Enantioseparation of 3-chlorophenylglycine enantiomers using Mandyphos-Pd as chiral extractant
2021-08-26XiongLiuShuhuanChenYuMaWenjieXiao
Xiong Liu *,Shuhuan Chen ,Yu Ma ,Wenjie Xiao
1 School of Chemistry and Chemical Engineering,Hunan University of Science and Technology,Xiangtan 411201,China
2 Hunan Provincial Key Laboratory of Controllable Preparation and Functional Application of Fine Polymers,Xiangtan 411201,China
Keywords:Enantioseparation Mandyphos 3-Chlorophenylglycine Response surface method Recognition mechanism
ABSTRACT Chiral extractant plays a key role in chiral extraction,and considerable efforts have been undertaken for the development of new and efficient chiral extractants in recent years.This work demonstrated for the first time that chiral ferrocenyl diphosphine ligand (Mandyphos-Pd) had considerable ability to enantioseparate 3-Chlorophenylglycine enantiomers with separation factor(α)of 2.64.Mandyphos-Pd concentration and pH had significant influences on enantioselectivity,while operating temperature showed less influence.The extraction experiments can be performed at room temperature(20°C)which had the advantage of energy saving.After optimization,the highest performance factor (pf,0.08376) was obtained at the condition of pH 7.8 and Mandyphos-Pd concentration 1.2 mmol·L−1.According to the experimental results,the possible recognition mechanism was discussed.
1.Introduction
The enantiomer of some chiral drugs has different pharmacology activity and different application.Thus,the acquisition of optically pure enantiomers is of interest to researchers in recent years[1–7].Generally,the catalyzed asymmetric synthesis and raceme separation are the main routes for preparation of pure enantiomers.The chiral ligands play the key roles in catalyzed asymmetric synthesis and racemic resolutions.In the past decades,many efforts have been made to develop new and efficient chiral catalyst.For instance,2,2'-Bis(diphenylphosphino)-1,1'-binaphthalene(BINAP)is a famous catalyst reported by Noyori in 1980[8].It appears to be both the most used and the most useful ligand for asymmetric catalysis.And many BINAP derivatives have been designed and synthesized to improve catalytic efficiency(turnover)and selectivity(ee)[9].At the same time,many efforts have also been made to find new and efficient chiral selectors in chiral resolution.Recent studies suggest that some catalysts used in asymmetric synthesis also showed good abilities to enantioseparate chiral compounds in chiral extraction.For instance,BINAP is the first diphosphine ligand that exhibited a good ability to separate amino acid enantiomers[10–16].And then,several diphosphine ligands,such as (S)-MeO-BIPHEP,(S)-SEGPHOS,(S,S)-DIOP and(S)-SDP,have also been proved to be effective chiral selectors in chiral extraction of amino acid enantiomers[12,17–21].The above results indicated that diphosphine ligands used in asymmetric catalysis may have good abilities to separate amino acids in chiral extraction.
In our previous work,the recognition mechanisms between diphosphine ligands and amino acid enantiomers had been analyzed[18–21].The mechanism analysis suggested that π-π interaction,coordination between metal and—COO−,and coordination between metal and—NH2are the three main acting forces in enantioseparation.The mechanism analysis provided an important basis to develop new chiral selectors in future work.As a continuous interest in finding chiral extractant with novel skeletal structure,we speculated that chiral ferrocenyl diphosphine ligand(Mandyphos)might be another ligand that has a good ability to enantioseparate amino acid enantiomers.
Mandyphos((S,S)-(-)-2,2′-Bis[(R)-(N,N-dimethylamino)(phenyl)methyl]-1,1′-bis(diphenylphosphino)ferrocene)is one of famous chiral ferrocenyl diphosphine ligand that is widely used in asymmetric hydrogenation and asymmetric allylic alkylations [22–25].The chemical structure of Mandyphos is shown in Fig.1.Mandyphos has a rigid backbone that could provide a stable stereoscopic configuration in chiral recognition.The phosphine atoms could coordinate with metal ion to form a stable Mandyphos-metal complex,which could coordinate with—COO−and —NH2in recognition.Moreover,the benzene rings in Mandyphos could provide steric effect and π-π interaction in recognition.Indeed,Mandyphos-Pd exhibited a good ability to separate 3-Chlorophenylglycine enantiomers with separation factor(α)of 2.64.Mandyphos-Pd had considerable enantioselectivity compared with(S,S)-DIOP-Pd(1.83)and(S)-MeO-BIPHEP-Cu(1.81).Encouragingly,the enantioselectivity of Mandyphos-Pd is insensitive to temperature.This means that the chiral extraction could be performed at room temperature,which had the advantage of energy saving.This work demonstrated for the first time that chiral ferrocenyl diphosphine ligand(Mandyphos-Pd) had considerable ability to separate 3-Chlorophenylglycine enantiomers,and broadened the library of chiral extractants,which was important for the field of chiral extraction.Herein,we reported the details of this work.

Fig.1.The chemical structures of diphosphine ligands used in previous works((S)-BINAP,(S)-MeO-BIPHEP,(S)-SEGPHOS,(S,S)-DIOP and(S)-SDP),and in this work(Mandyphos).
2.Experimental
2.1.Chemical reagents and samples
Mandyphos (>98%)was purchased from Bide Pharmatech Ltd.(China).Metal precursors,including Tetrakis(acetonitrile)copper([(CH3CN)4Cu]PF6,98%),bis(acetonitrile)dichloropalladium((CH3CN)2PdCl2,99%)and Bis(Triphenylphosphine)Nickel(II)Chloride([(C6H5)3P]2NiCl2,98%) were purchased from J&K Scientific Ltd.3-Chlorophenylglycine enantiomers(99%)were purchased from Adamas Reagent Co,Ltd.Organic solvents and other chemicals were analytically pure and used without further treatment.
2.2.Preparation of samples
Preparation of Mandyphos-metal complex:0.1 mmol Mandyphos and 0.1 mmol metal precursor(Pd(II),Cu(I)or Ni(II))were dissolved in 50 ml organic solvent(Dichloromethane,1,2-Dichloroethane,Chloroform or Chlorobenzene),then,the mixture was stirred at room temperature for 12 h under the atmosphere of nitrogen.The obtained mixture was then diluted to 100 ml with the same organic solvent.The Mandyphos-metal complex was obtained with concentration of 1.0 mmol·L−1.Preparation of 3-Chlorophenylglycine solution:0.1 mmol 3-Chlorophenylglycine was dissolved in 50 ml phosphate buffer(0.1 mol·L−1,pH 5–9)to obtain 3-Chlorophenylglycine solution with concentration of 2.0 mmol·L−1.
2.3.Enantioseparation
The enantioseparation experiment was performed as follows:2.0 ml Mandyphos-metal complex and 2.0 ml 3-Chlorophenylglycine solution were placed in a 10 ml centrifuge tube.The mixtures were shaken at 120 rpm for 12 h.Then,let it stand for 6 h.The water phase was suctioned by a syringe and filtered using 0.45 μm filter membrane.The concentrations of DL-3-Chlorophenylglycine in water phase were then detected by a high performance liquid chromatography using the method described in our previous work[17].Each test was performed three times and the mean was taken as the experimental result.
2.4.Calculations
The distribution ratios(kL,kD)of enantiomers in organic phase and water phase,separation factor(α),enantiomeric excess of enantiomer in organic phase(eeorg),fraction of D-3-Chlorophenylglycine in organic phase(fD)and performance factor(pf)were employed to evaluate the extraction behaviors of Mandyphos.The values of these parameters were calculated according to Eqs.(1)–(6),respectively[26,27].

where CL,organd CD,orgare the concentrations of L-3-Chlorophenylglycine and D-3-Chlorophenylglycine in the organic phase.CL,wand CD,ware the concentrations of L-3-Chlorophenylglycine and D-3-Chlorophenylglycine in the water phase.
2.5.Optimization method
Generally,metal precursor,organic solvent,pH,chiral extractant concentration and operating temperature affected stereoselectivity significantly in chiral recognition.In this paper,the influences of these factors on extraction were studied by individual factor experiment.Based on the results of individual factor experiment,the chiral extraction was optimized comprehensively by response surface method.To take performance factor (pf) as index,the effects of pH and Mandyphos-metal complex concentration were observed by a Central Composite Design.Quadratic polynomial model described in Eq.(7)is always employed to fit data that obtained in Central Composite Design.In Eq.(7),X1and X2are observed factors,β are interaction regression coefficients[28–30].
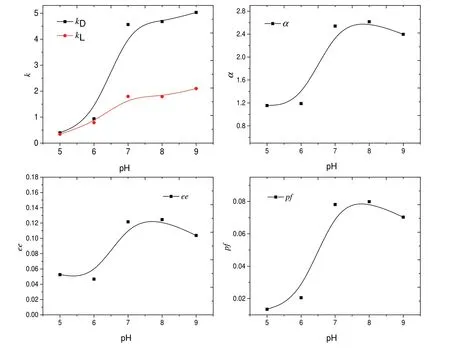
Fig.2.Effects of pH on extraction.Extraction condition:Temperature of 5°C,Mandyphos-Pd concentration of 1.0 mmol·L−1.

3.Results and Discussion
3.1.Effect of metal precursors
Metal precursor plays a key role in chiral extraction when metal complex used as chiral extractant.In this work,Pd(II),Cu(I)and Ni(II)were selected as metal precursors and the stereoselectivities of Mandyphos-Pd,Mandyphos-Cu and Mandypos-Ni were shown in Table 1.Obviously,Mandyphos-Pd had a good ability to separate 3-Chlorophenylglycine enantiomers while Mandyphos-Cu and Mandyphos-Ni had no recognition abilities in chiral extraction.The separation factor (α) for Mandyphos-Pd was 1.86.This result indicated that metal precursor had significant effect on stereoselectivity of Mandyphos.Generally,the 4-coordinate Pd(II) complex has a planar quadrilateral configuration while 4-coordinate Cu(I) and 4-coordinate Ni(II)complexes have the tetrahedral configurations [31,32].The difference of configurations between Mandyphos-Pd and Mandyphos-Cu/Ni is the possible reason why Mandyphos-Pd is more efficiency than Mandyphos-Cu and Mandyphos-Ni.In addition,kDwas bigger than kL,which implied that D-3-Chlorophenylglycine was the main enantiomer in organic phase.In chiral extraction,Mandyphos-Pd was preferred to combine with D-3-Chlorophenylglycine.Overall,Mandyphos-Pd had a considerable ability to separate 3-Chlorophenylglycine enantiomers.Pd(II)was chosen as the suitable metal precursor in the following extraction experiments.

Table 1Stereoselectivities of Mandyphos-Pd,Mandyphos-Cu and Mandyphos-Ni
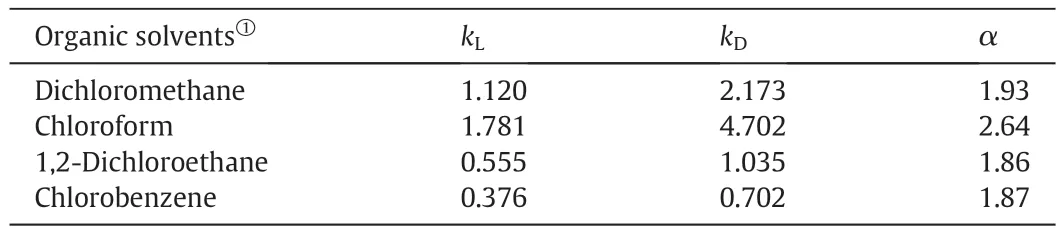
Table 2Stereoselectivities of Mandyphos-Pd in different organic solvents
3.2.Effect of organic solvents
The stereoselectivities of Mandyphos-Pd in different organic solvents were detected,and the results were tabulated in Table 2.Mandyphos-Pd exhibited good abilities to recognize 3-Chlorophenylglycine enantiomers in these four organic solvents.The highest α(2.64)was obtained when chloroform used as organic solvent.Interestingly,Mandyphos-Pd also showed a good ability to recognize 3-Chlorophenylglycine enantiomers in chlorobenzene.According to reports in the literature,the stereoselectivities of diphosphine ligands,such as(S)-BINAP and(S)-MeO-BIPHEP,are always very low when chlorobenzene selected as organic solvent.And previous studies suggested that π-π interaction between ligand and amino acid enantiomer had been weakened by chlorobenzene[16,17].The result of this work revealed that the chlorobenzene showed less influence on π-π interaction between Mandyphos-Pd and 3-Chlorophenylglycine.This is probably because that Mandyphos has a unique three-dimensional structure(sandwich structure)compared with other diphosphine ligands.In follow-up experiments,chloroform was picked as the organic solvent in chiral extraction.
3.3.Effects of pH
In chiral extraction,—COO−and—NH2in 3-Chlorophenylglycine could coordinate with Mandyphos-Pd to obtain a ternary complex.The complexation abilities of—COO−and—NH2would be significantly affected by pH of water phase.Therefore,the values of k,α,ee and pf at pH ranging from 5 to 9 had been detected and the results were depicted in Fig.2.The values of kLand kDwere both increased with increasing pH,which indicated that Mandyphos-Pd preferred to combine with anionic 3-Chlorophenylglycine.With the increasing of pH,there was more anionic 3-Chlorophenylglycine in water phase.Consequently,more 3-Chlorophenylglycine was extracted into organic phase and k values increased accordingly.The curve of α revealed that the stereoselectivity of Mandyphos-Pd increased at pH in range of 5–7,and then slightly decreased with pH further increased.When pH was low,the concentration of anionic 3-Chlorophenylglycine in water phase was low.Mandyphos-Pd was enough to combine with anionic enantiomers.And anionic Dand L-3-Chlorophenylglycine were both extracted into organic phase non-selectively.With pH increased to 7,more 3-Chlorophenylglycine existed as anionic form,and competition between D,L-enantiomers was high at high pH.More D-3-Chlorophenylglycine combined with Mandyphos-Pd in recognition.Thus,α values increased with increasing pH(5–7).However,α values slightly decreased when pH above 7.This is probably because that a second anionic 3-Chlorophenylglycine binded to the Mandyphos-Pd-enantiomer complex at high pH.The concentration of anionic 3-Chlorophenylglycine was low when pH below 7.Thus,anionic 3-Chlorophenylglycine combined with Mandyphos-Pd with 1:1 stoichiometry.When pH above 7,there was more and more 3-Chlorophenylglycine existed as anionic form.And a second anionic 3-Chlorophenylglycine combined with the Mandyphos-Pd-enantiomer complex [14].For the same reason,ee values also increased with increasing pH and then decreased with pH further increased.In chiral extraction,performance factor(pf)was always employed to comprehensively evaluate the extraction efficiency.High pf implied that D-3-Chlorophenylglycine was extracted into organic phase with high yield(kD)and high purity(ee)simultaneously[33].The curve of pf revealed that the optimal pH was in the range of 7.0–9.0.
3.4.Effects of Mandyphos-Pd concentration

Fig.3.Effects of Mandyphos-Pd concentration on extraction.Extraction condition:Temperature of 5°C,pH of 8.0.

Fig.4.Effects of temperature on extraction.Extraction condition:pH 8.0,Mandyphos-Pd concentration of 1.5 mmol·L−1.
The effect of Mandyphos-Pd concentration on extraction was shown in Fig.3.There was more Mandyphos-Pd that could combine with 3-Chlorophenylglycine at high extractant concentration.Thus,it is reasonable that k values were positively correlated with Mandyphos-Pd concentration.The curve of α revealed that the stereoselectivity of Mandyphos-Pd increased to the maximum and then decreased slightly with increase of Mandyphos-Pd concentration.In general,a small amount of 3-Chlorophenylglycine could be extracted into organic phase by physical dissolution with non-selectively [19].This phenomenon would decrease the enantiomeric excess of D-3-Chlorophenylglycine in organic phase when Mandyphos-Pd concentration was low.With the increase of Mandyphos-Pd concentration,more and more 3-Chlorophenylglycine was extracted into organic phase with high selectivity.Consequently,α value increased gradually with increase of Mandyphos-Pd concentration.When Mandyphos-Pd concentration was above 1.5 mmol·L−1,there was enough chiral extractant that could combine with 3-Chlorophenylglycine.The competition between D,L-enantiomers decreased with extractant concentration further increased.Thus,α value decreased slightly with Mandyphos-Pd concentration above 1.5 mmol·L−1.The result of Fig.3 showed that the highest pf was also obtained at Mandyphos-Pd concentration around 1.5 mmol·L−1.Thus,the optimal range of Mandyphos-Pd concentration in extraction was 1.0–2.0 mmol·L−1.
3.5.Effects of extraction temperature
The mass transfer performances of extractant and substrate in enantioseparation would be obviously affected by extraction temperature.Fig.4 revealed that k values increased with increasing temperature.This result implied that the physic solubility of 3-Chlorophenylglycine in organic phase increased with increasing temperature.The α values remained stable with operating temperature in range of 5–20 °C.This result indicated that the stereoselectivity of Mandyphos-Pd had been less affected by operating temperature.This phenomenon differed from the results reported in previous works.Generally,the stereoselectivities of chiral diphosphine ligands,such as(S)-BINAP,(S)-MeO-BIPHEP,(S)-SEGPHOS,(S,S)-DIOP and(S)-SDP,are all decreased sharply with increasing temperature[16–20,34].And the extractions need to be carried out at low temperature.Mandyphos-Pd is a sandwich coordination complex,which may partially explain why the stereoselectivity of Mandyphos-Pd is insensitive to temperature.The curves of ee and pf also revealed that the temperature had less effect on extraction.The above results suggested that the chiral extraction could be carried out at room temperature(20°C).
3.6.Optimization
In this work,the extraction condition was optimized using a Central Composite Design.According to the above experimental results,pH of water solution and Mandyphos-Pd concentration were selected as optimized variables,and pf was selected as response value.The optimization ranges of pH and Mandyphos-Pd concentration were 5.6–8.4 and 0.8–2.2 mmol·L−1,respectively.The codes and levels of experimental factors for Central Composite Design were shown in Table 3.Andexperimental design and results for Central Composite Design were tabulated in Table 4.Quadratic polynomial model described in Eq.(7)was used to fit these experimental data.And mathematical model for extraction was shown as Eq.(8).

Table 3Codes and levels of experimental factors for Central Composite Design
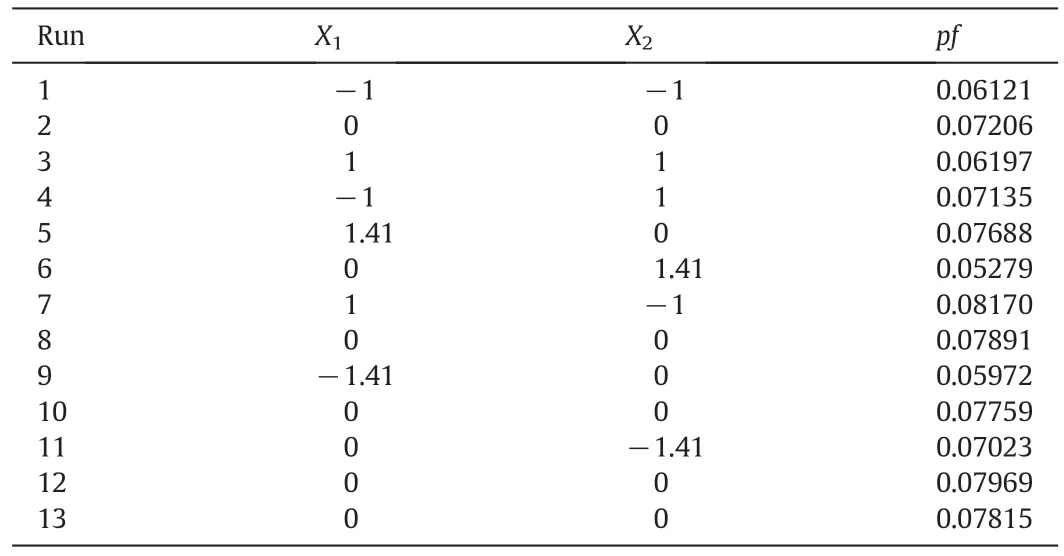
Table 4Experimental data in Central Composite Design
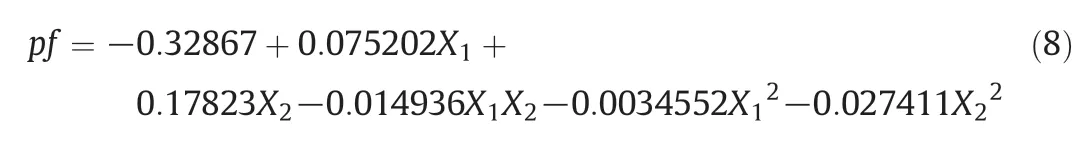
Table 5 showed the analysis of variance for the model.P value for the model was 0.0039,indicating that mathematical Eq.(8)fitted the experimental results well.P value for lack of fit was 0.1522,which indicated that the predicted values from Eq.(8) agreed well with the experimental results.Factors which affected pf significantly could be determined by P values.The P values of X1,X2,X1X2and X22were all below 0.05,which revealed that pH,Mandyphos-Pd concentration,interaction between pH and Mandyphos-Pd concentration,and quadratic levels of Mandyphos-Pd concentration were all affected pf significantly.Fig.5 showed the response surface plot of pf at different pH and extractant concentration.When Mandyphos-Pd concentration was low,pf showed a positive correlation with pH.However,pf had a negative correlation with pH at high Mandyphos-Pd concentration.Generally,pf increased at first,and then decreased with increasing of Mandyphos-Pd concentration.Based on Eq.(8),the predicted highest pf(0.08212)was obtained at the extraction condition of pH 7.8 and Mandyphos-Pd concentration 1.2 mmol·L−1.At this optimal extraction condition,the experimental pf was 0.08376.The relative error between predicted and experimental value was 1.95%,indicating that the model was suitable for the enantioseparation.

Table 5Variance analysis of model

Table 6The stereoselectivities for separation of 3-Chlorophenylglycine
3.7.Comparison of stereoselectivities

Fig.5.Response surface plot of pf at different pH and extractant concentration.
The stereoselectivities of separating 3-Chlorophenylglycine enantiomers using different chiral extractants were tabulated in Table 6.The α for Mandyphos-Pd,(S,S)-DIOP-Pd,(S)-SEGPHOS-Cu,(S)-MeO-BIPHEPCu were 2.64,1.83,2.94 and 1.81,respectively.Mandyphos-Pd had considerable ability to separate 3-Chlorophenylglycine compared with other chiral bisphosphine ligands.Most importantly,the stereoselectivity of Mandyphos-Pd is insensitive to temperature.This means that the chiral extraction could be performed at room temperature when Mandyphos-Pd used as extractant.It will help save energy in chiral extraction.In our previous work,the α for recognizing 3-Chlorophenylglycine has been increased to 3.68 by regulating the dihedral angle of(S)-MeO-BIPHEP.And(S)-C5TunaPhos-Cu exhibited excellent ability to separate 3-Chlorophenylglycine with α of 3.68 [21].Similarly,structural modification may be a promising method to improve the stereoselectivity of Mandyphos-Pd in future studies.
3.8.Recognition mechanism
Mechanism analysis is beneficial to develop new and efficient chiral extractant.According to the experimental results,the possible mechanism of Mandyphos-Pd recognizing 3-Chlorophenylglycine was proposed in Fig.6.The benzenes in Mandyphos-Pd provided steric effect and π-π interaction in chiral extraction.The coordination between metal precursor Pd(II) and —COO−was another important actingforce in chiral extraction.It could be verified by the fact that Mandyphos-Pd was conducive to combine with anionic 3-Chlorophenylglycine.When D-3-Chlorophenylglycine combined with Mandyphos-Pd,—NH2could coordinate with Pd(II)to generate a stable planar quadrilateral configuration.However,—NH2in L-3-Chlorophenylglycine could not coordinate with Pd(II)because of its unsuitable steric configuration.Thus,ternary complex Mandyphos-Pd-D-enantiomer (Fig.6A) is more stable than Mandyphos-Pd-L-enantiomer(Fig.6B).Accordingly,Mandyphos-Pd was preferred to combine with D-3-Chlorophenylglycine in extraction.

Fig.6.The possible recognition mechanism between Mandyphos-Pd and 3-Chlorophenylglycine.
4.Conclusions
This work demonstrated that Mandyphos-Pd was a good chiral extractant to separate 3-Chlorophenylglycine enantiomers.D-3-Chlorophenylglycine was the preferential enantiomer for Mandyphos-Pd in extraction.The stereoselectivity of Mandyphos-Pd was insensitive to extraction temperature(5–20°C)with α about 2.64.The extraction could be carried out at room temperature which had the advantage of energy saving.After optimizing by response surface method,the highest pf(0.08376)was obtained at the condition of pH 7.8,extractant concentration 1.2 mmol·L−1and temperature of 20°C.In addition,the mechanism analysis revealed that the steric hindrance,π-π interaction,coordination between Pd(II)and—COO−,and coordination between Pd(II) and —NH2played important roles in chiral recognition.This work broadened the library of chiral extractants,which was important for the field of chiral extraction.And the mechanism analysis provided a reference for developing new and efficient chiral ferrocenyl diphosphine ligands in future work.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was financially supported by National Natural Science Foundation of China(Grant No.51703060)and Hunan Provincial Innovation Foundation for Postgraduate(Grant No.CX2018B673).
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Comparison of catalyst-coated membranes and catalyst-coated substrate for PEMFC membrane electrode assembly:A review
- Experimental investigation of different brines imbibition influences on co-and counter-current oil flows in carbonate reservoirs
- Experimental investigation on the effect of surface characterization of electrodes on the gas bubble dynamics in electrolyte flow and performance of FLA batteries by using PIV
- Mechanically activated starch magnetic microspheres for Cd(II)adsorption from aqueous solution
- Robust,fluorine-free and superhydrophobic composite melamine sponge modified with dual silanized SiO2 microspheres for oil–water separation
- Efficient adsorption to hexavalent chromium by iron oxalate modified D301:Characterization,performance and mechanisms
