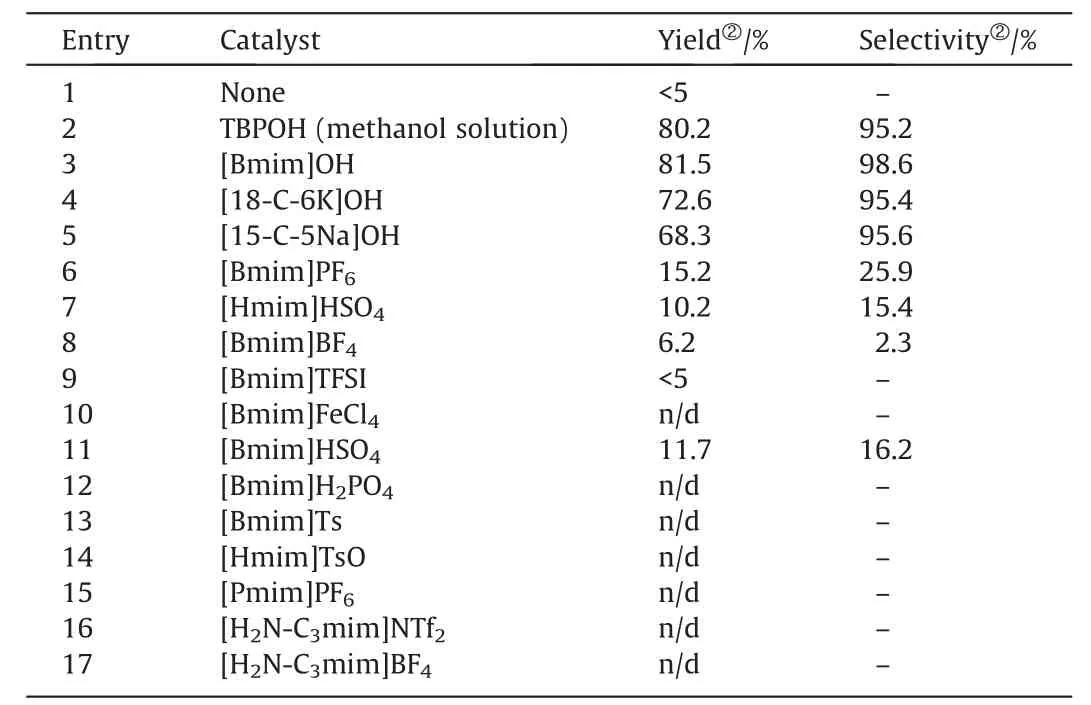
Table 1Catalytic activity screening of ILs for chalcone synthesis①
3.2.Effect of cations
Given the good performance of TBPOH for chalcone synthesis,various of basic ILs with hydroxyl anion,including [TBA]OH,[TEA]OH,[TMA]OH,[Emim]OH,[Bmim]OH,[18-C-6K] and[15-C-5Na] were prepared for this reaction.And the catalytic activity evaluation results are shown in Fig.3.The effect of cation on performance shows a decreasing order of[Emim] >[Bmim] >[TBP] >[TEA] >[TMA] >[TBA] >[15-C-5N a] >[18-C-6K],implying that the steric hindrance is an important factor in affecting the catalytic activity of ILs.Considering the economic and practical effects,[Bmim]OH is identified as the suitable catalyst for the reaction.
3.3.Optimization of reaction conditions
The effects of the molar ratio between benzaldehyde and acetophenone,catalyst amount,reaction time,and temperature on yield and selectivity of product were systematically investigated.The influence of the molar ratio of benzaldehyde to acetophenone on the catalytic performance is shown in Fig.4(a).When the molar ratio between benzaldehyde and acetophenone is increased from 0.5:1 to 3:1,the yield of chalcone can be enhanced from 58.7% to 85.4%with a selectivity of 100%.With further increase in the molar ratio from 3:1 to 5:1,both of the yield and selectivity become unchanged.Thus,the optimal molar ratio for this reaction is 3:1.The influence of catalyst amount on yield of chalcone is shown in Fig.4(b).When the concentration of [Bmim]OH is increased from 5 to 9 wt%,the yield tends to be stable with a selectivity of approximately 100%.Hence,the optimal catalyst amount is 5 wt%.The dependence of benzaldehyde conversion on reaction time is also studied.As shown in Fig.4(c),the selectivity of benzaldehyde increases rapidly in the initial stage and remained almost constant after 4 h.The highest yield of chalcone can reach as high as 86.7%after 4 h.Fig.4(d)shows the impact of reaction temperature,when the temperature is raised from 20 °C to 30 °C,it has a pronounced positive effect on both yield and selectivity of chalcone.However,the side reactions occur when the temperature is further increased,leading to the reduction in yield and selectivity.Thus,30 °C is the suitable reaction temperature.

Fig.3.Effect of cation on catalytic performance;reaction conditions:benzaldehyde:1 g,the molar ratio of acetophenone to benzaldehyde was 3:1,[Bmim]OH:5 wt%,reaction temperature:30 °C,reaction time:4 h.
As for the catalyst recovery,although it is relatively difficult to conduct due to the high sensitivity of basic IL to CO2,in our opinion,it is feasible to realize this process.As we know,the ionic liquid has no vapour pressure,so we can recover it through the distillation under reduced pressure.While we should be cautious about the distillation temperature and the CO2concentration,considering the thermal and chemical stability of ionic liquid catalyst during the process.
3.4.Mechanism and kinetic studies
The reaction pathway between benzaldehyde and acetophenone in the presence of [Bmim]OH is proposed based on the aldol reaction mechanistic study [36] and the experimental results of this catalytic system are shown in Fig.5.First,the α-H of acetophenone is abstracted by[Bmim]OH to form enol anionic intermediate B,followed by condensation with benzaldehyde to form D.D is highly reactive and easily protonates with water molecule to form β-hydroxyl ketone,which can be further dehydrated into chalcone[37–39].
The kinetic model was established on the basis of the derivation of the reaction mechanism.Given that the intermediate B reacts with H2O to generate acetophenone in an extremely rapid reaction,it can reach reversible equilibrium.Thus,CBcan be expressed by Eq.(1).

Considering that the intermediate D is reactive and the concentration is low in the reaction mixture,the instantaneous concentration can be considered as zero according to the steady-state approximation principle (Eq.(2)).

Fig.4.Optimization of reaction conditions;(a) effect of molar ratio of benzaldehyde to acetophenone,other conditions:[Bmim]OH:5 wt%,reaction temperature:30 °C,reaction time:4 h;(b)effect of catalyst amount,other conditions:reaction temperature:30°C,reaction time:4 h,molar ratio of benzaldehyde to acetophenone was 3:1;(c)effect of reaction time,other conditions:reaction temperature 30 °C,molar ratio of benzaldehyde to acetophenone was 3:1,[Bmim]OH:5 wt%;(d) effect of reaction temperature,other conditions:[Bmim]OH:5 wt%,molar ratio of benzaldehyde to acetophenone was 3:1.

Fig.5.Proposed reaction mechanism for the synthesis of chalcone from benzaldehyde and acetophenone.
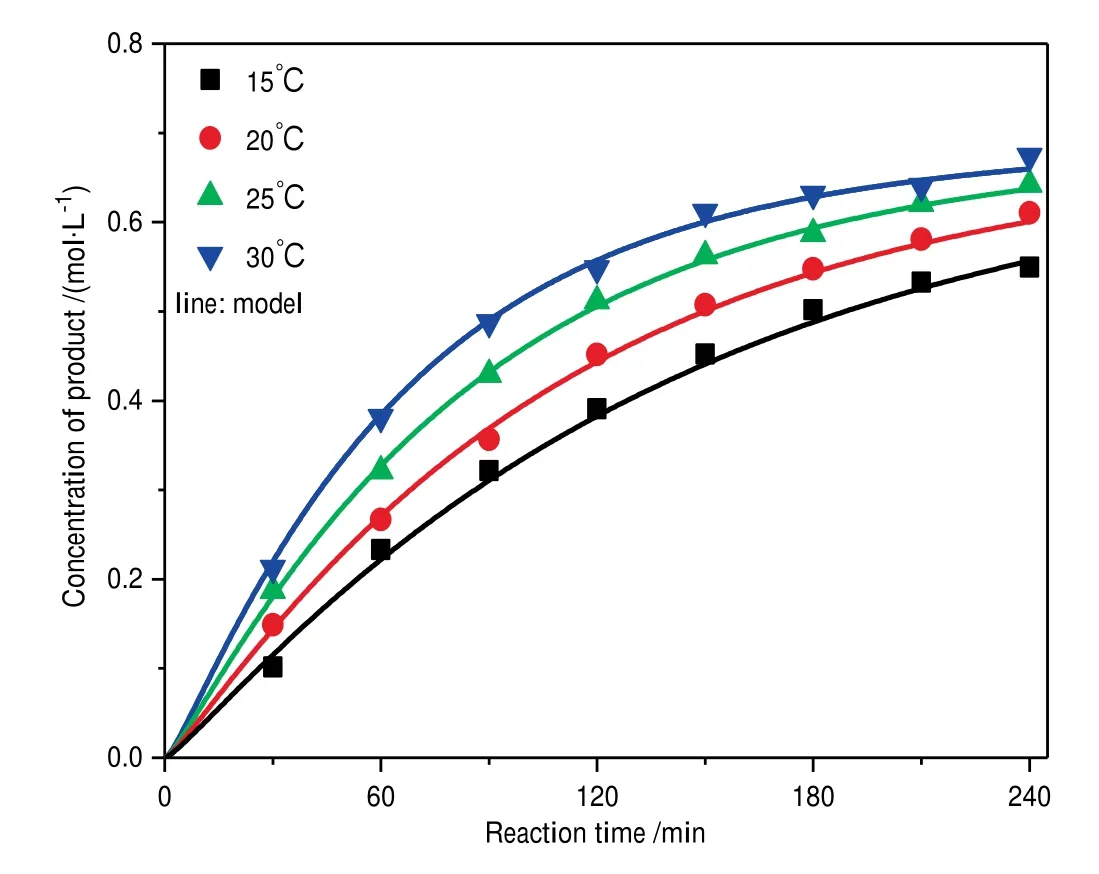
Fig.6.Comparison of the experimental concentration with computational simulation results of kinetic model.

Therefore,the concentration of substrate A and F can be expressed by Eqs.(4) and (5),respectively.

According to the above deduction,the kinetic equation of each substrate can be listed as Eqs.(6)–(8) based on the model.

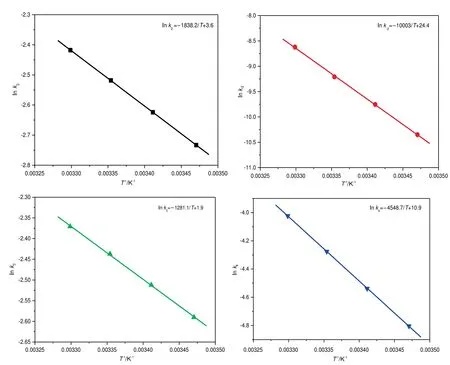
Fig.7.Arrhenius plot for the calculation of activation barrier of each reaction step.
Then the mechanism-based kinetic model was computationally simulated using the Runge-Kutta method on the MATLAB software,during which the temperature and concentration distribution under our reaction conditions were not considered.The obtained results were also compared with those for the experimental concentration used to determine the effects of reaction temperature and time as shown in Fig.6.The concentration of chalcone increases with the enhancement of reaction temperature and extension of time.The deviations between calculated data and experimental concentration are less than acceptable 5%,suggesting the established kinetic model is reliable.The obtained reaction rate constants and the equilibrium constants of the reversible reactions at different temperatures are listed in Table 2.The activation barrier and pre-exponential factor of each step can be calculated from Arrhenius plot,and the results are shown in Fig.7 and Table 3.For the equilibrium step,the activation barrier of the forward is lower than that of reverse reaction,which is advantageous for chalcone formation.By comparing these activation barriers,it can be observed that the dehydration is the rate-controlling step.Enthalpy change (ΔH) is one of the most important thermodynamic parameters of equilibrium reaction step,which can be calculated from the equilibrium constants at different temperatures using the van’t Hoff formula.The results shown in Fig.8 and Table 3 reveal this equilibrium reaction step is endothermic.

Table 3Pre-exponential factor,enthalpy and activation energy of each elementary reaction

Table 4Synthesis of chalcone derivates via aldol reaction with catalysis of [Bmim]OH①
3.5.Substrate extension
In addition to chalcone,other derivatives were synthesized from substituted benzaldehyde and acetophenone to test the universality of this IL-catalyzed solvent-free strategy.Experimental results in Table 4 show that the substitution of the electrondonating group in benzaldehyde or acetophenone is not beneficial for the product yield.However,when the methyl group is substituted in the ortho-position,the yield of related chalcone deriva-tives is higher than that in the para-position.While the electronwithdrawing groups can increase the yield,with the group on the para-position favoring the product yield the most.So it can be concluded that the distribution of electrons on the reactants will significantly affect the reaction activity and the yield of product can be improved by the electron-withdrawing effects.

Fig.8.van’t Hoff plot for the calculation of enthalpy change of equilibrium reaction step.
4.Conclusions
In this work,we developed a simple,green,solvent-free and efficient catalytic system that can effectively promote the conversion of benzaldehyde and acetophenone into chalcone and derivates via IL catalyzed aldol reaction under mild conditions.A series of functional ILs was screened and [Bmim]OH was determined as the optimal catalyst.The reaction conditions were optimized and the product yield could reached 86.7%–96.7% when the molar ratio of benzaldehyde to acetophenone was 3,the reaction temperature was 30 °C,the reaction time was 5 h,and the catalyst amount was 5 wt%.Dehydration with activation barrier of 37.8 kJ∙mol-1was determined as the rate-controlling step through the mechanism-based kinetic model simulation.Furthermore,this catalytic system can be extended for the synthesis of other chalcone derivatives with desirable yield ranging from 67% to 97%.
Acknowledgements
We thank the financial support of Major Program of National Natural Science Foundation of China (21890762),General Program of National Natural Science Foundation of China(21878293),National Natural Science Foundation of China(21676270),Key Research Program of Frontier Sciences,CAS(QYZDY-SSW-JSC011) and the K. C. Wong Education Foundation (GJTD-2018-04).
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Comparison of catalyst-coated membranes and catalyst-coated substrate for PEMFC membrane electrode assembly:A review
- Experimental investigation of different brines imbibition influences on co-and counter-current oil flows in carbonate reservoirs
- Experimental investigation on the effect of surface characterization of electrodes on the gas bubble dynamics in electrolyte flow and performance of FLA batteries by using PIV
- Mechanically activated starch magnetic microspheres for Cd(II)adsorption from aqueous solution
- Robust,fluorine-free and superhydrophobic composite melamine sponge modified with dual silanized SiO2 microspheres for oil–water separation
- Efficient adsorption to hexavalent chromium by iron oxalate modified D301:Characterization,performance and mechanisms
