Efficacy and safety of accelerated corneal collagen cross-linking in patients with keratoconus
2021-07-08
Abstract
•KEYWORDS:keratoconus; cross-linking; higher-order aberration; corneal densitometry; topometric index; keratometry
INTRODUCTION
Keratoconus is a progressive corneal ectatic disease. The characteristics of this disease are corneal thinning, corneal protrusion and irregular steepening resulting in progressive myopia and myopic astigmatism. Corneal collagen cross-linking is known to halt the disease progression by strengthening the corneal stiffness.
Considering the concept of corneal collagen cross-linking, riboflavin is used in combination with ultraviolet-A (UVA) irradiation. The wavelength of the irradiation is 365 nanometres[1-2]. Riboflavin acts as a photosensitizer for the production of singlet oxygen and the free oxygen radicals then trigger the interaction of riboflavin and UVA. As a result, there are formations of intra- and inter-fibrillar carbonyl-based covalent bonds. Consequently, the keratoconic corneal stroma becomes stiffer[2].
Conventional Dresden protocol collagen cross-linking was first reported by Wollensaketal[1]using 30min of 3 mW/cm2UVA irradiation. This deals with debridement of the central 8-9 mm zone of corneal epithelium after application of topical anesthetic eye drop, then instilling a solution of 0.1% riboflavin in 20% dextran every 2min for 30min. Then, UVA light is irradiated to the cornea for 30min at 3 mW/cm2irradiance (5.4 J/cm2total energy). This protocol shows promising results and long-term corneal stabilization. Accelerated protocol was developed to reduce the procedure time. Regarding the Bunsen-Roscoe law of reciprocity, equal photochemical effects on the cornea can be reached by using higher intensities but a shorter period of time in order to achieve the same amount of cumulative doses[3-4]. The proposed advantages include decreased exposure time, better patient comfort, and lower risk of infection. We studied the one-year efficacy and safety of accelerated collagen cross-linking in Thai patients diagnosed with keratoconus.
SUBJECTS AND METHODS
EthicalApprovalThis study was approved by the Institutional Review Board, Faculty of Medicine, Chulalongkorn University, Thailand and adhered to the tenets of Declaration of Helsinki. The study was conducted at King Chulalongkorn Memorial Hospital, Bangkok, Thailand.
StudyDesignThe study was retrospective chart review. Medical records of keratoconus patients underwent accelerated collagen cross-linking between April 2015 and August 2018 were reviewed by single investigator.
StudyPopulationInclusion criteria were all keratoconus eyes performed accelerated cornea collagen cross-linking in our center during the time period previously mentioned. The indication for collagen cross-linking in our study were age under 24 years or 2 progressive keratoconus which was defined by one of the following criteria: 1) Maximum keratometry value (Kmax) increased more than 1.0 diopter (D) in 6mo; 2) Mean keratometry value (Kmean) increased more than 1.5 D in 6mo; 3) Manifest cylinder increased more than 1.0 D in 6mo; 4) Thinnest pachymetry decreased more than 5% in 6mo. Exclusion criteria are medical charts of which patients failed to follow-up for at least 6mo. In addition, we have contraindications for corneal collagen cross-linking as follows: 1) Thinnest pachymetry less than 350 microns; 2) Previous herpetic ocular infection; 3) Severe corneal scarring; 4) Concurrent ocular infection; 5) Neurotrophic keratopathy; 6) Autoimmune disorders; 7) History of poor epithelial wound healing; 8) Pregnancy.
SurgicalTechniqueCorneal epithelium was removed at 8-9 millimeters in diameter using Hockey knife. One drop of 0.1% isotonic riboflavin in 1.1% hydroxypropyl methylcellulose solution (Medicross M, Behrensbrook, Neudorf, Germany) was instilled every 2min for ten times. The pachymetry was measured to ensure that the pachymetry value was more than 400 microns. If the values were less than 400 microns, 0.1% riboflavin in sterile water (Medicross H, Behrensbrook, Neudorf, Germany) was instilled every 2min until the pachymetry value was above 400 microns. The anterior chamber flare was then checked. UVA was irradiated for 5min with a fluence of 18 mW/cm2(CCL-365 Vario cross-linking system, Peschke Meditrade GmbH, Huenenberg, Switzerland). All eyes were covered with soft contact lens for 1wk. The patients were given a combination of topical 0.5% moxifloxacin and 0.1% dexamethasone eye drops four times daily for 1mo postoperatively. The cross-linking was performed by five experienced corneal specialists.
OutcomeMeasurementsBaseline characteristics of the patients, visual acuity [both uncorrected visual acuity (UCVA) and best corrected visual acuity(BCVA)] and refraction were recorded. Corneal tomography (Pentacam; Oculus, Inc., Wetzlar, Germany) was used to evaluate Kmax, Kmean, corneal astigmatism, Q-value, thinnest pachymetry, higher-order aberrations (HOA) at 6 mm zone from vertex, topometric indices and corneal densitometry. Corneal densitomtry, a method to measure the backscattered light from the cornea, divided into three zones (0-2, 2-6 and 6-10 mm diameter from corneal apex) and three depth layers (anterior 120 microns, posterior 60 microns and between). The topometric indices included index of surface variance (ISV), index of vertical asymmetry (IVA), keratoconus index (KI), central keratoconus index (CKI), index of height asymmetry (IHA), index of height decentration (IHD), the minimum radius of curvature (Rmin). Demarcation line was evaluated by slit lamp biomicroscopy and by anterior segment imaging using CASIA SS-1000 OCT (Tomey, Nagoya, Japan). All data were analyzed at 1, 3, 6mo and 1a. All the reported eyes at each time interval are the same. Sub-group analysis according to Amsler-Krumeich classification was analyzed. Moreover, we also studied the effectiveness of the collagen cross-linking regarding the characteristics of the eyes. Age of 24 and 30 years, Kmax of 55 D, and baseline BCVA of 20/40 (or 0.3 in LogMAR unit) were used as cut-off values to highlight the cross-linking effects. The association between the change of corneal densitometry and other factors including preoperative Kmax, Kmean, manifest refraction spherical equivalent (MRSE), visual acuity, thinnest pachymetry, the change in Kmax, and the change of those parameters were also analyzed. All complications were recorded.
StatisticalAnalysisBaseline characteristics of patients were described using mean±standard deviation (SD) for the continuous data and frequency and percentage for the categorical data. For visual acuity, manifest refraction spherical equivalent, Kmax, Kmean, corneal astigmatism, Q-value, thinnest pachymetry, HOA, topographic indices and corneal densitometry, multilevel data analysis (mixed linear model) were used to compare the difference of these values between each visit. The effect of age, preoperative Kmax and BCVA were analyzed by using Chi-squared test or Fisher’s exact test. Association factors were determined by Logistic regression analysis for categorical data and linear regression analysis for continuous data. Statistical analyses were performed with STATA®, Release 13.1 (StataCorp, 2013. College Station, TX, USA). Statistically significant change was considered whenPvalue was less than 0.05.
RESULTS
One hundred and fifty-five patients(185 eyes) were included. The demographic data were shown in Table 1 and the baseline values were shown in Table 2.

Table 1 Demographics data
VisualAcuityandManifestRefractionSphericalEquivalentUCVA and BCVA demonstrated significant improvement at 3mo postoperatively (P<0.05). At 1a, UCVA (LogMAR) improved from baseline value of 0.78±0.57 to 0.66±0.52 (P<0.05) but BCVA was not statistically different from baseline (P>0.05) (Table 3). However, the improvement of BCVA was found in stage 1 at 1a (P<0.05) (Figure 1). Manifest refraction spherical equivalent (MRSE) transiently increased at 1mo (P<0.05) and showed no significant change at 1a (P>0.05) (Table 3).
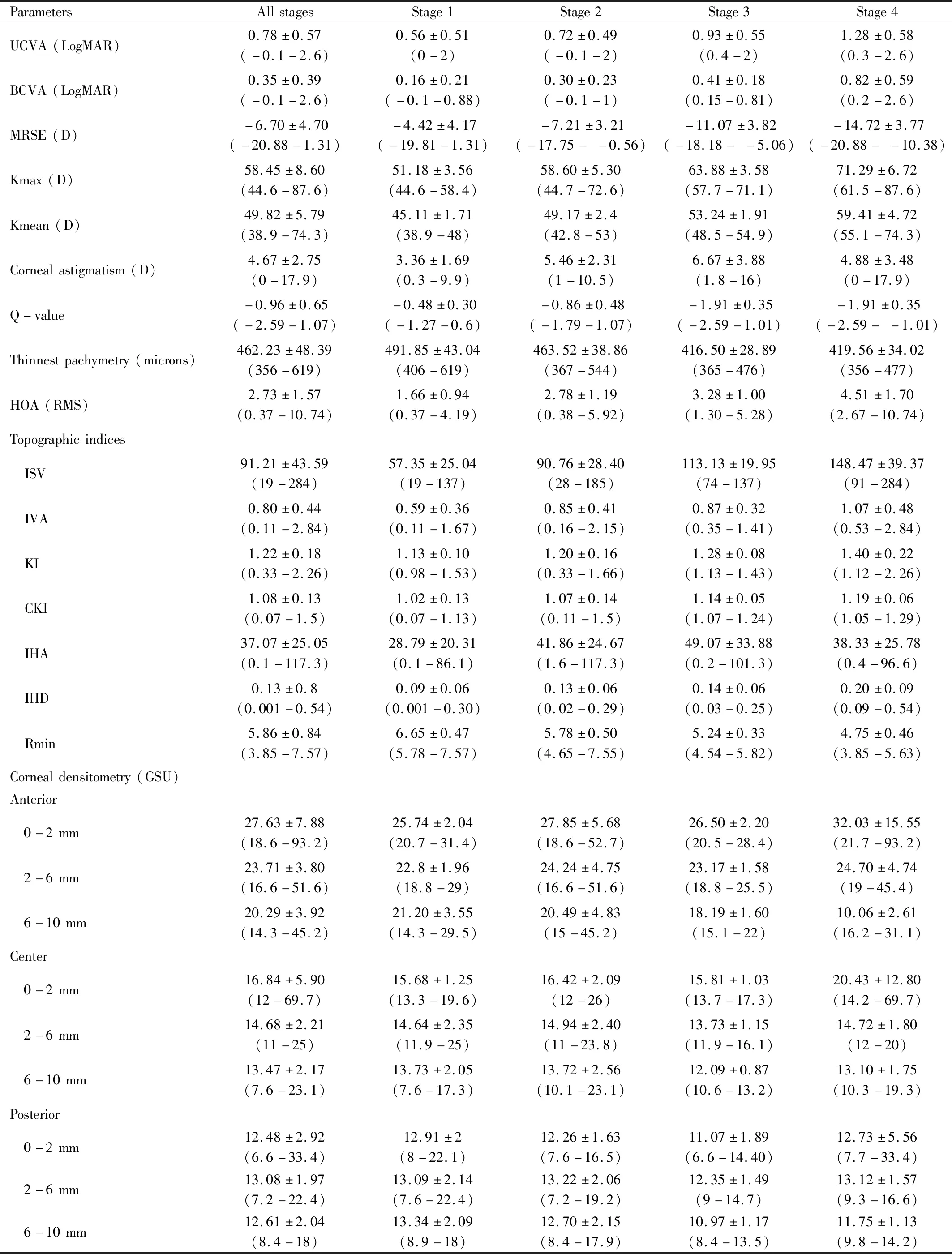
Table 2 Outcome parameters at baseline (min-max) mean±SD
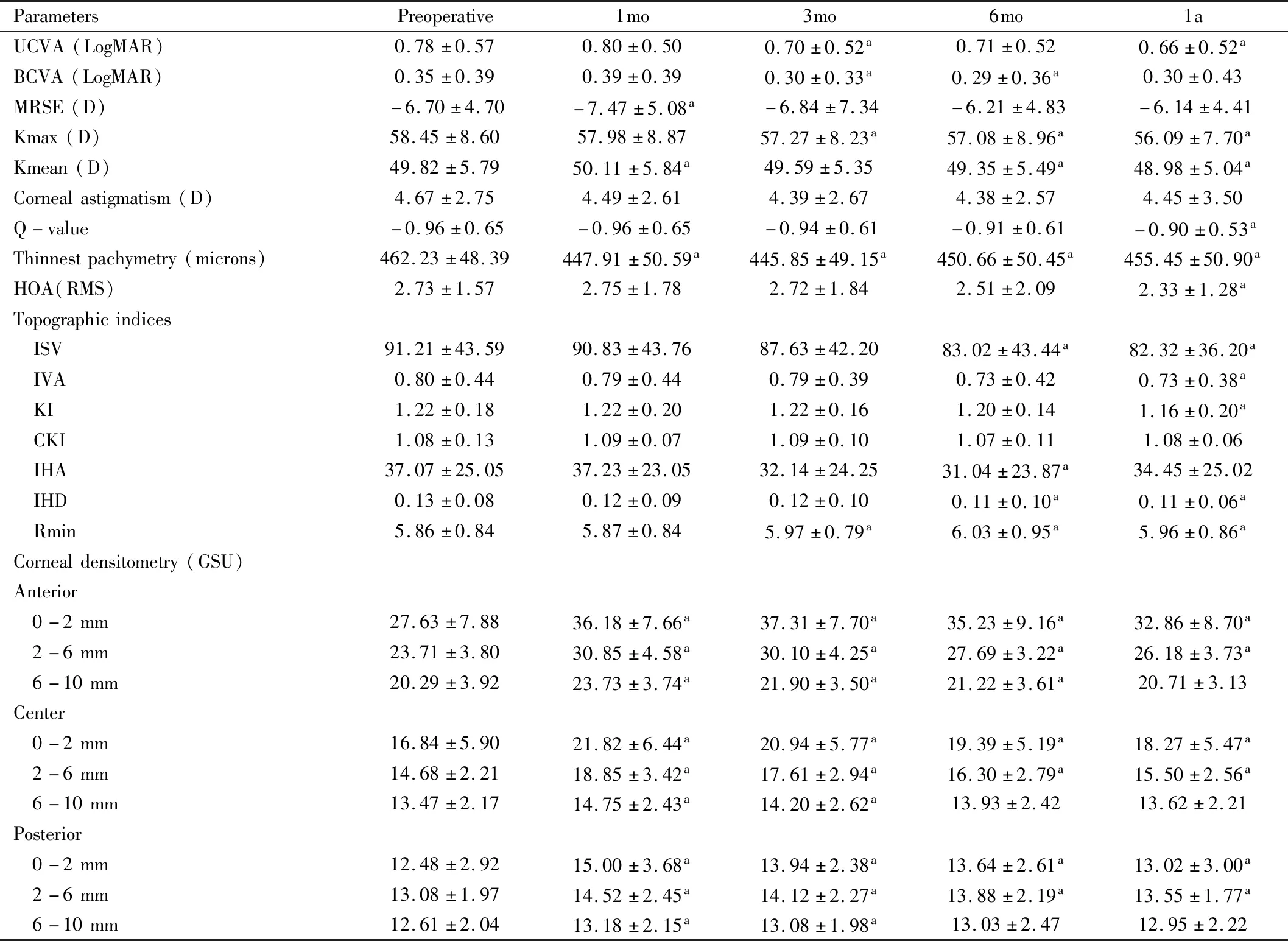
Table 3 Clinical measures in each visit (mean±SD)

Table 4 Kmax in each stage and 1a difference (mean±SD)
BestCorrectedVisualAcuityEffectWe used cut-off baseline BCVA value of 0.3 in LogMAR unit to count the number of the eyes of which BCVA improved more than 0.2 LogMAR at 1a. At 1a, the number of eyes of which BCVA improved more than 0.2 LogMAR was higher in the group of which preoperative BCVA worse than 0.3 compared to the group of which preoperative BCVA better than 0.3 (78.26%vs21.74%,P<0.001).
MaximumKeratometryValueandMeanKeratometryValueMaximum keratometry value (Kmax) statistically significantly decreased at 3, 6mo and 1a (P<0.001). At 1a, mean Kmax difference was 2.36 D compared to preoperative level (Table 3). Mean keratometry value (Kmean) transiently increased at 1mo (P=0.049) (Table 3). At 6mo, Kmean began to decrease statistically compared to baseline level (P=0.004). At 1a, Kmean was statistically significantly lower than baseline level by 0.84 D (P<0.001). In all stage subgroups, we found that Kmax and Kmean statistically decreased at 1a (P<0.05). Furthermore, the more the severity, the more decrease in Kmax value was observed (Figure 1; Table 4).
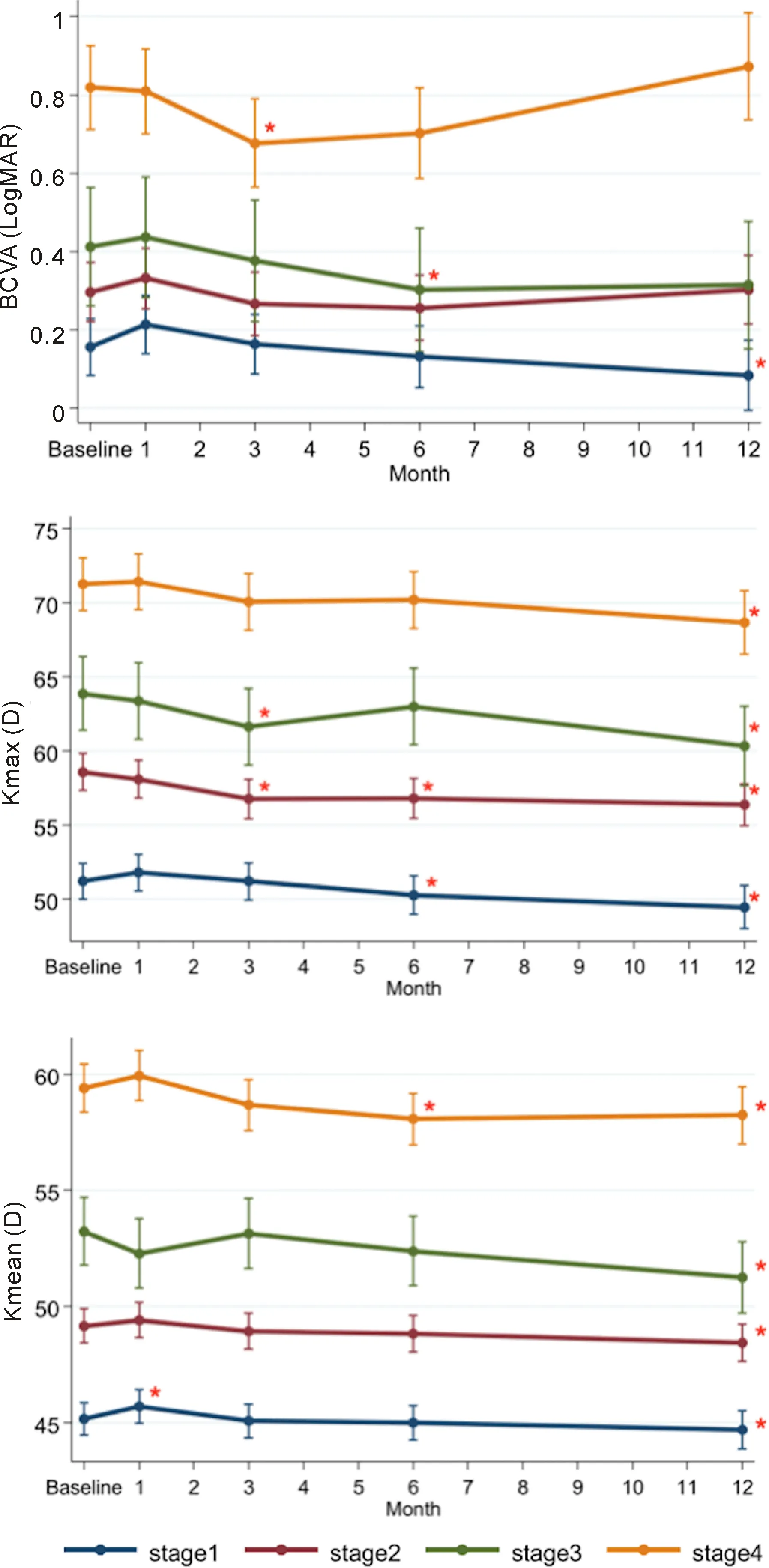
Figure 1 BCVA, Kmax, Kmean in each stage of the disease (compared to preoperative value P<0.05).
AgeandKmaxEffectIn those who had Kmax reduction ≥2.0 D at 1a postoperatively, the subgroup analysis was performed using the cut-off value of age at 24, 30 years and preoperative Kmax of 55.0 D. The results were shown in Table 5 and Table 6.
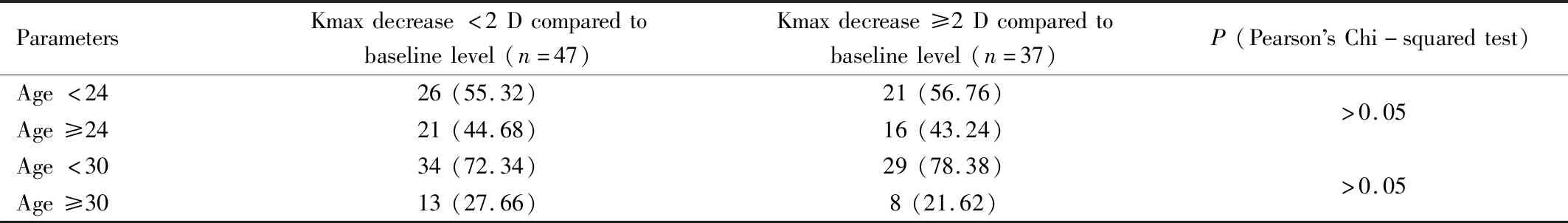
Table 5 Number and percentage of eyes of which Kmax decrease less or more than 2 D at 1a regarding cut-off age value of 24 and 30 and Kmax value of 55 D n(%)

Table 6 Number and percentage of eyes of which Kmax decrease less or more than 2 D at 1a regarding cut-off baseline Kmax value of 55 D n(%)
SuccessoftheProcedureSuccess rate after the procedure was defined as a decrease in the Kmax reading or increase not more than 1 D at the first postoperative year. Of eighty-two eyes of patients who had follow-up at 1a, there were seventy-four eyes (90.24%) that were defined as success.
CornealAsphericity(Q-value) Q-value in each visit was shown in Table 3 and was statistically decreased at 1a (P<0.05).
ThinnestPachymetryThe thinnest pachymetry began to statistically decrease at 1mo (P<0.001) as shown in Table 3. At 1a, the thinnest pachymetry was statistically lower than that baseline value by 6.78 microns (P<0.001). According to stage subgroup analysis, at 1a, only eyes in stage 1 had statistically decreased thinnest pachymetry compared to baseline level (P<0.001) while eyes in other stages showed no statistical change at 1a (Table 7).
Higher-orderaberrationThe HOA did not statistically change at 1, 3 and 6mo (P>0.05). However, HOA statistically decreased at 1a (P=0.022) as shown in Table 3.
TopographicIndicesThe mean and standard deviations of each topographic indices values were shown in Table 3. ISV (a unitless standard deviation of individual corneal sagittal radii from the mean curvature which is a general measure of corneal surface irregularity) and IVA (a measure of the difference between superior curvature and inferior curvature in the cornea) began to statistically decrease at 6mo and 1a (P<0.05) as shown in Table 3. KI and IHD calculated with Fourier analysis of corneal height to quantify the degree of vertical decentration, statistically decreased at 1a (P=0.001). IHA, a measure similar to the IVA but based on corneal elevation, statistically decreased at 6mo (P=0.023). CKI did not statistically change (P>0.05). Rmin statistically increased at 3, 6mo and 1a (P<0.05).
CornealDensitometryThe mean and standard deviations of corneal densitometry in each position of cornea were shown in Table 3. Anterior central cornea demonstrated the greatest densitometry. Densitometric declined towards posterior and peripheral area. Increase of the densitometry was found significant at 1mo postoperatively in almost all depth and area.

Table 7 Thinnest pachymetry in each stage (mean±SD)
Gradual reduction of densitometric values was evidenced but remained statistically higher than baseline level at 0-2 and 2-6 mm zone at 1a (P<0.05). Subgroup analysis regarding the stage of the disease showed similar results (Table 8).
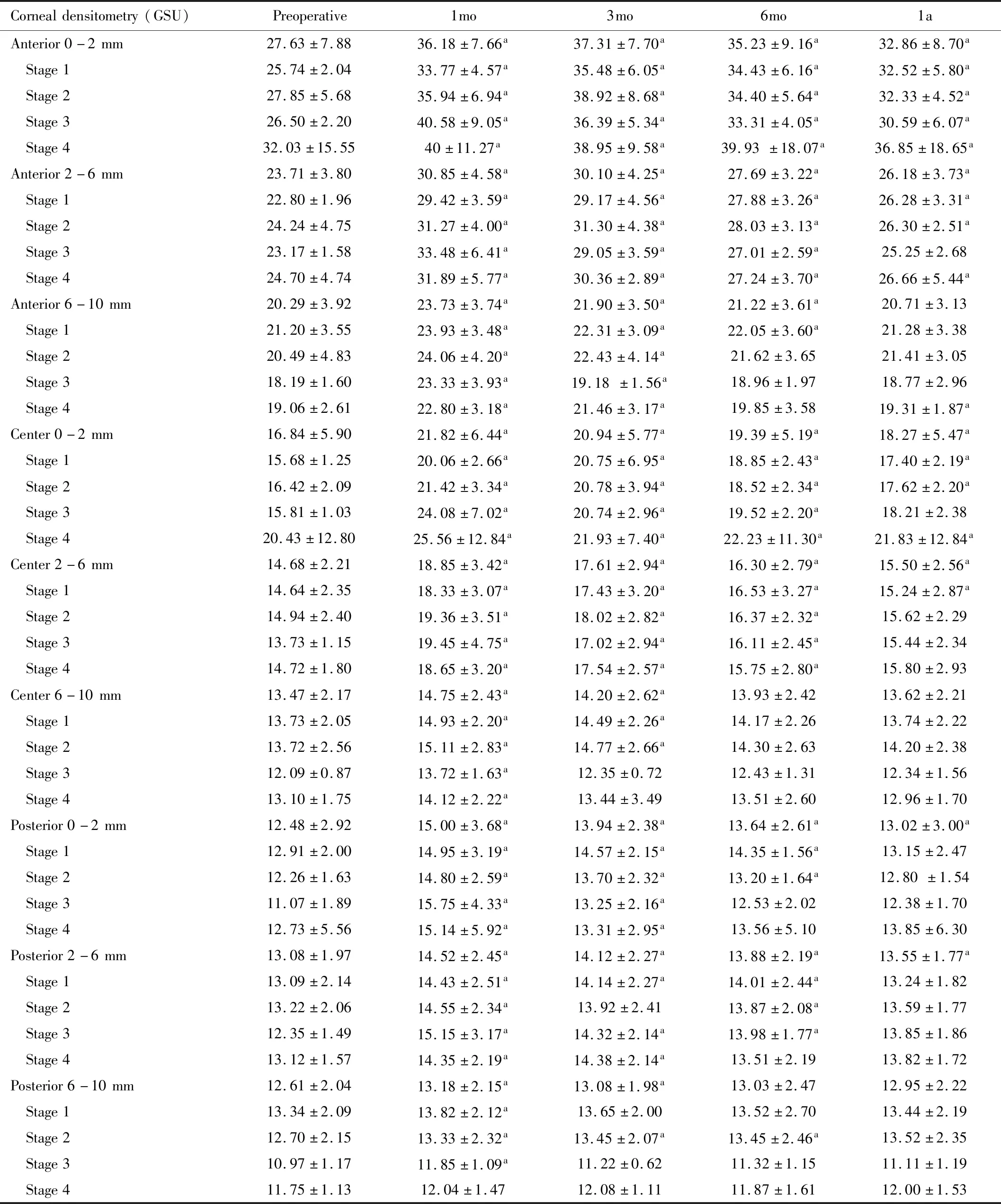
Table 8 Corneal densitometry in each stage (mean±SD)
The increase of densitometry at anterior and center layer of 0-6 mm zone was associated with the change of the thinnest pachymetry at 1a compared with baseline level (Linear regression analysis,P<0.05). The relationship of the increase in densitometric value and the decrease of thinnest pachymetry at 1a were in linear fashion as follows: “y=kx+c”, wheare “y” is the change of densitometric values at 1a (GSU); “x” is the change of the thinnest pachymetry at 1a (microns); if at anterior layer of the 0-2 mm zone; k= -0.016 and c=5.810; if at anterior layer of the 2-6 mm zone; k= -0.039 and c=2.046; if at central layer of the 0-2 mm zone; k=-0.058 and c=1.226; if at central layer of the 2-6 mm zone; k= -0.033 and c=0.687. However, preoperative Kmax, Kmean, MRSE, UCVA, BCVA, thinnest pachymetry and the change of these parameters were not associated with the densitometry change (Linear regression analysis,P>0.05).
ComplicationsPostoperative corneal haze was found 11.35%, 30.27%, 15.67%, 10.27% and 2.16% at 1wk, 1, 3, 6mo and 1a respectively. No eyes developed corneal edema. Ninety-eight percent of eyes were free of complications at 1a. There was one case of sterile keratitis after the collagen cross-linking. The infiltrate was multiple round white dots at the peripheral cornea both with and without epithelial defects occurred within one week and responded well to intensive topical steroid within a week. There was one case of presumed bacterial keratitis. The patient presented with red eye one week after the procedure. Empirical topical 0.5% moxifloxacin was initiated every hour for five days and then tapered to four times daily after clinical improvement. Culture of contact lens revealed negative for organisms.
DISCUSSION
Considering previous studies using accelerated protocol with 18 mW/cm2irradiation of UVA for 5min, our study showed greatest reduction of Kmax[5-8]. At 1-year result, our study showed that Kmax statistically decreased by 2.36 D whereas Hashemietal[6]found that Kmax decreased by 0.06 D at 18mo and Chowetal[5]found that Kmax decreased by 0.47 D at 12mo. At 1-year result, our study showed Kmean statistically decreased by 0.84 D. In contrast, Shettyetal[7]showed decrease in K1 and K2 by 0.29 and 0.52 D at 12mo. Hashemietal[6]and Chowetal[5]showed decrease in Kmean by 0.21 D at 18mo and by 0.19 at 12mo respectively. Our significant flattening of the cornea was comparable to reported results using conventional Dresden protocol[9-11]. This probably resulted from several reasons. One is that in our study the mean Kmax of patients included in the study was higher. The mean preoperative Kmax in study by Chowetal[5]and Hashemietal[6]were 51.96 D and 47.89 D respectively whereas that in our study was 58.45 D. This was in concordance with the Kmax effect that we found the number of eyes of which Kmax decreased more than 2.0 D was higher in baseline Kmax ≥55 D group. Another reason is our number of population was quite high enough to detect this result. According to the results that Kmax reduction more than 2.0 D at 1a was found higher in patients whose preoperative Kmax ≥55.0 D, this finding was consistent with results from conventional Dresden protocol studied by Greensteinetal[12]. This would provide information for ophthalmologists to predict the outcome from preoperative Kmax and Kmean readings.
Regarding visual acuity, we found that UCVA statistically decreased significantly at 1a. This was similar to previous studies[5-6]. This probably resulted from flattening effect of corneal cross-linking. However, at 1a, BCVA showed no statistical difference compared with baseline. This was also consistent with previous studies[5-6]. This was probably because the MRSE remained statistically unchanged in both our results and the results of previous studies[5-6]. We also found that eyes with worse preoperative BCVA were more likely to have an improvement. This was consistent with previous study by Greensteinetal[12]. Consequently, it might be reasonable to state that eyes with worse vision would allow more chance of improvement.
Considering the Q-value, we found that collagen cross-linking could improve the corneal asphericity. This showed that cornea shape was less prolate. This was probably because the flattening effect of the procedure.
Regarding the thinnest pachymetry, we found that collagen cross-linking made the thinnest cornea thinner by 6.78 microns at 1a. Although this difference was statistically significant, the amount of the change was little and less than 5% which is one of the criteria for progressive disease[3]. This result is consistent with previous studies[1,3,5-7,11]. This thinning effect may be the consequence of increase keratocyte apoptosis. The decrease in corneal thickness has been reported up to 36mo postoperatively[13]. Change in corneal thickness could also be due to epithelial remodeling.
Considering HOA, the effect of collagen cross-linking remained controversial. Uysaletal[14]and Greensteinetal[15]found that collagen cross-linking effect could improve HOA at 1a. Ghanemetal[16]also found similar results at 2a. However, Wisseetal[17]found that HOA remained unchanged at 1a. All mentioned studies used standard Dresden protocol for collagen cross-linking[14-17]. To date, our study was the first to evaluate the HOA after accelerated protocol of collagen cross-linking. We found that HOA statistically decreased at 1a. This improvement of HOA might be associated with UCVA. Nevertheless, further studies to show the association or cause-and-effect result are yet to be performed. Further studies involving the glare test or questionnaire dealing with HOA are needed.
Considering topographic indices, in keratoconus there were increases in all topographic indices (ISV, IVA, IHD, IHA, KI, CKI) except Rmin. Rmin decreases in keratoconus. We found that 1a after collagen cross-linking, ISV, IVA, KI, IHD decreased whereas CKI and IHA remained unchanged at 1a after cross-linking. Decrease in ISV reflects a decrease in variation of corneal curvature compared with the mean curvature. Decrease in IVA means there was decrease in difference of superior and inferior curvature of cornea. Since KI was derived from the ratio of the mean superior radius of curvature to the inferior radius of curvature and KI usually increases in keratoconus, decrease in KI implies that postoperative topography of the cornea become more normal. IHD was based on Fourier analysis of the elevation data and shows the amount of vertical decentration. This index tends to be steeper in keratoconus. Decrease in IHD reflects less degree of vertical decentration. Moreover, in our study Rmin increased as we expected. All five parameter improvements after collagen cross-linking may be attributable to the flattening and reshaping effect of the procedure. We can infer that after collagen cross-linking the cornea becomes more symmetric, regular and flattening. Previous studies also showed some improvements in common. Kolleretal[18]found improvements in CKI, KI, IHA and Rmin 1a after cross-linking while Greensteinetal[19]found improvements in ISV, IVA, KI and Rmin 1a after Dresden protocol cross-linking. Omaretal[20]found significant reduction in ISV, IVA, and KI but not in IHA and IHD 1a after accelerated protocol. However, the protocol that Omaretal[20]used was different from ours. 0.1% Riboflavin drops were instilled over the de-epithelized cornea four times every 2min while in our protocol with ten times every 2min. Exposure to 365 nm UVA was performed with a total surface dose of 7.2 joules which was pulsed (1s on, 1s off) for 5min and 20s, achieving a total delivery of 120 mWatt which were higher than our energy[20].
Considering the corneal densitometry, Greensteinetal[21]found a significant increase in densitometry at 1mo and a significant reduction between 6mo and 12mo. On the contrary, Kimetal[22]found that the densitometry peaked at 1mo and returned to baseline level after 6mo. However, in our study, most of the areas of the cornea reached their maximum density at 1mo after the procedure and decreased over time. At 1a, the densitometry values at 0-6 mm zone were still statistically higher than those at baseline regardless of the depth of the cornea whereas the densitometry values at 6-10 mm zone were not statistically different from baseline level. We also found that decrease of the thinnest pachymetry was associated with the increase in densitometry. This provided ophthalmologist instant useful information to indirectly expect the increase of the densitometry by notice the alteration of the thinnest pachymetry. This evidence was novel. Corneal densitometry provides a quantitative method of haze measurement. Increase in densitometry might affect vision. Previous studies estimated that after cross-linking, the keratocyte apoptosis might lead to lacunar edema[22-24]. Studies usinginvivoconfocal microscopy found that there were increase in density of the extracellular fibrillar matrix and reduce in density of anterior keratocytes[25-26]. This might lead to transient haze after cross-linking. In our study, only 4 eyes had clinical corneal haze at 1a while the other eyes were free of haze at 1a. This means that the clinical haze was undetected while the corneal densitometry still remained.
Opting between epithelium-off and transepithelial cross-linking, we took both efficacy and safety of the procedures into account. Soetersetal[27]in 2015 found that although transpithelial cross-linking was a safe procedure without epithelial healing problems, 23% of cases showed a continued keratoconus progression after 1a. Therefore, at that time, they did not recommend replacing epithelium-off cross-linking by transepithelial cross-linking for treatment of progressive keratoconus[27]. Also, a recent systematic review and Meta-analysis by Nathetal[28]in 2020 found that the efficacy of transepithelial cross-linking remained inferior to the epithelium-off approach, although it was significantly safer. They found that the gap in decrease in maximum keratometric value between the two protocols was widening over time. The incidence of disease progression at 12mo after treatment was noted to be significantly higher with transepithelial cross-linking when compared with conventional cross-linking. Regarding usual adverse events, haze in cross-linking was encountered commonly but did not impact visual function substantially. Moreover, haze after cross-linking was often self-limiting. Focusing their attentions on more severe adverse events, such as corneal melt, persistent epithelial defects, and visually significant, non-resolving haze, they found that although these complications were determined to be more likely a result of epithelium-off cross-linking (RR, 0.22), the overall rate of complications remained low at 4% within the epithelium-off group and 2% in the transepithelial group[28]. All things considered, we continued using epithelium-off technique rather than transepithelial technique in our study.
Since the number of the experienced specialists performing collagen cross-linking in our study was five, there was still a chance that could bias the outcomes. However, we included all cases performed in our center during that period of time in order to reduce the bias as much as possible. Another limitation was that in our study was a retrospective before-after study. Long-term outcomes of randomized controlled trials remain to be studied.
In conclusion, the accelerated corneal collagen cross-linking in progressive keratoconus and in patients under 24 years of age was effective to flatten, reshape the cornea, and also improve the unaided visual acuity and HOA. Also, the cross-linking improved most of the topographic indices. However, the corneal densitometry at one year after the surgery is still higher but tends to decrease over time.
