乳癌病人血清sB7-H3表达及其临床意义
2021-08-18于超周小凤吕志栋毛艳韩清昕吴琍
于超 周小凤 吕志栋 毛艳 韩清昕 吴琍

[摘要] 目的
探讨乳癌病人血清可溶性人B7同源体3(sB7-H3)表达及其临床意义。
方法 采用ELISA法,检测106例乳癌病人(乳癌组)和43例健康体检者(健康组)血清中sB7-H3表达,并分析乳癌病人血清sB7-H3表达与病理参数的关系。
结果 乳癌组血清sB7-H3表达明显高于健康组,差异有显著意义(t=8.530,P<0.01);血清sB7-H3最佳诊断截断值为21.73 μg/L。单因素分析显示,血清中sB7-H3表达与乳癌病人术后的病理分期(pTNM)、是否有淋巴结转移以及肿瘤浸润淋巴细胞表达高低有关(t=-2.219~2.067,P<0.05),而与肿瘤大小、组织学分级、分子分型等无关(P>0.05)。
结论 血清sB7-H3可能成为筛选乳癌的重要肿瘤标志物,高表达的血清sB7-H3可能与乳癌预后不良有关。
[关键词] 乳房肿瘤;sB7-H3;血清;诊断;病理学,临床
[中图分类号] R737.9
[文献标志码] A
[文章编号] 2096-5532(2021)03-0361-04
doi:10.11712/jms.2096-5532.2021.57.087
[開放科学(资源服务)标识码(OSID)]
[网络出版] https://kns.cnki.net/kcms/detail/37.1517.R.20210426.1111.003.html;2021-04-26 16:17:22
EXPRESSION OF SERUM SOLUBLE HUMAN B7 HOMOLOG 3 AND ITS CLINICAL VALUE IN BREAST CANCER PATIENTS
YU Chao, ZHOU Xiaofeng, L Zhidong, MAO Yan, HAN Qingxin, WU Li
(Breast Center, The Affiliated Hospital of Qingdao University, Qingdao 266100, China)
[ABSTRACT]Objective To investigate the expression of serum soluble human B7 homolog 3 (sB7-H3) and its clinical va-
lue in breast cancer patients.
Methods ELISA was used to measure the expression of sB7-H3 in the serum of 106 breast cancer patients (breast cancer group) and 43 healthy subjects who underwent physical examination (healthy group), and the association between serum sB7-H3 expression and pathological parameters was analyzed for breast cancer patients.
Results The breast can-
cer group had significantly higher expression of serum sB7-H3 than the healthy group (t=8.530,P<0.01), and the optimal cut-off value for diagnosis was 21.73 μg/L. The univariate analysis showed that the expression of sB7-H3 in serum was associated with postoperative pathological staging, presence or absence of lymph node metastasis, and expression of tumor-infiltrating lymphocytes (t=-2.219 to 2.067,P<0.05), while it was not associated with tumor size, histological grade, and molecular typing (P>0.05).
Conclusion Serum sB7-H3 is expected to become an important tumor marker for breast cancer screening, and highly expressed serum sB7-H3 may be associated with the poor prognosis of breast cancer.
[KEY WORDS]breast neoplasms; sB7-H3; serum; diagnosis; pathology, clinical
目前,乳癌已成为全球女性癌症死亡的第二大原因[1],并占全球女性癌症病例总数的1/4[2]。在中国,乳癌的致死人数逐年增加,已成为导致45岁以下女性死亡的主要原因和发病率较高的女性疾病[3-5]。乳癌早期诊断不理想[6]。近几年研究发现,血清中可溶性人B7同源体3(sB7-H3)在多种癌症病人中异常高表达[7-9],并与肿瘤恶性进展和预后密切相关[10-11]。sB7-H3对于癌症诊断和预后评价可能有重要价值。本研究探讨乳癌病人血清中sB7-H3表达及其临床意义,为乳癌早期诊断提供依据。
1 资料和方法
1.1 对象与分组
2018年8月—2019年4月,选取我院收治乳癌病人106例作为研究对象(乳癌组),均为女性。纳入标准:①自愿参加本次研究;②病历及术后病理资料完整;③穿刺及术后病理诊断为浸润性乳癌。排除标准:①伴有其他恶性肿瘤、自身免疫系统疾病和炎症性疾病;②术前有新辅助治疗史;③近3个月有药物应用史。以同期来我院体检中心体检的健康者43例作为对照组,所有对象均为女性。乳癌组病人年龄25~76岁,平均(53.42±10.26)岁;对照组年龄为19~62岁,平均(44.53±11.28)岁。本文研究取得医院伦理委员会批准和病人知情同意,并签署知情同意书。
1.2 检测指标及方法
采集受检者清晨空腹静脉血5 mL,静置0.5 h后,以3 000 r/min离心5 min,取血清并将其保存于-80 ℃冰箱内备用。应用ELISA法测定标本中sB7-H3表达,试剂盒购自江苏宝莱生物科技有限公司,操作参照说明书进行。
1.3 统计学方法
使用SPSS 21.0软件进行统计学分析,计量资料数据以±s表示,两组数据间比较采用t检验,多组数据间比较采用单因素方差分析。采用Med Calc 19.0.2软件绘制ROC曲线。以P<0.05为差异有统计学意义。
2 结 果
2.1 两组血清sB7-H3表达比较
乳癌组、对照组血清sB7-H3表达量分别为(26.27±4.21)、(19.44±4.91) μg/L,两组比较差异有显著性(t=8.530,P<0.01)。
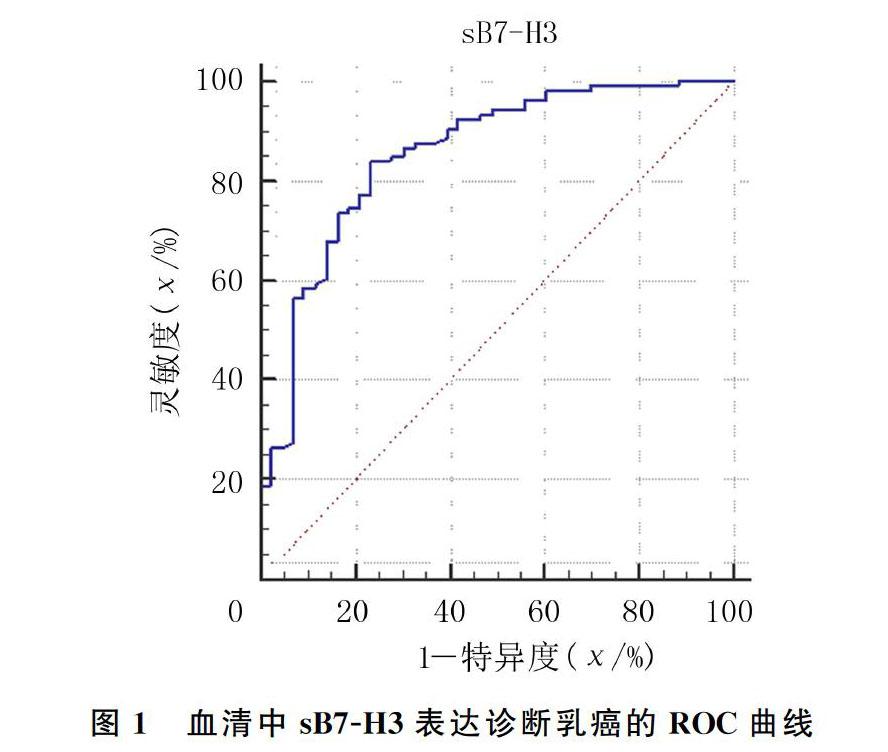
2.2 血清中sB7-H3表达筛选乳癌的最佳截断值
ROC曲线分析显示,曲线下面积为0.854(95%CI=0.787~0.907,P<0.01); 最佳诊断截断值为21.73 μg/L,灵敏度为84.0%,特异度为76.7%,约登指数0.607。即以血清sB7-H3水平21.73 μg/L作为区分乳癌和健康人的筛选界值时,诊断价值最高。见图1。
2.3 乳癌病人sB7-H3表达与临床病理参数的关系
pTNM分期较高(Ⅲ期和Ⅳ期)的乳癌病人血清中sB7-H3表达高于分期较低乳癌病人(Ⅰ期和Ⅱ期),差异有统计学意义(t=-2.219,P<0.05)。淋巴结有转移的病人血清sB7-H3明显高于无淋巴结转移病人,差异有统计学意义(t=-2.205,P<0.05)。肿瘤浸润淋巴细胞(TILS)≤5%的病人sB7-H3表达高于TILS>5%的病人,差异有统计学意义(t=2.067,P<0.05)。而肿瘤大小、组织学分级、分子分型等病理特征与sB7-H3表达无关(P>0.05)。见表1。
3 讨 论
近年来,人们致力于与肿瘤的诊断和预后评价具有较高临床相关性的血液生物标志物研究[12]。B7-H3为一种表达于人体各类免疫细胞膜上的Ⅰ型跨膜蛋白,愈来愈多研究证实其具有免疫相关作用[13]。对B7-H3生物学功能的研究发现,B7-H3在调节T细胞活化过程同时具有共刺激和共抑制作用[14-16];同时有研究显示,B7-H3在特异性和非特异性免疫调节中均具有重要意义[17]。但B7-H3的作用机制仍不明确。有研究显示,B7-H3与T细胞上的髓样细胞(TREM-)样转录物(TLT-2)分子上表达的受体结合可增强T细胞增殖、細胞因子产生和细胞毒性[18]。但这一观点并没有被普遍接受。一些研究人员认为,T细胞上可能有其他潜在受体[19-20]。B7-H3抑制T细胞活化的相关机制尚需进一步研究。
有研究显示,B7-H3在人体体液中的可溶性形式sB7-H3能够与B7-H3受体结合。正常人血清,甚至人的成骨细胞和骨髓基质细胞的上清液和前列腺分泌物中均可检测到sB7-H3的表达[21-22],多种疾病病人体内检测到sB7-H3表达水平与健康人群不同[23]。对于sB7-H3的来源,绝大多数研究认为,其是通过基质金属蛋白酶(MMP)切割单核细胞、树突细胞、活化的T细胞和肿瘤细胞细胞膜上的B7-H3而释放出来,其支持证据是MMP抑制剂能够增强B7-H3在细胞膜上的表达[21]。
关于sB7-H3的生物学功能,有研究认为在肿瘤的发生、发展中,外周血中的游离sB7-H3可以竞争性地与T细胞表面上的B7-H3受体结合,阻断B7-H3的T细胞活化作用,从而具有与B7-H3相反的免疫调节功能[24-25]。CHEN等[9]研究结果显示,剪切型sB7-H3能够抑制T细胞增殖,减少细胞因子的分泌。
而XIE等[12]研究结果证明,sB7-H3通过TLR4/NF-κB途径促进胰腺癌细胞的侵袭和转移。提示sB7-H3可能与人体免疫调节有关,并可能在肿瘤发生、发展过程中发挥重要作用。
本研究结果显示,乳癌组血清sB7-H3表达明显高于健康组,提示血清sB7-H3检测可作为乳癌筛选和辅助诊断的潜在标志物;同时对血清sB7-H3与临床病理参数的关系分析显示,血清中sB7-H3表达与乳癌病人pTNM分期、是否有淋巴结转移以及TILS表达高低等有关,而与年龄、肿瘤大小及组织学分级等无关,sB7-H3高表达可能提示乳癌分期更高,预后更差。
大量研究认为,TILS是人体对肿瘤抗原进行免疫反应的标志,其具有潜在预测乳癌病人预后的价值[26-27]。尤其在三阴性与Her-2阳性乳癌病灶中TILS浸润程度较高,可能与这两种类型肿瘤的免疫原性相对较高有关[28]。值得注意的是,本研究结果显示,血清sB7-H3表达与乳癌组织中TILS水平有关,提示sB7-H3可能参与调节T细胞对乳癌肿瘤细胞的免疫反应,并有可能抑制T细胞活化,这与SUN等[25]研究结果类似。此外有研究发现,MMP除可能参与切割mB7-H3产生sB7-H3,还介导组成肿瘤微环境的细胞外基质的降解,使乳癌组织间质中T细胞穿透细胞外基质形成的屏障,促进其免疫活性[29],提示血清sB7-H3可能与肿瘤浸润微环境形成存在某种联系。其具体作用机制需要进一步研究。
綜上所述,sB7-H3在乳癌病人中高表达,具有成为筛查乳癌血清标志物的良好前景;sB7-H3与乳癌病人术后病理参数存在密切相关性,提示其具有预测乳癌病人临床预后的潜力。sB7-H3可能通过抑制B7-H3对T细胞的活化功能,促进肿瘤细胞的免疫逃逸,其具体机制尚需进一步研究证实。
[参考文献]
[1]LI M Q, LAO Y H, MINTZ R L, et al. A multifunctional mesoporous silica-gold nanocluster hybrid platform for selective breast cancer cell detection using a catalytic amplification-based colorimetric assay[J]. Nanoscale, 2019,11(6):2631-2636.
[2]BRAY F, FERLAY J, SOERJOMATARAM I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA: a Cancer Journal for Clinicians, 2018,68(6):394-424.
[3]CHEN W, ZHENG R, BAADE P D, et al. Cancer statistics in China, 2015[J]. CA: A Cancer Journal for Clinicians, 2016,66(2):115-132.
[4]KHAN S I, AUMSUWAN P, KHAN I A, et al. Epigenetic events associated with breast cancer and their prevention by dietary components targeting the epigenome[J]. Chemical Research in Toxicology, 2012,25(1):61-73.
[5]JIA M M, ZHENG R S, ZHANG S W, et al. Female breast cancer incidence and mortality in 2011, China[J]. Journal of Thoracic Disease, 2015,7(7):1221-1226.
[6]LUO L, DONG L Y, YAN Q G, et al. Research progress in applying proteomics technology to explore early diagnosis biomarkers of breast cancer, lung cancer and ovarian cancer[J].Asian Pacific Journal of Cancer Prevention: APJCP, 2014,15(20):8529-8538.
[7]YE Z M, ZHENG Z J, LI X D, et al. B7-H3 overexpression predicts poor survival of cancer patients: a meta-analysis[J]. Cellular Physiology and Biochemistry, 2016,39(4):1568-1580.
[8]ZHANG G B, XU Y H, LU X D, et al. Diagnosis value of serum B7-H3 expression in non-small cell lung cancer[J]. Lung Cancer (Amsterdam, Netherlands), 2009,66(2):245-249.
[9]CHEN W W, LIU P X, WANG Y D, et al. Characterization of a soluble B7-H3 (sB7-H3) spliced from the intron and ana-
lysis of sB7-H3 in the sera of patients with hepatocellular carcinoma[J]. PLoS One, 2013,8(10): e76965. doi:10.1371/journal.pone.0076965.
[10]MASUDA A, ARAI K, NISHIHARA D, et al. Clinical significance of serum soluble T cell regulatory molecules in clear cell renal cell carcinoma[J]. BioMed Research International, 2014, 2014:396064.
[11]ZHAO L, XIE C, LIU D Q, et al. Early detection of hepatocellular carcinoma in patients with hepatocirrhosis by soluble B7-H3[J]. Journal of Gastrointestinal Surgery: Official Journal of the Society for Surgery of the Alimentary Tract, 2017,21(5):807-812.
[12]XIE C, LIU D Q, CHEN Q J, et al. Soluble B7-H3 promotes the invasion and metastasis of pancreatic carcinoma cells through the TLR4/NF-κB pathway[J]. Scientific Reports, 2016,6:27528.
[13]WANG L, KANG F B, ZHANG G C, et al. Clinical significance of serum soluble B7-H3 in patients with osteosarcoma[J]. Cancer Cell International, 2018,18:115.
[14]STEINBERGER P, MAJDIC O, DERDAK S V, et al. Mole-
cular characterization of human 4Ig-B7-H3, a member of the B7 family with four Ig-like domains[J]. Journal of Immunology (Baltimore, Md:1950), 2004,172(4):2352-2359.
[15]KANG F B, WANG L, JIA H C, et al. B7-H3 promotes aggression and invasion of hepatocellular carcinoma by targeting epithelial-to-mesenchymal transition via JAK2/STAT3/Slug signaling pathway[J]. Cancer Cell International, 2015,15:45.
[16]KREYMBORG K, HAAK S, MURALI R, et al. Ablation of B7-H3 but not B7-H4 results in highly increased tumor burden in a murine model of spontaneous prostate cancer[J]. Cancer Immunology Research, 2015,3(8):849-854.
[17]LIU F, ZHANG T, ZOU S T, et al. B7-H3 promotes cell migration and invasion through the Jak2/Stat3/MMP9 signaling pathway in colorectal cancer[J]. Molecular Medicine Reports, 2015,12(4):5455-5460.
[18]PODOJIL J R, MILLER S D. Targeting the B7 family of co-stimulatory molecules: successes and challenges[J]. BioDrugs, 2013,27(1):1-13.
[19]ZHANG S S, TANG J, YU S Y, et al. Expression levels of B7-H3 and TLT-2 in human oral squamous cell carcinoma[J]. Oncology Letters, 2015,10(2):1063-1068.
[20]LEITNER J, KLAUSER C, PICKL W F, et al. B7-H3 is a potent inhibitor of human T-cell activation: no evidence for B7-H3 and TREML2 interaction[J]. European Journal of Immunology, 2009,39(7):1754-1764.
[21]LEE Y H, MARTIN-OROZCO N, ZHENG P L, et al. Inhibition of the B7-H3 immune checkpoint limits tumor growth by enhancing cytotoxic lymphocyte function[J]. Cell Research, 2017,27(8):1034-1045.
[22]ZHANG G B, HOU J Q, SHI J F, et al. Soluble CD276 (B7-H3) is released from monocytes, dendritic cells and activated T cells and is detectable in normal human serum[J]. Immuno-
logy, 2008,123(4):538-546.
[23]CHEN L W, ZHANG G B, SHENG S Q, et al. Upregulation of soluble B7-H3 in NSCLC-derived malignant pleural effusion: a potential diagnostic biomarker correlated with NSCLC staging[J]. Clinica Chimica Acta; International Journal of Clinical Chemistry, 2016,457:81-85.
[24]LEE H, KIM J H, YANG S Y, et al. Peripheral blood gene expression of B7 and CD28 family members associated with tumor progression and microscopic lymphovascular invasion in colon cancer patients[J]. Journal of Cancer Research and Clinical Oncology, 2010,136(9):1445-1452.
[25]SUN J, LAI H J, SHEN D, et al. Reduced sB7-H3 expression in the peripheral blood of systemic lupus erythematosus patients[J]. Journal of Immunology Research, 2017, 2017:5728512.
[26]JIANG J A, JIANG J H, LIU C P, et al. Enhancement of membrane B7-H3 costimulatory molecule but reduction of its soluble form in multiple sclerosis[J]. Journal of Clinical Immunology, 2013,33(1):118-126.
[27]袁静萍,袁修学,阎红琳. 肿瘤浸润淋巴细胞在乳腺癌肿瘤微环境中的角色[J]. 临床与实验病理学杂志, 2018,34(12):1356-1359.
[28]黄佳慧,陈小松,沈坤炜. 肿瘤浸润淋巴细胞在乳腺癌中的研究进展[J]. 中华外科杂志, 2015,53(9):714-717.
[29]COHEN I J, BLASBERG R. Impact of the tumor microenvironment on tumor-infiltrating lymphocytes: focus on breast cancer[J]. Breast Cancer: Basic and Clinical Research, 2017,11. https://doi.org/10.1.1178223417731565.
(本文編辑 黄建乡)
