Strong interfacial charge transfer between hausmannite manganese oxide and alumina for efficient photocatalysis
2021-08-26AishaKanwalShamailaSajjadSajjadAhmedKhanLeghariMuhammadNaeemKhan
Aisha Kanwal ,Shamaila Sajjad, *,Sajjad Ahmed Khan Leghari ,Muhammad Naeem Khan
1 International Islamic University,H-10 Islamabad,Pakistan
2 Pakistan Institute of Engineering and Applied Sciences,Islamabad 44000,Pakistan
3 Department of Chemistry,Government College of Science,Lahore,Pakistan
Keywords:Hausmannite Mn3O4 Alumina Band gap tuning Interfacial charge transfer Nanomaterials Catalysis
ABSTRACT Well crystalline manganese oxide (Mn3O4) nanoparticles anchored on gamma alumina (γ-Al2O3) have been successfully tailored via a proficient and cost effective chemical process as an efficient material for photo catalysis.XRD indicated the composite formation of γ-Al2O3 and hausmannite structure of Mn3O4.SEM and TEM revealed that hetero structure of Mn3O4/γ-Al2O3 exhibits an amalgam of aggregated nanoparticles and nanorods.XPS demonstrated the chemical states of binary nanocomposite.The band gap tuning has been performed with γ-Al2O3 nanoparticles by assimilating hausmannite Mn3O4 particles into flower like microstructure of Al2O3.The photoluminescence spectra affirmed the enhancement in charge separation in Mn3O4/γ-Al2O3 binary hybrid photocatalyst.The band gap becomes narrow with the increase in concentrations of Mn3O4.The narrowing of band gap is concorded with crystalline domains of primary aggregated particles.To elucidate the mechanism of the photocatalytic activity linear sweep voltammetry was performed.The results showed that Mn3O4/γ-Al2O3 nanocomposite revealed the enhancement in current density as compared to pure γ-Al2O3 which confirmed the electron transfer from Mn3O4 to γ-Al2O3 through the interfacial potential gradient in conduction bands.The optimum concentration of 6.0%Mn3O4/γ-Al2O3 for hybrid structure showed an excellent photocatalytic activity under visible light due to narrow band gap energy.High degree distribution of Mn3O4 nano architects overlying on γ-Al2O3 induces a significant synergic effect between γ-Al2O3 and hausmannite phase of manganese oxide(Mn3O4).This strong interfacial contact between γ-Al2O3 and Mn3O4 endures the quick transfer of photo generated charge carriers across interface.
1.Introduction
Ecological,health and natural issues emerging because of micro-organism and expeditious industrialization have proposed redundant contingency of illnesses and water defilements.In addition to substantial metal contaminants,different precarious contaminants found in the atmosphere are organic tints,release from the textile industries and other manufacturing process into water.These issues have compelled scientists to evolve new ecological friendly materials to deal with global health hazard and for the eradication of organic pollutants from water.Currently prime focus of researchers is to find a solution for clean and pure water polluted by tints [1,2].
Extensive work has been done on the expulsion of the contaminants from wastewater by coagulation,precipitation,adsorption,flocculation,filtration,oxidation,electrochemical process are conventional methods recorded for the expulsion of tints from effluents [3].But all these techniques have numerous disadvantages including high working cost and inadequacy to degrade dye completely.Nowadays photo catalysis is considered to be an extraordinary technique as compared to other strategies for removing dye from waste water due to the capability of the technique to totally mineralize the target contaminations.Eminent features of photo catalysis is the utility,high purification efficiency,low working temperature,recoverable heat and absence of optional contaminations and colossal oxidative intensity of OH-radicals,which are fundamental and major oxidative factor in the process [4–6].In photo catalysis,particularly heterogeneous catalytic incineration has been remunerated the most consideration due to its final disposal and energy saving process.Heterogeneous photo catalyst not only acquired acid-base or redox properties,but also have diverse pore structure.During the synthesis,these properties can be changed according to their applications [7].
Metals,non-metals and transition metal oxides have considered imperative materials due to their photonic,electronic,optical and catalytic properties [1,8].Among different techniques,extraordinary consideration has been paid to aluminium oxide (Al2O3)as a supportive catalyst in the field of photo catalysis because of photochemical inertness and thermal endurance.Alumina has an amphoteric character with high surface area,wide band gap,magnificent mechanical properties and substantial capability of Al2O3to make strong cooperation with transition metals [9,10].However,Al2O3is being researched as a prime photo catalyst because of its splendid properties of expanding the degree of dispersion of catalytically active constituents,hindering their grain development and hence enhancing the strength and activity of supported catalyst.
Additionally,the combination of transition metal oxide with Al2O3may result in modification in their thermal behavior,geometric structure and electronic properties.Because of insulating nature,alumina is utilized as energy barrier to lessen the recombination rate of e/h pair that prompt the change in catalytic activity[9–11].Recently significant endeavors have been dedicated to form cost effective synthetic route for tailoring Al2O3with different textural peculiarities because of its various applications [12].For instance,copious techniques are revealed to accomplish particular texture with high surface area,morphologies and controlled pore volume [13–16].
Manganese and its oxides belongs to class of economical compound with their high capacity for fabricating nanostructures which makes them captivating candidate for supercapacitor electrode,Li-ion batteries and catalysis.They exhibit high synergist activity for various reduction and oxidation reactions because of the assorted variety in their Mn+cation oxidation states [17].Among the series of manganese oxide,Hausmannite (Mn3O4) has drawn particularly colossal research attention due to its stability,excellent electro-chemical performance,environmental compatibility,natural abundance,distinctive structural attributes combine with fascinating physiochemical properties.Hausmannite(Mn3O4)seems to be a potential material for pseudo-capacitors and catalysis [18–20].
A plethora of papers and researches revealed that metals,nonmetals and transition metal oxides in coalesce with each other exhibit tremendous and marvelous enhancement in photocatalytic performance.Rekha et al.synthesized Al2O3and Mn3O4supported on Al2O3by precipitation impregnation method and observed catalytic performance which depend upon reaction parameter such as temperature and fabricated catalysts showed meritorious reusability[21].Asif et al.prepared wire shape Al2O3doped Mn3O4by low temperature stirring method and studied the photo deterioration of organic toxins under sunlight for eminent environmental applications.He reported that Al2O3doped Mn3O4as a catalyst showed high decomposition of cresyl blue under sunlight radiations in photo catalysis [8].Iqbal et al.prepared novel heterojunction of γ-Al2O3and GO as a potent photo catalyst under solar light.They found γ-Al2O3/GO showed a diverse spectral response and revealed lessen electron hole pair recombination probabilities because of inclusion of GO sheets [9].More categorically the activity of Mn3O4diffused in Al2O3and silica has been investigated in catalytic ozonation of acetone by Oyama et al.[22].Due to matching band potentials,when metal oxides are mixed,this results in improvement of charge carrier separation,protracted charge carrier life time and quick charge transfer takes place.So far variety of conjugated metal,non-metal and transition metal oxide systems including TiO2-Al2O3[23],AOMO [24],Pd-Mn3O4[25],WO3/Mn3O4/N doped Graphene [26],Guar Gum/Al2O3[27],Cu-Al2O3[28],CdO-Al2O3-NiO [10],ZrO2/Al2O3[29] and Fe3O4/Mn3O4[30]have been studied as efficient visible light photo catalysts.
To the best of our knowledge,the current study explores the influence of hausmannite(Mn3O4)on the role of γ-Al2O3in the fast photo degradation of MO for the first time.In recent research,we have fabricated Mn3O4/γ-Al2O3heterostructure with different concentration of Mn3O4vs.γ-Al2O3by wet impregnation method and evaluated the photocatalytic performance of the Mn3O4/γ-Al2O3samples under visible light irradiation.The composition,structural,morphological,optical and catalytic properties of Mn3O4/γ-Al2O3as compared to pristine Mn3O4and γ-Al2O3have been investigated comprehensively.Bulk γ-Al2O3has wide band gap of 7.0–9.5 eV.This report shed the light on the strategies which successfully tuned the band gap to remarkable value of 3.4 eV.Moreover,we illustrate the relationship between the transfer behavior of photo generated charges at the surface of heterostructure and interface among the Mn3O4and γ-Al2O3particles and photo degradation activity of an organic pollutant (MO).It is demonstrated that the formation of a hetero-interface Mn3O4/γ-Al2O3shows the meritorious photocatalytic performance as compared to single Mn3O4and γ-Al2O3due to the presence of Mn3O4,low band gap energy and strong interfacial contact between γ-Al2O3and Mn3O4enduring the quick transfer of photo created charge carriers across interface.
2.Experimental
2.1.Materials
Aluminum foil,poly ethylene glycol,sodium hydroxide pellets,manganese nitrate and methylene orange were used in experiment.All the chemicals were of analytical grade and procured from Sigma Aldrich.Deionized water was used as solvent throughout experiment.
2.2.Catalyst preparation
2.2.1.Preparation of aluminum oxide
Aluminum foil was used as a precursor for the synthesis of Al2O3nanoparticles by following sol–gel method.6.0 g Al foil and 5.0 g sodium hydroxide(NaOH)was deliquesce into 150 ml of double distilled water having pH nearly equal to 14 at room temperature under the continuous robust stirring till the Al foil was completely dissolved into solvent.Another solution of 0.5 g polyethylene glycol (PEG) were deliquesced in 20 ml double distilled water to make a solution.The suspension was kept at room temperature and stirred it for 30 min.Now this solution was inculcated to first solution dropwise at 35–40 °C under vigorous stirring for 150 min.The prepared solution was left for 48 h at room temperature.After being aged,the greyish precipitates were settled down in beaker.The precipitates were washed with deionized water 10–12 times through centrifugation to discharge the excess impurities from solution.The washed precipitates were finally dried at 50 °C for 5 h in drying oven to achieve the powder sample.As prepared sample was calcined at 800 °C for 4 h and finally characterized by various techniques.
2.2.2.Preparation of hausmannite Mn3O4
In order to synthesize Hausmannite(Mn3O4),solvothermal process was adopted.Initially 8.9 g of manganese nitrate was mixed in 70 ml of deionized water with continuous stirring for 30 min to get homogenized solution.Second solution was prepared by adding 4.7 g NaOH in distilled water under robust stirring for 30 min.Subsequently,second solution was dropwise added into first solution.The mixed solution was stirred for 90 min.Finally,chocolate brown precipitates were extracted from the solution by centrifugation.Several washing with deionized water were carried to remove impurities and to adjust the pH level.The prepared specimen was dried in a drying oven at 40 °C for 5 h followed by the calcination for 400 °C 3 h.
2.2.3.Mn3O4/Al2O3 composite fabrication
Samples with 3.0%,6.0% and 9.0% Mn3O4were tailored via wet chemical impregnation method.Aluminum oxide powder (Al2O3)and Mn3O4were dissolved in deionized water and stirred for about 4 h.The synthesized samples were then marked as x% Mn3O4/Al2O3,where x% refers to Mn3O4mass ratios.
2.2.4.Possible growth mechanism for nanocomposite fabrication
The fabrication of Mn3O4/γ-Al2O3nanocomposite may be manifested by following chemical reactions;
2.3.Catalyst characterization
The crystalline nature,structure and phase of prepared samples(pure Mn3O4,pure Al2O3and Mn3O4/γ-Al2O3) were identified through X-ray diffraction source (Rigaku D/Max 2550 VB/PC).Xray diffractograms were collected in an angle range from 20° to 80° using (Cu Kα1 Radiation,λ=0.15406 nm) calibrated at 100 mA and 40 KV.Scanning electron microscope (JEOL-JAD-2300) was carried out to elucidate the morphology of prepared samples.Elemental composition of all fabricated specimens was investigated by Energy dispersive X-ray spectroscopy (EDS).Fourier transform infrared spectroscopy was performed in the range 500–3500 cm-1to record the functional groups,chemical bonding and IR spectra present in the specimens.FT-IR spectrometer(Nicolet 740)assembled with the beam splitter of KBr and TGS detector was used for collecting the spectra of samples.The meticulous surface peculiarities were investigated by transmission electron microscopy (TEM,JEOL JEM 2010).The optical properties and absorption characteristics were extracted from diffused reflectance measurements using UV–Vis spectrophotometer Cary 100 assembled with an integrating sphere assembly by taking BaSO4as reference reflectance sample.The diffused reflectance spectra was then converted into absorption spectra by employing Kubelka-Munk function.X-ray photoelectron spectroscopy (XPS) spectra were recorded by Perkin-Elmer PHI 5000C ESCA system operated at 250 W with Al Kα radiations.The photoluminescence properties were examined by luminescence spectrometer (Perkin Elmer LS-50B).The degradation products of MO were studied by GCMS(Hewlett-Packard 6890) having HP-1MS capillary column(30 m × 0.25 mm × 1.0 μm film thickness).The electrochemical measurements were obtained by using core test potentiostat.
2.4.Photocatalytic degradation
The photocatalytic activity of the fabricated specimens were examined by using methyl orange (MO) dye under visible light.To perform the photo catalysis,0.05 g of each sample were deliquesce in 50 ml of dye.For adsorption–desorption phenomena,the solution was kept in dark room for 30 min under vigorous continuous stirring.These solution were simultaneously irradiated by visible light with constant stirring.All the experiments were carried out at room temperature in similar environment.At regular intervals,4.0–5.0 ml aliquot were extracted from suspended solution and centrifuging was performed to separate photo catalyst for further examination.UV–visible spectrophotometer was used to determine the absorption of 2.0 ml of irradiated sample solutions.
3.Result and Discussion
3.1.X-ray diffraction
Fig.1(A–C) delineates the XRD profile for investigation of crystallinity,crystalline size and structure of fabricated samples.XRD diffractogram of the pristine γ-Al2O3in(Fig.1A)exhibited the most intense diffraction peak at 67.0° (4 4 0) and other peaks at 19.7°,32.0°,37.5°,39.0°,46.0° and 60.0° corresponds to (1 1 1),(2 2 0),(3 1 1),(2 2 2),(4 0 0) and (5 1 1) crystal planes.All of the peaks are matched with diffraction data of cubic structure of γ-Al2O3[9,31].The observed peaks are visibly distinguishable in pure Al2O3as well as in hetero structure(Fig.1C).No peaks of impurities and other phases have been observed.Thus the synthesized sample is of high purity and very well crystalline in nature.The average crystalline size of γ-Al2O3has been computed by Debye Scherer formula and is found to be approximately 6.87 nm.(Fig.1B)shows the diffractogram of pure hausmannite(Mn3O4)sample composed of three predominant sharp and intense peaks centered at 2θ value of 36.0°and 32.0°associated with crystal planes(2 1 1)and(1 0 3)respectively.The other peaks with low and moderate intensities found at 2θ value of 28.8°,44.4°,50.6°,58.5° and 64.5° associated with(1 1 2),(2 2 0),(1 0 5),(3 2 1)and(4 0 0)crystal planes which are in good accordance with JCPDS data card No.80-0382.Therefore,body centered tetragonal structure of hausmannite has been confirmed through XRD pattern [32,33].No impurity peak have been detected in synthesized sample.Thus the average crystallite size for Mn3O4is found to be approximately 15.8 nm.XRD diffraction pattern of Mn3O4/γ-Al2O3composite (3.0%,6.0%,9.0%) are illustrated in (Fig.1C).The major peaks displayed at 2θ value of 28.8°,32.0°,36.0°,46.0°,60.0°and 67.0°.All the peaks corresponds to tetragonal Mn3O4and γ-Al2O3phases.The diffraction peaks at 28.8°,32.0° and 36.0° (marked with asterisk symbol) depicted the existence of tetragonal Mn3O4[8]whereas characteristic peaks at 46.0°,60.0° and 67.0° (marked with bullet symbol) confirmed the presence of γ-Al2O3in heterostructure and well coincided with JCPDS card 29-0063 [9].No peak shift has been observed in XRD pattern of Mn3O4/γ-Al2O3supports the fact that Mn3O4is uniformly distributed in Al2O3structure without acquiring any strong chemical interaction between two species and broadening of peaks in hybrid structure is evident for modification of γ-Al2O3by amalgamation of Mn3O4.No impurity peaks are observed in all samples which is suggestive of high grade purity of samples and fully crystalline nature due to absence of diffraction halo [8].XRD findings are summarized in Table 1.
3.2.Scanning electron microscopy (SEM)
SEM images for pure γ-Al2O3,hausmannite (Mn3O4) and Mn3O4/γ-Al2O3are displayed in Fig.2(A–D).Fig.2(A and B) represents elongated cauliflower like micro-structure of pure γ-Al2O3and keen observation reveals that γ-Al2O3holds leaf like architect along with some cubic crystallites[9].Fig.2C illustrates the Mn3O4textural peculiarities of pure Hausmannite (Mn3O4).It is observed that particles are nano sized which have plate like morphology and are agglomerated due to high surface energy.Due to aggregated structure significant quantity of interparticle voids are created and these voids play a vital role in photocatalytic reaction[8,33,34].The textural profile of the hetrostructure Mn3O4/γ-Al2O3is shown in (Fig.2D).It is perceived that in contrast to both pure samples,the heterostructure consists of nanorods and cumulated form of aggregated distorted spherical shape nanoparticle.It is clearly noticeable that there is drastic change in morphology of γ-Al2O3due to the inculcation of manganese oxide nanoparticle into it.Moreover due to homogeneous distribution of Mn3O4onto the surface of γ-Al2O3leads to provide the active site for oxidation reaction for the efficient removal of perilous substances from water.
3.3.Energy dispersive X-ray spectroscopy
Elemental analysis and chemical composition has been determined by Energy dispersive X-ray spectroscopy.EDX spectra of pristine γ-Al2O3,pristine hausmannite (Mn3O4) and 9.0% Mn3O4/γ-Al2O3binary nanocomposite are displayed in Fig.3(A–C),respectively.According to EDX spectra in (Fig.3A),the predominant peaks of Al and O are distinctively noticeable in pure γ-Al2O3and dominant peaks of Mn and O are observed in(Fig.3B)for hausmannite phase of manganese oxide.EDX spectrum (Fig.3C) of 9.0%Mn3O4/γ-Al2O3hybrid structure composed of Al,Mn and O.Furthermore,no remnant of impurities and surrounding elements have been observed in EDX spectrum which affirm the high grade purity level of all fabricated samples.The results are very well coincided with XRD and SEM analysis.Table 2 represents the compositional analysis for pure γ-Al2O3,hausmannite (Mn3O4) and Mn3O4/γ-Al2O3heterostructure.
3.4.Fourier transform infrared spectroscopy
FT-IR study was accomplished to acclaim the vibrational transitions of numerous bands,chemical information and major functional groups in our as grown sample which is elucidated in Fig.4(A–C).FT-IR spectra of γ-Al2O3powder recorded in KBr pellet is illustrated in (Fig.4A).The bands lie in the range of 400–800 cm-1corresponds to γ-Al2O3phase.The most intense peak centered at 645 cm-1is accredited to stretching vibrational mode of Al—O and Al—O—Al [35,36].The absorption band displayed at 1032 cm-1is associated to Al—O bending vibration of Al—OH group.The bending vibration of Al—O bond is confirmed by band appearing at 1000 cm-1[37,38].A peak at 1062 cm-1are assigned due to the symmetrical bending mode of Al—O—H group [39].FTIR pattern of γ-Al2O3revealed the appearance of relatively weak band at 2979 cm-1due to C—H vibration mode of precursor molecule in the synthesis process.This does not affect the purity of material as reported in literature [35].Fig.4B represents that FTIR spectra of pure hausmannite (Mn3O4) exhibits significant and predominant peaks in the range of 400–650 cm-1[33,34].The absorption peak displayed at 609 cm-1which may be attributed to pairing modes between Mn—O stretching modes of tetragonal sites [40].The vibrational frequencies at 510 cm-1and 525 cm-1is ascribed to the distortion of Mn—O in octahedral site.No corresponding peaks of OH are found in FT-IR spectra which suggest the removal of water molecules during calcination.The examined results are well consonance with XRD results and affirmed the structure of hausmannite phase of manganese oxide [33,34,41].Fig.4C represents FT-IR spectra of Mn3O4/γ-Al2O3composite structure.The bands centered at 556 cm-1and 664 cm-1corresponding to Al—O—Al stretching bands (marked as *) [38] whereas peaks at 520 cm-1and 595 cm-1are the qualitative peaks of O—M—O and M—O vibration stretching mode (marked as ◆) [8].It is distinctively observable in pure sample as shown in Fig.4(A and B)as well as in composites (Fig.4C) bands are narrow and peaks are intense and sharp supports the fact that samples possess an excellent quality and increase in functional groups which leads the enhance photocatalytic activity [18].The FT-IR results are encapsulated in Table 3.

Table 1XRD parameters and band gap energies of synthesized pure and composite samples
3.5.Transmission electron microscopy (TEM)/high resolution TEM
Transmission electron microscope analysis was carried out to affirm the close interfacial cooperation between Mn3O4and γ-Al2O3,texture and shape of prepared nanocomposite.Fig.5 exhibits the low and high magnification photographs of as prepared Mn3O4/γ-Al2O3hetrostructure.Fig.5 illustrates the formation of nanorods on to surface of γ-Al2O3with high accumulation.The average length and diameter of nanorods is found to be 70.0 nm and 17.5 nm.Furthermore the images clearly delineates the random orientation of nanorods as shown in(Fig.5).TEM images indicates that high degree distribution of Mn3O4nano architects overlying on gamma alumina(γ-Al2O3)induces a significant synergic effect between γ-Al2O3and hausmannite phase of manganese oxide (Mn3O4).This strong interfacial contact between γ-Al2O3and Mn3O4enduring the quick transfer of photo created charge carriers across interface.TEM image of Mn3O4/γ-Al2O3hybrid structure is concomitant with the results of SEM,which also spectacles the nanorods along with attached agglomerated plate like shaped nanoparticles.
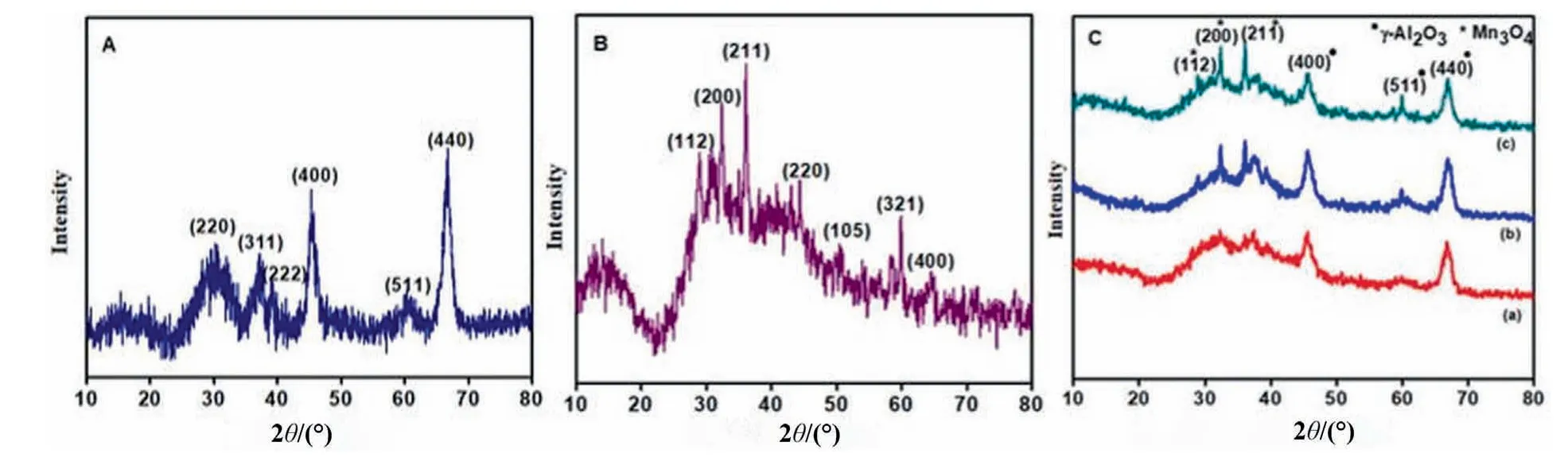
Fig.1.XRD patterns of(A)γ-Al2O3 calcined at 800°C;(B)Hausmannite;(C)Mn3O4/γ-Al2O3 heterostructure with various%ratios;(a)3.0%Mn3O4/γ-Al2O3;(b)6.0%Mn3O4/γ-Al2O3 and (c) 9.0% Mn3O4/γ-Al2O3.
3.6.Diffused reflectance spectroscopy (DRS)
The UV–Vis diffuse reflectance spectra (DRS) are employed to examine the optical peculiarities of the specimens.It can be seen from Fig.6A that the introduction of different concentrations of Mn3O4has a significant effect on the optical light absorption property for the as-prepared Mn3O4/γ-Al2O3.UV–Vis diffuse reflectance spectra of prepared specimens are evaluated by the modified Kubelka Munk function.The Kubelka Munk function is given as F(R)=(1 -R)2/2R,where R is reflectance of material.
The optical energy band gap value can be calculated by extrapolation of linear part to the hυ axis as delineated in Fig.6(A–E).The band gap value for bare γ-Al2O3(Fig.6A) is found to be approximately 3.4 eV which is according to the literature [9] whereas for pure hausmannite (Mn3O4) the estimated band gap is 1.3 eV(Fig.6B)which is much smaller than bulk Mn3O4due to crystallite size and morphological change in structure[42].Fig.6(C–E)represents the estimated band gap values of the samples are 1.84,1.8,1.76 eV,corresponding to 3.0% Mn3O4/γ-Al2O3,6.0% Mn3O4/γ-Al2O3and 9.0% Mn3O4/γ-Al2O3,respectively [8].This implies a band gap narrowing of the γ-Al2O3due to the insertion of Mn3O4on to the surface of γ-Al2O3,which can be accredited to the synergistic cooperation between γ-Al2O3and Mn3O4support.The observed results are compiled in Table 1.

Table 2Compositional analysis for pure γ-Al2O3,pure hausmannite and Mn3O4/γ-Al2O3

Table 3Infrared band areas of functional groups in various samples
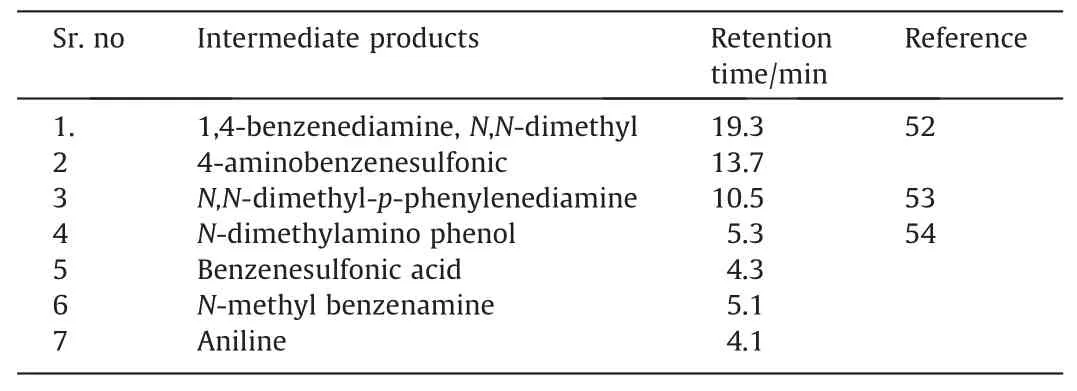
Table 4Intermediates products after GCMS analysis of a photodegraded sample of MO
3.7.Photoluminescence spectroscopy

Fig.2.SEM images of pristine γ-Al2O3 (A,B),pristine Mn3O4 (C) and 6.0% Mn3O4/γ-Al2O3 binary nanocomposite (D).
Photolumincsece spectroscopy was used to analyze the separation between the photo induced electron–hole pairs,transferance of charges and rate of recombination between electron hole pair in pristine γ-Al2O3nanoparticles and Mn3O4/γ-Al2O3binary nanocomposite.A higher PL intensity illustrates the increase in recombination rate between electrons and holes [43].Fig.7(a and b) depicts the PL spectra of as fabricated bare γ-Al2O3and Mn3O4/γ-Al2O3binary hybrid structure at an excitation wavelength of 290 nm.As displayed in Fig.7a strong peak is evident at 348 nm and broad shoulders at 390 nm,426 nm and 489 nm which is in good accordance with reported literature.The band at 348 nm is associated with F+-centers i.e.oxygen vacancy with trapped electron.The peak at 426 nm exists because of radiative relaxation of F-center.Band at 489 nm is assigned to hydroxyl group attached to surface of Aluminum ion (Al—OH) centers.It can clearly noticed that relative PL peak intensity is decreased remarkably by inclusion of Mn3O4into γ-Al2O3as displayed in Fig.7b [44,45].This noticeable reduction in peak intensity implies the decrease in recombination rate of photo induced e-–h+pair and easy transfer of electrons from Mn3O4and γ-Al2O3.This result suggests that synergistic effect occur between Mn3O4and γ-Al2O3which promotes the electron migration and photocatalytic ability.
3.8.X-ray photoelectron spectroscopy
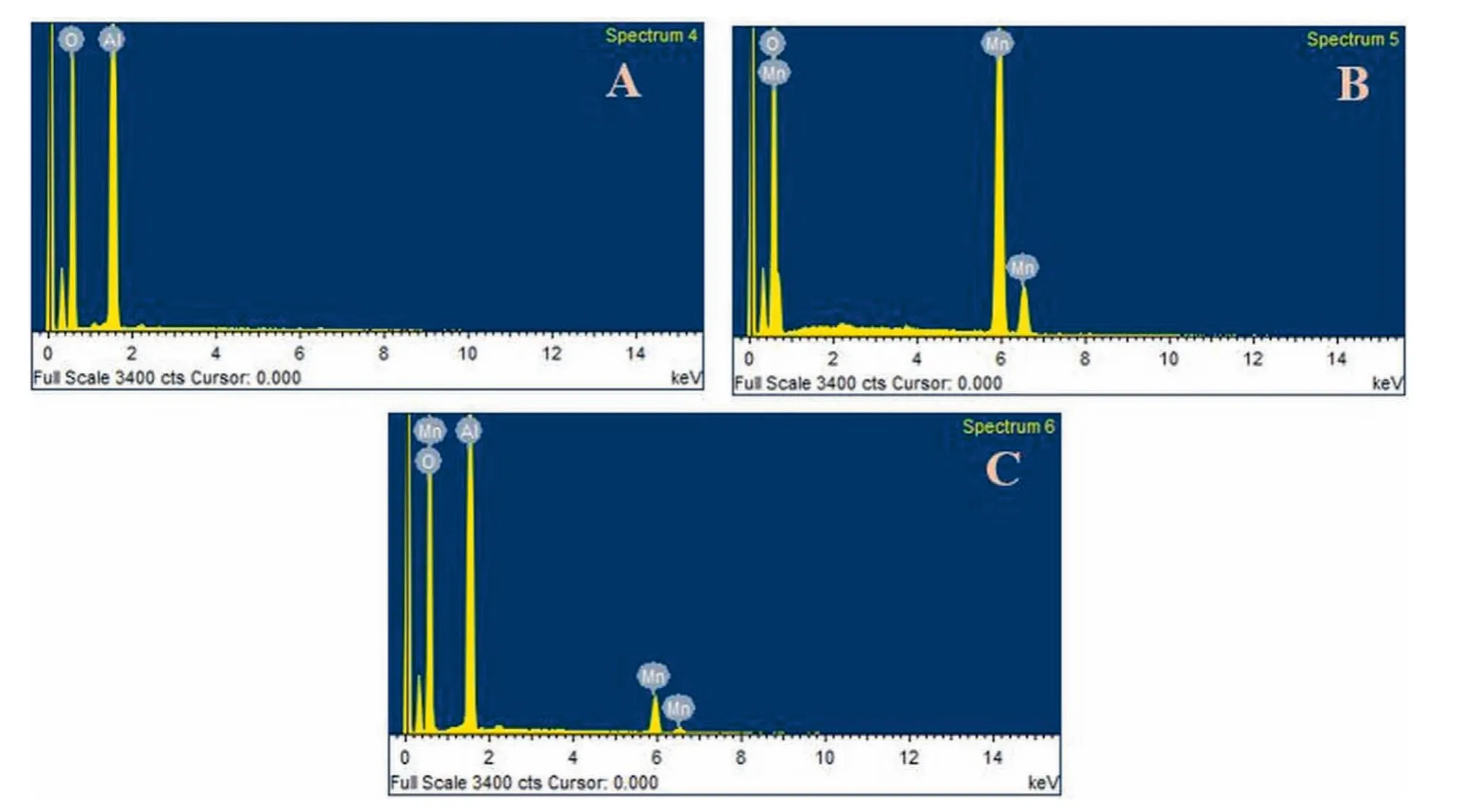
Fig.3.Energy dispersive spectra of pristine and binary composite materials;(A)γ-Al2O3;(B) Mn3O4;(C) Mn3O4/γ-Al2O3 composite structure.

Fig.4.FTIR spectra of various specimens;(A) pristine γ-Al2O3 (B) pristine Hausmannite Mn3O4 (C) 6.0% Mn3O4/γ-Al2O3 nanocomposite structure.
The oxidation states,composition and atomic concentration on the surface of Mn3O4/γ-Al2O3was affirmed by XPS.Fig.8(A–D)delineates the XPS spectrum of hybrid nanostructure Mn3O4/γ-Al2O3which substantiate the existence of Al,O,Mn and C.It displays full survey scan (Fig.8A) and high resolution spectra for Al 2p,Mn 2p,O 1s and C 1s Fig.8(B–D).The binding energies centered at 641.3 eV and 652.7 eV were accredited to Mn 2p3/2and Mn 2p1/2respectively as shown in Fig.8B.The binding energy demonstrate the difference of 11.4 between the Mn 2p3/2and Mn 2p1/2peaks which arose from spin orbitcoupling which corresponds well with previousreports for Mn3O4[46].Therefore,XPS spectra illustrated that all Mn species in synthesized sample were in the form of Mn3O4.The core level binding energy of Al 2p as displayed in(Fig.8C)was located at characteristic peak of 74 eV which affirmed the presence of γ-Al2O3as previously reported [47].(Fig.8D) enlightened the existence of shoulder peak at 532 eV corresponds to binding energy of O 1s which may present because of chemisorbed oxygen[8].C 1s can be attributed to the adventitious hydrocarbon from XPS instrument itself[48].XPS full survey scan(Fig.8A)assuring that composite consisted of Mn3O4and γ-Al2O3,therefore,hausmannite (Mn3O4) have successfully substituted on the surface of γ-Al2O3.
3.9.Linear sweep voltammetery
Linear sweep voltammetry (LSV) was carried out to evaluate electron-hole pair separation,rate of recombination and the charge transfer efficiencies in bare and binary nanocomposite.Fig.9(a and b)depicts the typical LSV curves of different electrodes in 1 mol∙L-1NaOH(electrolyte)at a scan rate of 10 mV/s sweeping the potential ranging from 0.6 to 1.8 V.As shown in Fig.9b Mn3O4/γ-Al2O3binary hybrid structure illustrates a good stability in electrolyte solution and increase in photocurrent density as compared to pure γ-Al2O3(Fig.9a).The results of photocurrent test illustrates that the photocatalytic activity of the Mn3O4/γ-Al2O3binary hybrid are several folds greater than those of pristine sample.This improved photocatalytic activity could be ascribed to the transfer of electron from Mn3O4to γ-Al2O3by interfacial potential gradient in the binary nanocomposite conduction bands.This transference of electron in the binary heterojunction promotes the charge separation and decreases the recombination rate of electron hole pair.Moreover,interfacial hybridization provides high surface area which has more active sites which further leads an enhancement in photocurrent and resulting in enhanced photocatalytic activity[49].
3.10.Photocatalytic testing
Photocatalytic degradation of organic compound (MO) is used to investigate the photoactivity of pure alumina,pure Mn3O4and Mn3O4/γ-Al2O3,hybrid structures.Preliminary control experimentconducted for the self-degradation of MO and was found almost negligible under visible light irradiation after 1 h.This result suggest a good photo stability of methyl orange under the visible light irradiation.In the absence of light,photocatalytic decomposition of organic dye was negligible.Moreover there is no change in the amount of the MO with time in dark.Furthermore photocatalytic process in not only due to ordinary adsorption.Hence,photocatalyst and presence of light are the mandatory factors in the photocatalytic process.Fig.10 illustrates the time dependent comparative study of photocatalytic performance of all specimens.Due to amassed particle,γ-Al2O3showed a diminutive photocatalytic activity 8.0% under visible light.In contrast to pure Al2O3,Mn3O4showed 31.0% degradation of methyl orange (MO) due to fact that structure is highly crystalline and exhibit the plate like morphology,these factors perform as vital role for attaining high degradation rate [50].Under visible light irradiation,3.0% Mn3O4/γ-Al2O3nanocomposite exhibited 68.0% photocatalytic activity for organic dye.6.0% Mn3O4/γ-Al2O3reveal 93.0% degradation and 9.0%Mn3O4/γ-Al2O3showed 85.0%degradation of organic pollutant.The amount of Mn3O4in x%Mn3O4/γ-Al2O3nanocomposites affects the photocatalytic activity.The photocatalytic activity enhances as the concentration of Mn3O4is raised up to the optimal level.The decreased photocatalytic activity beyond the optimum loading of Mn3O4is due to the dispersion and less penetration of light in solution for reaction.After the optimal amount of Mn3O4,the turbidity of the medium increases by the deactivation of activated molecules by collision and increases the agglomeration which leads to the decrease in photocatalytic efficiency.Clearly,the addition of an appropriate amount of Mn3O4can affect the photo activity effectively.6.0% Mn3O4/γ-Al2O3heterostructure illustrate the good photo activity and found to be far excellent to that of bare γ-Al2O3and pure hausmannite Mn3O4.This higher photocatalytic degradation of MO for optimum concentration 6.0%composite is due to textural change and inherit photocatalytic features of Mn3O4and presence of Mn3O4in hybrid structure amplified the light absorption ability of photo catalyst (Mn3O4/γ-Al2O3) and enrich dye molecules in the channel to react with Mn3O4nanoparticles which serve as active catalytic sites to decompose contaminations [8].

Fig.5.TEM and HRTEM images of 6.0% Mn3O4/γ-Al2O3 nanocomposite structure.

Fig.6.Kubelka Munk function (KMF) of various samples;(A)γ-Al2O3;(B) Hausmannite (Mn3O4);(C) 3.0% Mn3O4/γ-Al2O3 (D) 6.0% Mn3O4/γ-Al2O3 (E) 9.0% Mn3O4/γ-Al2O3.

Fig.7.PL spectra of pristine and binary nanocomposite;(a) pristine γ-Al2O3,(b)Mn3O4/γ-Al2O3 binary composite at an excitation wavelength of 290 nm.

Fig.8.XPS studies of 6.0% Mn3O4/γ-Al2O3 composite structure;(A) Full XPS spectra;(B) High resolution XPS spectra of Mn 2p;(C) Al 2p;(D) O 1s.

Fig.9.LSV studies of(a)pure γ-Al2O3;(b)6.0%Mn3O4/γ-Al2O3 composite structure.
3.10.1.Photo catalysis mechanism
The basic phenomena of photo catalysis under visible light irradiation is displayed in Fig.11.The valence band electrons promoted to the conduction band when the energy greater than the band gap energy supplied to the heterostructure.This leads to charge separation process and created electron hole pair.To approach the phenomena of the enhanced photocatalytic activity of the Mn3O4/γ-Al2O3nanocomposite,the relative band positions of the Mn3O4and γ-Al2O3were investigated,since the band-edge potential levels play a pivotal role in determining the flow sheet of photo excited charge carriers in a heterojunction.
Photo generated electrons are transferred with high efficiency from conduction band of Mn3O4to conduction band of Al2O3because of the intimate contact between the two materials.In such a way,the photo induced holes and electrons can be quickly separated and the recombination of electron/hole pairs can be reduced(Eq.(7)).Therefore,the Mn3O4/γ-Al2O3nanocomposite exhibits marvelous photocatalytic performance as compared to bare γ-Al2O3and Mn3O4.The electrons transferred react with atmospheric oxygen and transformed into super oxide anion radical (O2-·) (Eq.(8)).Consequently superoxide anion radical combines with proton producing hydrogen peroxide H2O2(Eq.(9)) and hydroxyl radical·OH (Eq.(10)).The holes in the valence band of hausmannite(Mn3O4) created hydroxyl radical from water solvent (Eq.(11)).Such an energetic oxidizing (·OH) act as pillar for degradation of organic compound to safe and harmless compounds [9,51].
Now synergistically O2-·and·OH radicals continuously strikes on the methyl orange leads to the decomposition of dye molecule to carbon dioxide in a secondary auto catalytic dark reaction (Eq.(12)).This phenomena enrich the separation of electron hole pair to speed up the process and increases the photoactivity of composite material.The optimum loading of the nanocomposite (6.0%Mn3O4/γ-Al2O3) shows enhanced photocatalytic activity due to its narrow band gap energy.On the above manifestations,the proposed mechanism for the photocatalytic deterioration of inimical dye methyl orange over Mn3O4/γ-Al2O3is as follows;
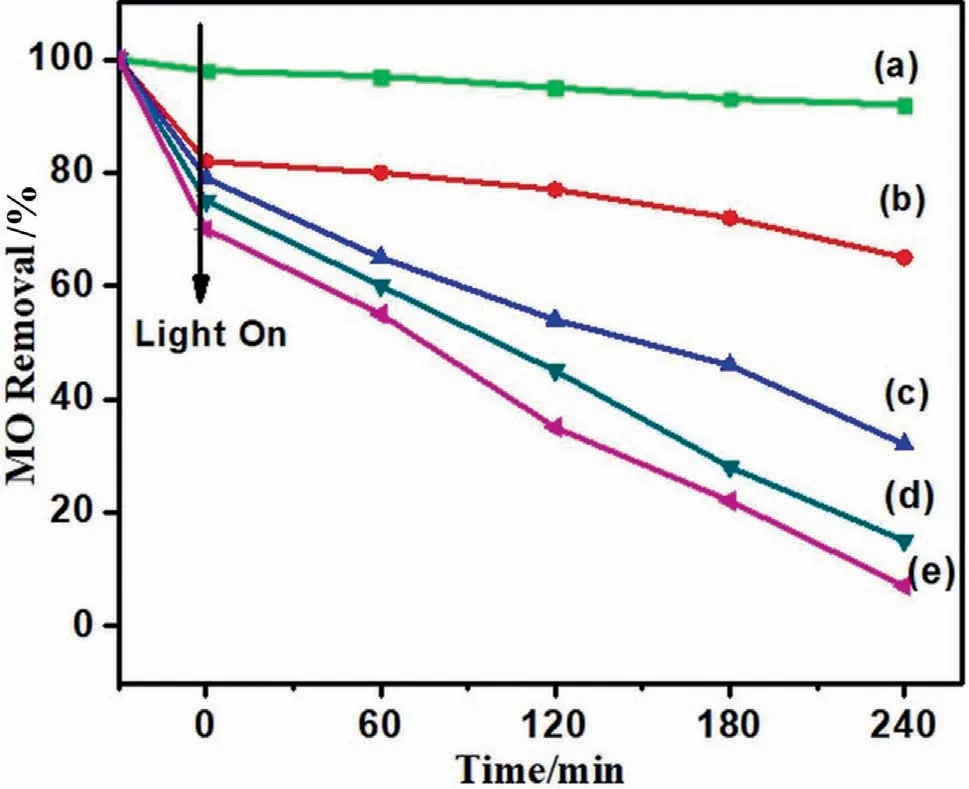
Fig.10.Time profile of photocatalytic degradation of MO under visible light (a)pure Al2O3 (b) pure Mn3O4 (c) 3.0% Mn3O4/Al2O3 (d) 6.0% Mn3O4/Al2O3 (e) 9.0%Mn3O4/Al2O3 composite materials.

Fig.11.Suggested mechanism for decomposition of Methyl Orange over Mn3O4/γ-Al2O3 nanocomposite structure.

Fig.12.Proposed pathway for degradation of methyl orange.

3.10.2.GC–MS analysis
GC–MS technique was employed to determine the intermediate product formed during the photocatalytic degradation of methyl orange.Various degradation intermediates at different retention times are shown in Table 4.Decomposition proceed through symmetric cleavage of azo bond.A proposed pathway for the decomposition of organic dye MO is shown in Fig.12.The intermediate products observed are 1,4-benzenediamine,N,N-dimethyl(RT=19.3),4-aminobenzenesulfonic (RT=13.7) [52],N,N--dimethyl-p-phenylenediamine (RT=10.54)[53],Ndimethylamino phenol (RT=5.378),benzenesulphonic acid(RT=4.3),N-methyl benzenamine (RT=5.1) and aniline(RT=4.1) [54].All the identified intermediate byproducts were determined using the NIST98 library with the matching degree of 92% (Table 4).The results corroborate well with the literature in the reference.GC–MS spectrum of MO during degradation process is shown in Fig.13.
4.Conclusions

Fig.13.GC–MS spectrum of methyl orange after degradation process.
Aluminum foil (Al) is used as a cost friendly precursor in the presence of PEG as template for the fabrication of high quality γ-Al2O3microstructures.Several Mn3O4/γ-Al2O3composites are prepared by facile and simple wet chemical technique.Various techniques are employed to interpret the detailed investigation of synthesized samples.Optical properties of the as synthesized sample are determined by diffused reflectance spectroscopy.The estimated band gaps value for pure γ-Al2O3,pure hausmannite(Mn3O4),3.0% Mn3O4/γ-Al2O3,6.0% Mn3O4/γ-Al2O3and 9.0%Mn3O4/γ-Al2O3are 3.40,1.30,1.84,1.80 and 1.76 eV.It is found that Mn3O4/γ-Al2O3hybrids represent marvelous photocatalytic performance under visible light irradiations.Due to interfacial charge transfer phenomena and small crystallite size,the binary nanocomposite has large surface to volume ratio which creates more photo excited e--h+pairs under visible light.This might have boost its photo catalytic activity.It is found to be increased for Mn3O4/γ-Al2O3heterostructure than pure γ-Al2O3.Mn3O4decorated on the surface of γ-Al2O3serves as sink which can nab an electron,prolonging the electron-hole pair life times which leads to enrich photocatalytic activity.The enhancement can be ascribed to the increased mobility of photogenerated charge carriers,decrease in recombination rate of e-/h+pair and enhanced separation efficiency verified by PL spectroscopy and linear sweep voltammetry curve.The current investigation illustrates that 6.0%Mn3O4/γ-Al2O3heterostructure (with 1.8 band gap energy) is promising material with magnificent and distinguished photocatalytic properties and can be effectively applied for the removal of contaminations and organic dye degradation in water treatment and textile industry.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The work is acknowledged to H-10,Islamabad,Higher Education of Commission of Pakistan (NRPU Grant No.3660),International Islamic University,H-10,Islamabad,and Pakistan Institute of Engineering and Applied Sciences.We are very much grateful to School of Environmental and Chemical Engineering,Shanghai Jiao Tong University,Shanghai,China,University of the Punjab Lahore,Government College University Lahore,National Centre of Physics,Institute of Space Technology and Allama Iqbal Open University Islamabad for analysis.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Comparison of catalyst-coated membranes and catalyst-coated substrate for PEMFC membrane electrode assembly:A review
- Experimental investigation of different brines imbibition influences on co-and counter-current oil flows in carbonate reservoirs
- Experimental investigation on the effect of surface characterization of electrodes on the gas bubble dynamics in electrolyte flow and performance of FLA batteries by using PIV
- Mechanically activated starch magnetic microspheres for Cd(II)adsorption from aqueous solution
- Robust,fluorine-free and superhydrophobic composite melamine sponge modified with dual silanized SiO2 microspheres for oil–water separation
- Efficient adsorption to hexavalent chromium by iron oxalate modified D301:Characterization,performance and mechanisms
