Total flavonoids from Saussurea involucrata attenuate inflammation in lipopolysaccharide-stimulated RAW264.7 macrophages via modulating p65, c-Jun,and IRF3 signaling pathways
2021-05-20LiShanYanLiWangBrianChiYanChengYuDingJingKongQingGaoWangXiuQiongFuShuoFengZhangGanLuoYiZhang
Li-Shan Yan, Li Wang, Brian Chi-Yan Cheng, Yu Ding, Jing Kong, Qing Gao Wang, Xiu-Qiong Fu,Shuo-Feng Zhang, Gan Luo✉, Yi Zhang✉
1School of Chinese Materia Medica, Beijing University of Chinese Medicine, Beijing, China
2College of Professional and Continuing Education, Hong Kong Polytechnic University, Hong Kong, China
3The First Affiliated Hospital, Guangxi University of Chinese Medicine, Guangxi, China
4Centre for Cancer and Inflammation Research, School of Chinese Medicine, Hong Kong Baptist University, Hong Kong, China
ABSTRACT
KEYWORDS: Saussurea involucrata; Cytokines; Proinflammatory;Macrophages; Flavonoids
1. Introduction
The immune system is our host defense against various adverse conditions, such as tissue injury from xenobiotic insult[1]. However,over-activated or dysregulated immune responses may conversely result in molecular dysregulation, cellular injury, and tissue damage[2]. In spite of the complexity of immune pathogenesis,molecular evidence has indicated that toll-like receptors (TLRs),especially TLR4, play a key role in various infectious diseases, such as sepsis, plague, asthma, and pneumonia[3,4]. Increased activity of TLR4 has been detected in many types of immune cells[4], such as macrophages[5]. Upon ligand binding to external stimuli [e.g.lipopolysaccharide (LPS)], TLR4 interacts with its co-receptor MD2 and adaptors, and subsequently initiates MyD88-dependent and-independent signaling[6]. The subsequent kinase cascades result in the activation of transcription factors, such as nuclear factor κB (NF-κB), activator protein 1 (AP-1), and interferon regulatory factor 3(IRF3), leading to increased production of inflammatory mediators which trigger immune responses. Therefore, blocking NF-κB, AP-1,and IRF3 signaling pathways emerges as an attractive therapeutic approach for inhibiting inflammatory actions[7].
Because of the chronic nature of some inflammatory diseases,patients may need long-term treatment using drugs like nonsteroidal anti-inflammatory drugs, which may cause serious adverse effects[8].Seeking a safe and effective anti-inflammatory agent has been attracting intensive interest of researchers. Herbal medicines may provide an ideal source of safe and effective agents for prevention and/or treatment of inflammatory diseases[9]. However, one of the most important obstacles for Chinese medicinal herbs to be included in mainstream medicine is unclear active constitutes and mechanism of action. Saussurea involucrata (S. involucrata) (Xue Lian Hua in Chinese) is a traditional Chinese herbal medicine and has been widely used for the treatment of rheumatoid arthritis, stomachache,and dysmenorrhea for hundreds of years in China[10]. It has been reported that the extracts of S. involucrata possessed a wide array of pharmacological actions, including anti-inflammatory, antihypoxic, and anti-fatigue activities[11-13]. The major constituents isolated from S. involucrata include flavonoids, amino acids,polysaccharides, and sesquiterpene lactone[14-18]. Recent studies reported that as natural polyphenolic compounds, flavonoids could inhibit regulatory enzymes and transcription factors involved in inflammatory actions[19]. Thus, we hypothesized that flavonoids from S. involucrata might possess anti-inflammatory properties.To verify our hypothesis, we isolated the total flavonoids from S.involucrata and evaluated the anti-inflammatory effects and the underlying mechanisms of total flavonoids from S. involucrata in LPS-stimulated RAW264.7 cells.
2. Materials and methods
2.1. Chemicals, reagents, and antibodies
Chlorogenic acid (batch number: Y24J7K16726) and rutin (batch number: Y16M9S61523) were purchased from Shanghai Yuanye Biotechnology Co. Ltd. (Shanghai, China). The purity of each standard was higher than 98%. AB-8 macroporous resin was obtained from the chemical plant of Nankai University (Tianjin, China).Polyamide resin was bought from Sinopharm Chemical Reagent Co.Ltd. (Shanghai, China). 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), LPS (Escherichia coli 055:B5), and modified Griess reagent were obtained from Sigma Chemical Co. (St. Louis, MO, USA). Fetal bovine serum was bought from Biological Industries (Beth-Haemek, Israel). Dulbecco’s Modified Eagle Medium was obtained from Corning Cellgro (Manassas, VA,USA). Penicillin-streptomycin solution was bought from Caisson labs (Smithfield, UT, USA). Tumor necrosis factor-alpha (TNF-α),interleukin 10 (IL-10), macrophage inflammatory protein-1α (MIP-1α), monocyte chemoattractant protein-1 (MCP-1), and RANTES ELISA kits were purchased from Thermo Fisher Scientific (San Diego, CA). Prostaglandin E(PGE) ELISA kit was provided by Enzo Life Sciences (Exeter, UK). IκB kinase alpha (IKKα) and SP1 monoclonal antibodies were supplied by Santa Cruz Biotechnology(Santa Cruz, CA, USA). IRF3 and anti-mouse IgG HRP linked antibody were purchased from Abcam (Cambridge, UK). IRF3 (Ser 396)was provided by ABNOVA (Taiwan, China). Phospho (p)-NF-κB/p65 (Ser536), NF-κB/p65, p-c-Jun (Ser73), c-Jun, cyclooxygenase-2(COX-2), inducible nitric oxide synthase (iNOS), p-IRF3 (Ser396),p-IκBα (Ser32), IκBα, p-IKKα/β (Ser176/177), extracellular-signalregulated kinases (ERK), p-ERK (Thr202/Tyr204), c-Jun N-terminal kinase (JNK), p-JNK (Thr183/Tyr185), p-p38 (Thr180/Tyr182), p38,TANK-binding kinase 1 (TBK1), p-TBK1 (Ser172), anti-rabbit IgG HRP linked antibody, and Alexa Fluor 488-conjugated secondary antibody were bought from Cell Signaling Technology (Boston, MA,USA).
2.2. Preparation of ethanol extract of S. involucrata
S. involucrata was bought from local market of Xinjiang and authenticated by Lecturer Guang Xi-ren (School of Chinese Materia Medica, Beijing University of Chinese Medicine). Voucher specimens were deposited at the Department of Pharmacology,School of Chinese Medicine, Beijing University of Chinese Medicine. S. involucrata was cut into small pieces. Then the pieces(70 g) were homogenized with 700 mL of 70% ethanol (v:v) at room temperature. Subsequently, heating under reflux was performed 3 times and 1 h for each at room temperature. The extraction solvent was slightly boiled across the whole extraction process and the boiling point of the solvent was 80.2 ℃. Then combined extracting solution was filtrated, and vacuum-evaporated to remove the solvent.Finally, ethanol extract of S. involucrata (17.82 g) was obtained.
2.3. Preparation of flavonoids of S. involucrata powder
A chromatographic column (3.5 cm × 100 cm) wet-packed with AB-8 macroporous resin (chemical plant of Nankai University,Tianjin, China) was used to carry out the enrichment experiment.The ratio of diameter to height was 1:8 and the bed volume (BV) was 270 mL. Briefly, 16 grams of ethanol extract of S. involucrata were dissolved by 50 mL of water and the mixture was then loaded on the chromatographic column. The flow rate was 2 BV/h. Next, 4 BV of water was used to remove the aqueous impurities. The column was then eluted with 6 BV 15% and 40% methanol aqueous solution,respectively. The 40% ethanol eluate was collected and vacuum dried at 60 ℃ to afford a residue (1.3 g flavonoid enrichment of S.involucrata).
The purification was conducted on a glass column (2.6 cm × 50 cm)wet-packed with polyamide resin (Sinopharm Chemical Reagent Co., Ltd, Shanghai, China). The ratio of diameter to height was 1:8 and the BV was 110 mL. One gram of flavonoid enrichment was dissolved in 50 mL of water and loaded onto the column with a flow rate of 2 BV/h. Next, 4 BV of water and 4 BV of 20% ethanol were used to remove impurities. The column was then eluted with 6 BV 50% ethanol. The eluates were collected, merged, and evaporated under reduced pressure using a rotator evaporator and vacuum dried at 60 ℃. Finally, 305 mg of flavonoid of S. involucrata was obtained. For ultra-performance liquid chromatography (UPLC)analysis, ethanol extract and flavonoid enrichment were diluted by 50% methanol-water solution, and flavonoids of S. involucrata were diluted by 70% methanol. All samples were filtered through a millipore membrane filter with an average pore diameter of 0.22 μm,and each sample (5 μL) was injected for UPLC analysis.
2.4. Characterization of the ethanol extract, flavonoid enrichment, and flavonoids of S. involucrata
To control the quality of flavonoids of S. involucrata, UPLC analysis was conducted using a Waters Acquity UPLC system (Waters Corporation, Milford, MA, USA), consisting of a binary solvent delivery pump, an autosampler, and a photodiode array detector. The chromatographic separation was performed by an Acquity UPLCBEH Ccolumn (1.7 μm, 100 mm × 2.1 mm) with a Van GuardPre-Column (1.7 μm, 5 mm × 2.1 mm). The gradient mobile phase consisted of solvent A (acetonitrile) and solvent B (0.2% formic acid in water). The UPLC elution profile was as follows: 0-6 min, 5%-9% A; 6-12 min, 9%-13% A; 12-19 min, 13%-16% A; 19-28 min, 16%-17% A; 28-40 min, 17%-28% A. The flow rate was 0.3 mL/min and the column temperature was set at 40 ℃. The chromatograms were monitored with the PDA detector at a wavelength of 340 nm to detect chlorogenic acid and rutin.
2.5. Detection of total flavonoids content
The total flavonoid contents in three samples were determined according to a previous study with some modifications[20]. Each mixture containing 0.2 mL of 5% (w/v) NaNOand 0.1 mL of test compound was incubated for 6 min at 22 ℃ before adding 0.2 mL of 10% (w/v) Al(NO). After additional 6 min incubation, 2 mL of 4% NaOH and 2.7 mL of 70% methanol were added and the absorbance was read at 505 nm. The experiment was performed in three replicates.
在“寿怡红群芳开夜宴”那回,在场的丫鬟小姐全都占了花名,惟独没写晴雯占,林黛玉已经掣了一枝“芙蓉签”在手。
2.6. Cell lines and cell culture
The RAW264.7 cell line was obtained from American Type Culture Collection (Manassas, VA, USA). Cells were cultured in Dulbecco’s Modified Eagle Medium supplemented with penicillin/streptomycin antibiotics (1%) and heat-inactivated fetal bovine serum (10%) in a 5% COincubator at 37 ℃. Flavonoids of S. involucrata were dissolved in dimethyl sulfoxide to prepare a stock solution and then freshly diluted with cell culture medium to various concentrations.
2.7. MTT assay
RAW264.7 cells were seeded (6 × 10cells/well) into 96-well plates.Cells were treated with 1 μg/mL lipopolysaccharides (LPS) in the presence or absence of various concentrations of flavonoids of S.involucrata (6.25-400 μg/mL) for 24 h at 37 ℃. Then, cells were incubated with 10 μL of the MTT solution (5 mg/mL) for 3 h at 37 ℃. The medium was carefully aspirated and MTT formazan precipitate was dissolved in 100 μL dimethyl sulfoxide. Then the absorbance was measured at 570 nm using a microplate spectrophotometer (BMG SPECTROstar Nano, Germany). Cell viability in the control group was set as 100%. Six replicates of experimental data were used.
2.8. Griess assay
As nitric oxide (NO) plays important roles in LPS-induced inflammatory responses and is implicated in the pathogenesis of various inflammatory conditions[21,22], we determined the content of NO in the culture medium after LPS treatment by Griess assay.Briefly, RAW264.7 cells were seeded at 2 × 10cells/mL on 24-well culture plates for 24 h. Cells were treated with 1 μg/mL LPS in the presence or absence of various concentrations of flavonoids of S. involucrata (25-200 μg/mL) for 24 h at 37 ℃. NO production was determined by measuring the accumulated nitrite (a stable degradation product of NO) in the culture medium with Griess reagent. Absorbance at 540 nm was measured with a NaNOstandard curve. NO production was then determined.
2.9. ELISA assays
Macrophages activated by endotoxin initiate the release of pro-inflammatory cytokines and chemokines in the early stage of inflammatory response[5,23]. Hence, we detected several imflammatory cytokines and chemokines in the culture medium after LPS exposure using the corresponding ELISA kits in this study. RAW264.7 cells were seeded (2 × 10cells/well) into 24-well plates. Cells were treated with 1 μg/mL LPS in the presence or absence of various concentrations of flavonoids of S. involucrata (25-200 μg/mL) for 24 h at 37 ℃. Cell-free supernatants were obtained for the determination of PGE, TNF-α, IL-10, MCP-1, MIP-1α,and RANTES using ELISA kits according to the manufacturer’s instructions. Four replicates of experimental data were used.
2.10. Immunofluorescence staining
The nuclear localization of p65, c-Jun, and IRF3 was detected with Nikon A1R Eclipse Ti confocal microscope (Nikon Corp.,Tokyo, Japan) as previously described[24]. Briefly, cells were seeded into chamber slides for 12 h and then treated with flavonoids of S. involucrata (100 and 200 μg/mL) for 1 h. After LPS (1 μg/mL)stimulation for 1 h, cells were fixed by 4% paraformaldehyde for 10 min and then permeabilized with Triton X-100 (0.25%) for 30 min at 37 ℃. Cells were then blocked for 1 h with 2% bovine serum albumin and incubated with target antibodies including p65, c-Jun,and IRF3 diluted in cold PBS containing 2% bovine serum albumin at 4 ℃ overnight. Then, the cells were incubated with FITC-labeled anti-rabbit secondary antibody (Cell Signaling Technology, catalog no. 4412; 1:500 dilution) in dark under room temperature for 1 h.After incubation, cells were washed 3 times by cold PBS. 4′,6-diamidino-2-phenylindole (DAPI, YESEN, Shanghai, China) was used to stain nucleus right before performing assay.
2.11. Cytosolic and nuclear protein separation
RAW264.7 cells were seeded (5 × 10cells/well) into 60 mm diameter culture dishes and cultured for 24 h. Cells were pretreated with flavonoids of S. involucrata (100 and 200 μg/mL) for 1 h and LPS for additional 30 min. Cells were collected and nuclear extraction kit (Solarbio, Beijing, China) was used to separate nuclear and cytosolic protein according to the manufacturer’s instructions.After that, samples were dissolved in lysis buffer for Western blotting assay.
2.12. Immunoblotting
The quantified cell lysates were assessed by Western blotting analysis as described previously[25]. Briefly, cells (5 × 10) were seeded into 60-mm culture dishes and then treated with flavonoids of S. involucrata at concentrations of 100 and 200 μg/mL. LPS(1 μg/mL) was added 1 h after pre-treatment with flavonoids of S. involucrata and the cells were incubated at 37 ℃ for 30 min.Subsequently, cells were collected and lysed with RIPA buffer(Beyotime Biotechnology, Beijing, China). The supernatant protein from each sample was heated with 4 × sodium dodecyl sulfate (SDS)sample buffer at 95 ℃ for 8 min. An aliquot of 30 μg of protein was subjected to 10% SDS-PAGE and electro-transferred onto polyvinylidene fluoride membrane. After blocking with 5% non-fat milk in TBST, the membrane was incubated with indicated primary antibodies overnight at 4 ℃. Antibodies against the following molecules were used: p65 (Cell Signaling Technology, catalog no.8242; 1:1 000), p-p65 (Ser536; Cell Signaling Technology, catalog no. 3033; 1:1 000), c-Jun (Cell Signaling Technology, catalog no. 9165; 1:1 000), p-c-Jun (Ser73; Cell Signaling Technology,catalog no. 9164; 1:1 000), COX-2 (Cell Signaling Technology,catalog no. 12282; 1:1 000), iNOS (Cell Signaling Technology,catalog no. 13120; 1:1 000), p-IRF3 (Ser396; Abnova, catalog no.PAB31634; 1:1 000), IκBα (Cell Signaling Technology, catalog no. 4814; 1:1 000), p-IκBα (Ser32; Cell Signaling Technology,catalog no. 2859; 1:1 000), p-IKKα/β (Ser176/177; Cell Signaling Technology, catalog no. 2078; 1:1 000), ERK (Cell Signaling Technology, catalog no. 4695; 1:1 000), p-ERK (Thr202/Tyr204;Cell Signaling Technology, catalog no. 4370; 1:1 000), JNK (Cell Signaling Technology, catalog no. 9252; 1:1 000), p-JNK (Thr183/Tyr185; Cell Signaling Technology, catalog no. 4668; 1:1 000), p38(Cell Signaling Technology, catalog no. 8690; 1:1 000), p-p38 (Thr180/Tyr182; Cell Signaling Technology, catalog no. 4511; 1:1 000), TBK1(Cell Signaling Technology, catalog no. 3013; 1:1 000), p-TBK1(Ser172; Cell Signaling Technology, catalog no. 5483; 1:1 000), IRF3(Abcam, catalog no. ab68481; 1:1 000), IKKα (Santa Cruz, catalog no. sc-7607; 1:500). β-actin (Cell Signaling Technology, catalog no.4970; 1:1 000) and SP1 (Santa Cruz, catalog no. sc-420; 1:500) were used as internal control. The membranes were washed three times and incubated with the anti-rabbit IgG secondary antibody (Cell Signaling Technology, catalog no. 7074; 1:2 000) or anti-mouse IgG HRP-linked antibody (Abcam, catalog no. ab6789; 1:2 000)for 1 h. Visualization was performed using Tanon 5200 Multi chemiluminescent imaging system (Tanon Science & Technology Co., Ltd., Shanghai, China) with an enhanced-chemiluminescence substrate, and the blots were analyzed using Image J software[National Institutes of Health (NIH), Bethesda, MD, USA]. Protein levels were corrected with values determined on β-actin blots. Three replicates were used.
2.13. Statistical analysis
All values were expressed as mean ± standard deviation (SD).Data were analyzed by one-way ANOVA using SPSS (version 16.0) statistical analysis program followed by Dunnett’s multiple comparisons. P<0.05 was considered statistically significant.
3. Results
3.1. Characterization of ethanol extract, flavonoid enrichment,and flavonoids of S. involucrata powders
UPLC chromatogram showed that the contents of chlorogenic acid and rutin were different among the ethanol extract, flavonoid enrichment, and flavonoids of S. involucrata powders (Figure 1). The mean contents of chlorogenic acid/rutin in these three samples were 15.4/10.7, 3.2/136.7, and 0/506.5 mg/g, respectively. Moreover, the contents of total flavonoids in the three extracts were 130.7, 646.9,and 751.5 mg/g, respectively.
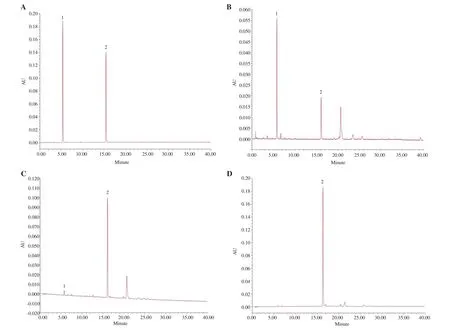
Figure 1. UPLC chromatograms of chlorogenic acid (peak 1) and rutin (peak 2) in different samples of Saussurea involucrata (S. involucrata). (A) Chlorogenic acid and rutin; (B) ethanol extract of S. involucrata; (C) flavonoid enrichment of S. involucrata; (D) flavonoids of S. involucrata.
3.2. Effect of flavonoids of S. involucrata on cell viability
Compared with the control group, the cell viability of RAW264.7 cells did not change when up to 400 μg/mL of flavonoids of S.involucrata was added with LPS for 24 h (Figure 2). We therefore chose the sub-toxic concentrations of flavonoids of S. involucrata(25-200 μg/mL) in the following experiments.
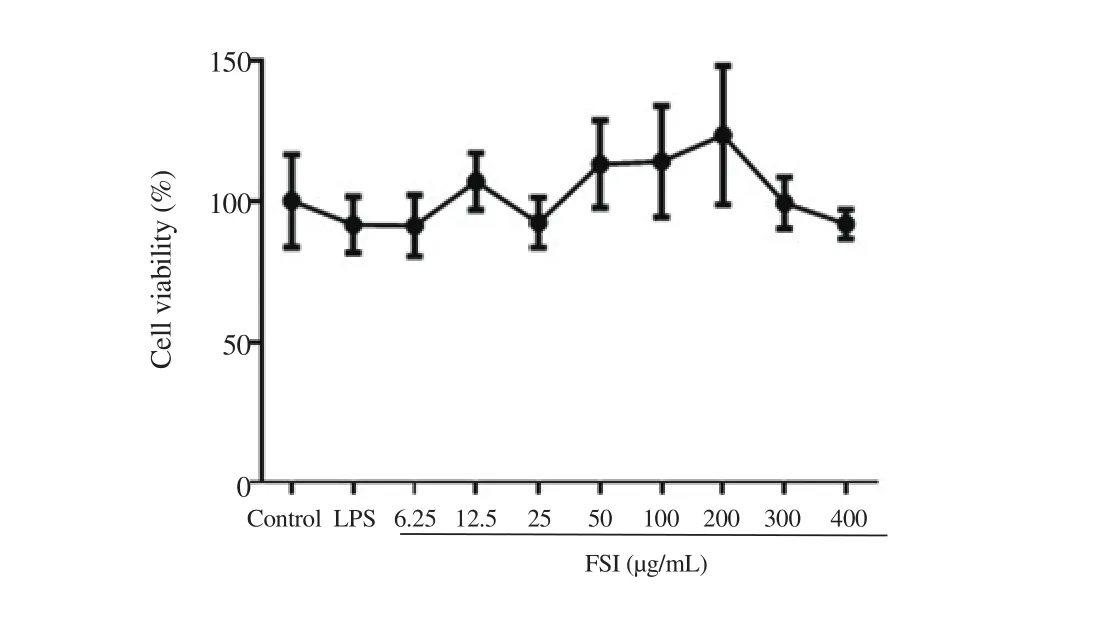
Figure 2. Effect of flavonoids of S. involucrata (FSI) on cell viability of LPS-stimulated RAW264.7 macrophages. Cells were treated with FSI (0-400 μg/mL) in the presence of lipopolysaccharides (LPS) (1 μg/mL) for 24 h. The cell viability was compared with cells without FSI and LPS stimulation using MTT assay. Data are expressed as mean ± SD of six independent experiments.
3.3. Effects of flavonoids of S. involucrata on the production of NO and PGE2 and the expression of iNOS and COX-2
Figures 3A and 3B show that the production of NO and PGEwas significantly increased in LPS-stimulated RAW264.7 cells.Flavonoids of S. involucrata (25-200 μg/mL) concentrationdependently suppressed the release of NO and PGE. In addition, the expressions of iNOS and COX-2 were also suppressed by flavonoids of S. involucrata in a concentration-dependent manner (Figure 3C).
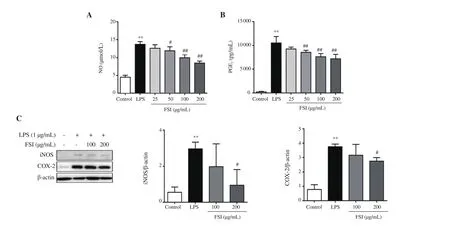
Figure 3. Effect of FSI on the production of NO and PGE2 and the expression of iNOS and COX-2 in LPS-stimulated RAW264.7 macrophages. The production and expression of NO (A) and PGE2 (B) and enzymes (C) were detected by Griess assays, ELISA, and Western blotting, respectively. Data are expressed as mean ± SD. Four replicates were used in ELISA assays and three independent experiments in Western blotting. **P<0.01 vs. the control group; #P<0.05,##P<0.01 vs. the LPS group. Statistical analysis was performed using one-way ANOVA followed by Dunnett’s multiple comparison test. NO: nitric oxide, PGE2:prostaglandin E2, iNOS: inducible nitric oxide synthase, COX-2: cyclooxygenase-2.
3.4. Effects of flavonoids of S. involucrata on the production of TNF-α, IL-10, MCP-1, MIP-1α and RANTES
As shown in Figures 4A and 4E, the release of inflammatory cytokines, including TNF-α and IL-10 was obviously elevated after LPS stimulation in RAW264.7 cells. Flavonoids of S. involucrata inhibited the secretion of these inflammatory cytokines in a concentration-dependent manner. The same trend was observed in the production of MCP-1, MIP-1α, and CCL5/RANTES after treatment with flavonoids of S. involucrata in LPS stimulated RAW264.7 cells (Figures 4B-D).
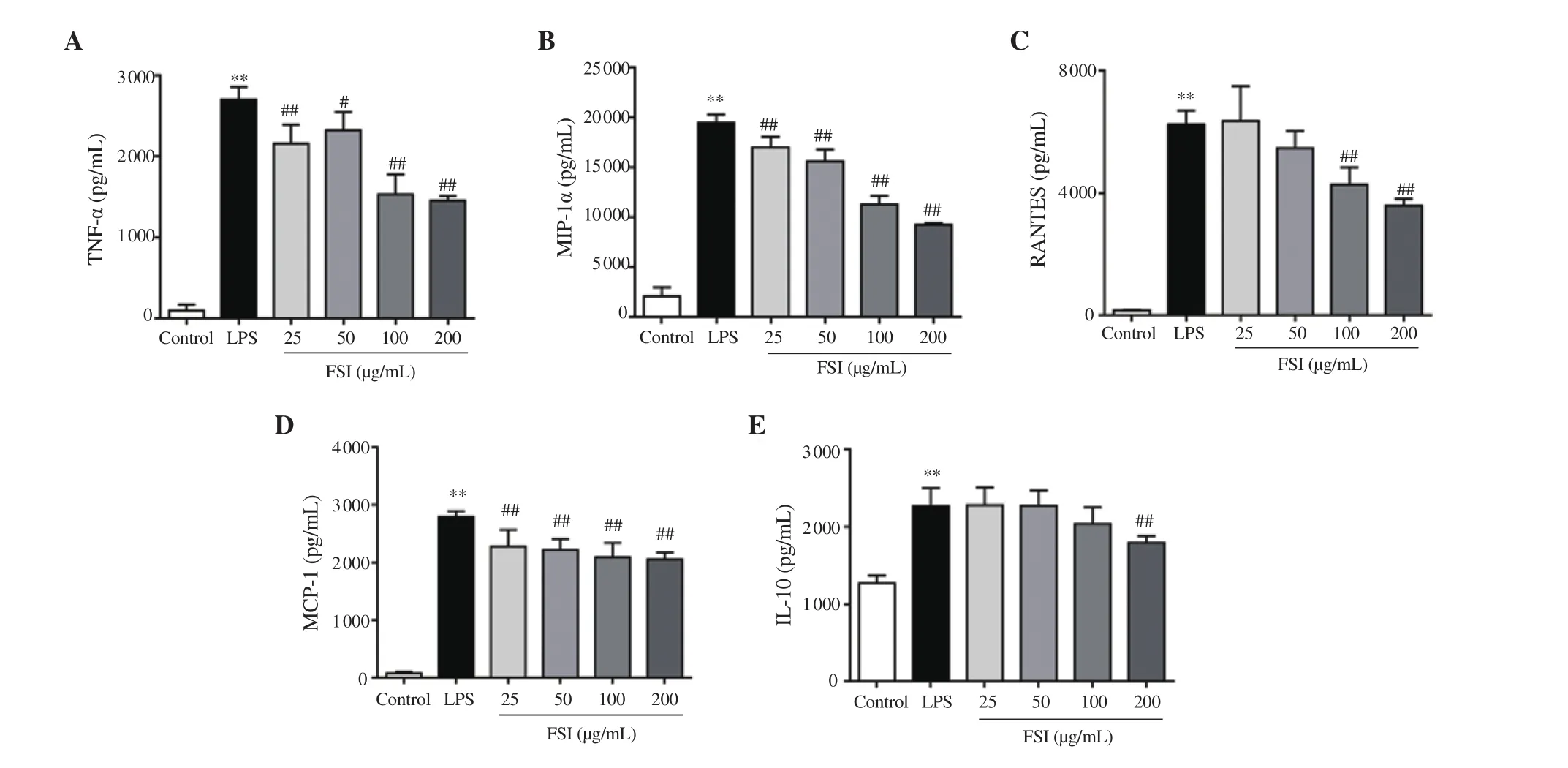
Figure 4. Effect of FSI on the production of pro-inflammatory cytokines and chemokines in LPS-stimulated RAW264.7 macrophages. The production of cytokines (A and E) and chemokines (B-D) in the culture medium were detected by ELISA assay. Data are expressed as mean ± SD of four independent experiments. **P<0.01 vs. the control group; #P<0.05, ##P<0.01 vs. the LPS group. Statistical analysis was performed using one-way ANOVA followed by Dunnett’s multiple comparison test. CCL5/RANTES: C-C motif chemokine ligand 5, TNF-α: tumor necrosis factor-alpha, IL-10: interleukin 10, MIP-1α:macrophage inflammatory protein-1α, MCP-1: monocyte chemoattractant protein-1.
3.5. Effect of flavonoids of S. involucrata on the nuclear localization of p65, c-Jun, and IRF3
Immunofluorescence was employed to detect the nuclear localization of p65, c-Jun, and IRF3. Figure 5 shows that the LPS exposure promoted the translocation of these transcriptional factors from the cytoplasm to the nucleus in RAW264.7 macrophages.Flavonoids of S. involucrata remarkably reduced the nuclear localization of p65, c-Jun, and IRF3, demonstrating potential ability to regulate transcription factor activity in inflammatory response.
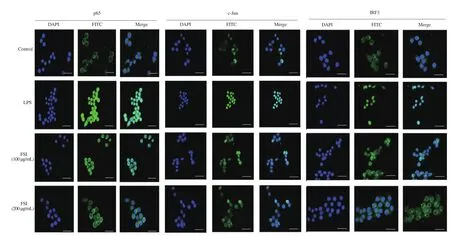
Figure 5. Effect of FSI on the nuclear localization of p65, c-Jun, and IRF3. The nuclear localization of p65, c-Jun, and IRF3 was detected by immunofluorescence analyses. Bar scale=33 μm.
3.6. Effects of flavonoids of S. involucrata on the expression of p65, c-Jun, and IRF3
We further confirmed the nuclear translocation of these key transcriptional factors using Western blotting. As shown in Figure 6,exposure of RAW264.7 cells to LPS for 30 min significantly elevated the nuclear protein levels of p65, c-Jun, and IRF3. Flavonoids of S. involucrata concentration-dependently decreased the nuclear protein levels of these three transcriptional factors. Furthermore, the cytoplasmic protein levels of p65, c-Jun, and IRF3 were decreased in LPS-stimulated cells, whereas no significant changes in cytoplasmic proteins of these transcriptional factors were observed after treatment with flavonoids of S. involucrata. Consistent with the data from Figure 5, flavonoids of S. involucrata inhibited p65, c-Jun, and IRF3 nuclear translocation, thus reducing their target gene expression.
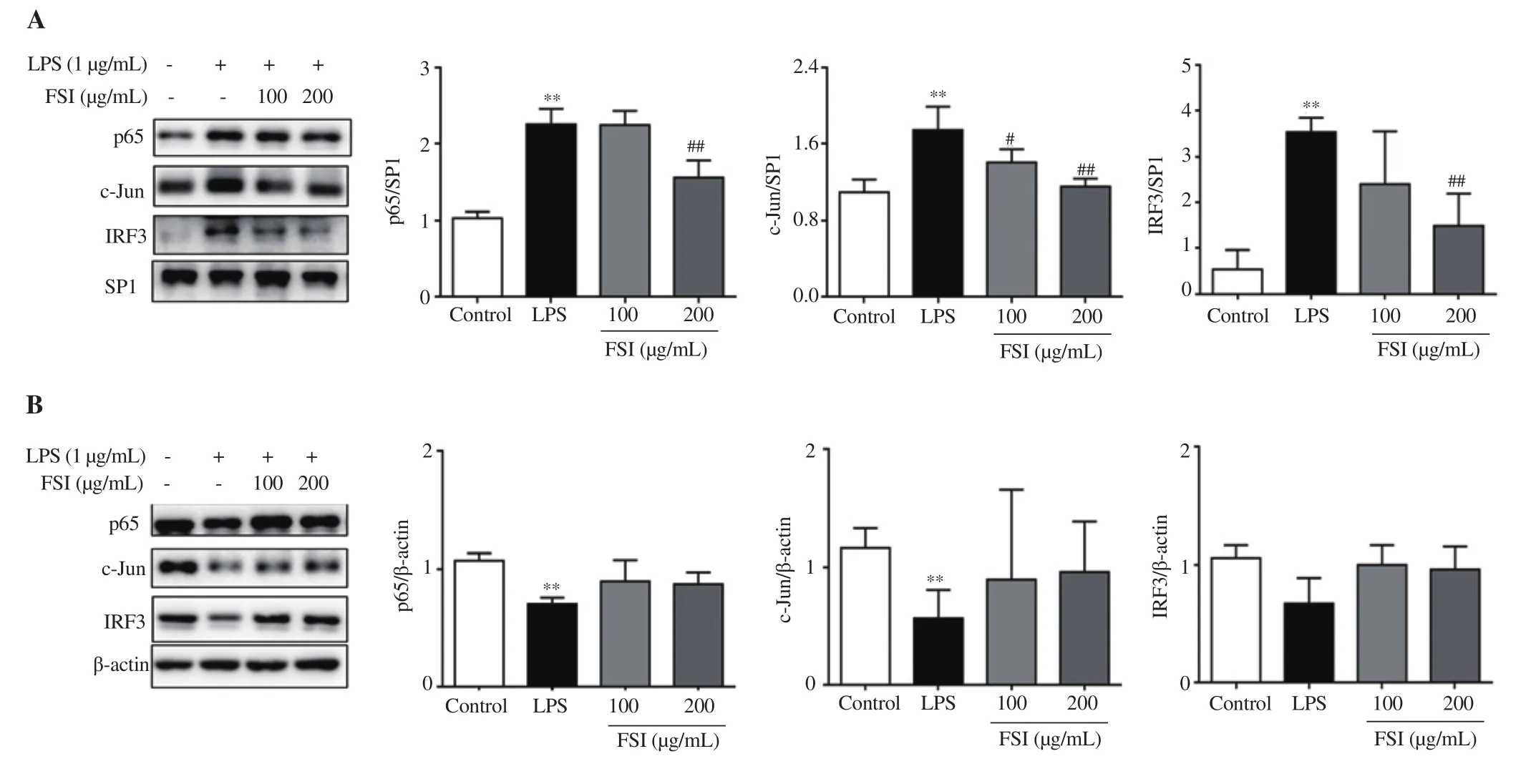
Figure 6. Effect of FSI on the expression of p65, c-Jun, and IRF3. The expression levels of nuclear (A) and cytoplasmic (B) proteins of p65, c-Jun, and IRF3 were detected by Western blotting. The nuclear proteins were normalized against SP1 levels, and the cytoplasmic proteins were normalized for β-actin levels.Data are expressed as mean ± SD of three independent experiments. **P<0.01 vs. the control group; #P<0.05, ##P<0.01 vs. the LPS group. Statistical analysis was performed using one-way ANOVA followed by Dunnett’s multiple comparison test. SP1: specificity protein 1.
3.7. Effect of flavonoids of S. involucrata on the p65, c-Jun,and IRF3 related signaling pathways
To elucidate the underlying mechanism of the inhibitory effect of flavonoids of S. involucrata on the nuclear translocation of these three transcriptional factors, we tested the effect of flavonoids of S.involucrata on the related molecular signaling pathways. As shown in the Figure 7, the expression of phosphorylated p65, c-Jun, and IRF3 was significantly elevated after exposure to LPS. Flavonoids of S.involucrata suppressed the phosphorylation of p65, c-Jun, and IRF3 in a concentration-dependent manner. Moreover, the phosphorylated MAPKs, including p38, ERK, and JNK were markedly increased after LPS stimulation and these effects were prevented by flavonoids of S. involucrata in a concentration-dependent manner. Meanwhile,the phosphorylation of IκBα, IKKα/β, and TBK1 was significantly upregulated by LPS treatment, which was downregulated by flavonoids of S. involucrata (Figure 7). Taken together, these data showed that IKKα/β/NF-κB, MAPKs/AP-1, and TBK1/IRF3 signaling pathways were involved in the inhibitory effect of flavonoids of S. involucrata on the nuclear translocation of p65,c-Jun, and IRF3, thereby reducing the secretion of inflammatory mediators.
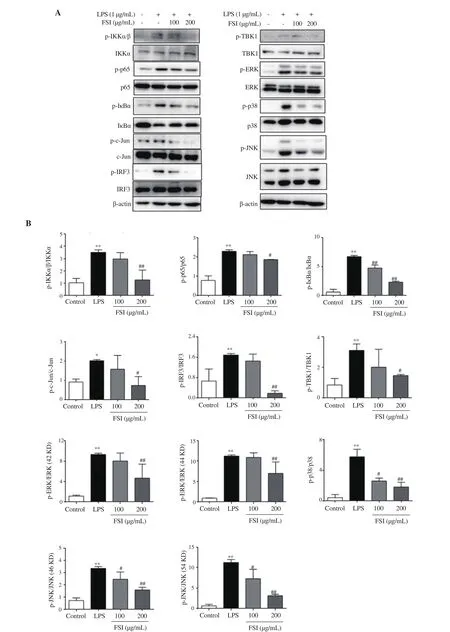
Figure 7. Effect of FSI on p65, c-Jun, and IRF3 signaling pathways. (A) Representative bands of proteins; (B) Quantification results. The expression levels of phosphorylated and total IκBα, IKKα/β, TBK1, p38, ERK, JNK, p65, c-Jun, and IRF3 were determined by Western blotting. Data are expressed as mean ± SD of three independent experiments. *P<0.05, **P<0.01 vs. the control group; #P<0.05, ##P<0.01 vs. the LPS group. Statistical analysis was performed using oneway ANOVA followed by Dunnett’s multiple comparison test. TBK1: TANK-binding kinase 1, IRF3: interferon regulatory factor 3, c-JNK: c-Jun N-terminal kinase, ERK: extracellular signal-regulated kinase, p38: p38 mitogen-activated protein kinase, IKKα/β: IκB kinase α/β.
4. Discussion
In recent years, traditional Chinese medicine has increased in popularity, especially for preventing and curing inflammatory diseases[26,27]. Flavonoids are a large group of polyphenolic compounds found in almost all natural supplements and exhibit strong anti-inflammatory properties via modulating a broad range of inflammation-associated signaling pathways[28]. Among these flavonoids, rutin is one of the most popular phenolic compounds known to have a variety of biological activities, including antiallergic, anti-inflammatory, anti-proliferative, and anti-carcinogenic effects[29]. In our present study, UPLC analysis was performed and rutin was used as a marker to estimate the contents of flavonoid compounds in the ethanol extract, flavonoid enrichment, and flavonoids of S. involucrata. The results showed that the contents of rutin and chlorogenic acid (a marker of phenolic acid) were progressively increased and decreased after purification, respectively,indicating the total flavonoid was successfully separated from S. involucrata. Given that rutin has been reported as a good antiinflammatory agent, the anti-inflammatory effect of flavonoids of S.involucrata may be related to the presence of rutin. Further studies will be conducted to verify the role of rutin in the anti-inflammatory effects of flavonoids of S. involucrata and identify other active components (e.g. phenolic acid) responsible for the action.
During the initial phase of the inflammatory response, NO and PGEact as vasodilators to facilitate the influx of neutrophils,macrophages, and mast cells from the bloodstream, leading to swelling and edema at the site of infection or tissue injury[30,31].It has been implicated that iNOS and COX-2 are the key enzymes of NO and PGE, respectively[31,32]. Elevated activities of iNOS and COX-2 promote the production of NO and PGE, thereby amplifying inflammatory responses and even contributing to tissue destruction[2]. Our current research showed that flavonoids of S.involucrata suppressed the production of NO and PGEas well as their synthetizing enzymes. We also observed that the inhibitory concentrations (25-200 μg/mL) of flavonoids of S. involucrata against these inflammatory mediators did not cause cytotoxicity.These results suggested that flavonoids of S. involucrata inhibited the NO and PGEproduction via decreasing the activities of iNOS and COX-2 in LPS-stimulated RAW264.7 cells.
Cellular response to LPS is initiated by the interaction of LPS and its receptor complex composed of LBP, CD14, MD2, and TLR4[33].This activates their down-stream proteins IKK complex, which contains two catalytic subunits (IKKα and IKKβ), triggering the degradation of IκBα by phosphorylation[34]. Subsequently, NF-κB is phosphorylated and translocates into the nucleus, promoting transcription of pro-inflammatory mediators such as NO, PGE,TNF-α, and IL-10[35]. According to previous studies, the extracts of S. involucrata ameliorated infiltration of inflammatory cells and reduced the elevation of TNF-α in synovial tissue as well as serum TNF-α, IL-1β, and IL-6 in arthritic rats[36,37]. Moreover,S. involucrata and its main component rutin could decrease the expression of COX-2 via downregulation of NF-κB activity[38].Consistent with these findings, in our present study, we found that the production of TNF-α and IL-10 were suppressed by flavonoids of S. involucrata in LPS-stimulated RAW264.7 cells. We also observed that the phosphorylation of the upstream intermediary proteins of these molecules, including p65, IκBα, and IKKα/β was inhibited by flavonoids of S. involucrata, revealing that flavonoids of S. involucrata effectively inhibited the p65 signaling pathway.Besides, AP-1 and IRF3 also play an important role in the regulation of inflammation[33,39]. Once AP-1 is activated, it translocates into the nucleus and binds to the target DNA, triggering the secretion of pro-inflammatory chemokines, such as MCP-1 and MIP-1α. This attracts inflammatory cells to enter the tissue during inflammatory response[40]. MAPKs (p38, ERK, and JNK) are serine-threonine kinases that regulate the transcriptional activation of AP-1 and its DNA-binding activity which mediates transcription of a wide array of AP-1 target genes[41]. We found that the production of MCP-1 and MIP-1α was reduced after treatment with flavonoids of S.involucrata. The nuclear-phosphorylated protein levels of the main component of AP-1 (c-Jun) were also inhibited by flavonoids of S.involucrata. It also reduced the phosphorylated upstream proteins including p38, ERK, and JNK. That may attribute to the presence of rutin which has been implied as a potent inhibitor of p38 and JNK activity[42]. These results indicated that flavonoids of S. involucrata suppressed the MAPKs/AP-1 signaling transduction. In addition, in a TRIF-dependent manner, activated TLR4 promotes the binding of TRIF to TBK1/IKKε, which then initiates the cascade of activation of IRF3 and facilitates the nuclear translocation of IRF3, thereby increasing the production of chemokines, such as RANTES[43].Our study revealed that the release of RANTES was increased after LPS exposure and decreased by flavonoids of S. involucrata in RAW264.7 cells, showing that IRF3 signaling might also be a target of flavonoids of S. involucrata. Therefore, the expression levels of the upstream proteins were detected. The results showed that the nuclear translocation of IRF3 was inhibited in LPS-stimulated RAW264.7 cells after treatment with flavonoids of S. involucrata.The phosphorylation of IRF3 and TBK1 was also suppressed. These data suggested that inhibition of IRF3 signaling may also contribute to the effects of flavonoids of S. involucrata on adaptive immune responses.
In summary, the current study showed that flavonoids of S.involucrata downregulated the LPS-induced activities of proinflammatory mediators in RAW264.7 cells. Our work also proved that flavonoids of S. involucrata suppressed the nuclear translocation of NF-κB/p65, AP-1/c-Jun, and IRF3 in LPS-treated RAW264.7 cells. The underlying mechanism may be related to inhibition of the related signaling pathways. However, lack of in vivo data is a major limitation of this study. Our further investigation will employ inflammatory animal models, such as mouse model for arthritis,to confirm the anti-inflammatory properties of flavonoids of S.involucrata. Taken together, the results may provide a chemical and pharmacological justification for the use of flavonoids of S.involucrata in the treatment of patients with inflammatory diseases.
Conflict of interest statement
The authors declare that they have no competing interests.
Funding
This work was supported by the National Natural Science Foundation of China (Grant Number 81803793 and 82003957), the Young Scientist Program by Beijing University of Chinese Medicine,and the Fundamental Research Funds for the Central Universities(Grant Number 2018-JYBZZ-XJSJJ008).
Authors’ contributions
YZ contributed to the conceptualization, funding acquisition and project administration. YZ, LSY, LW and YD conducted the biological investigation. GL and JK performed UPLC analysis. GL,LSY, BCC, QGW and SFZ analyzed data. YZ and XQF performed data visualization. YZ and GL drafed the original manuscript. BCC revised the manuscript.
猜你喜欢
杂志排行
Asian Pacific Journal of Tropical Biomedicine的其它文章
- Potential of polyphenols in curbing quorum sensing and biofilm formation in Gramnegative pathogens
- Caffeic acid and protocatechuic acid modulate Nrf2 and inhibit Ehrlich ascites carcinomas in mice
- Ginseng ameliorates pulmonary toxicity induced by silicon dioxide nanoparticles in rats
- Immunostimulatory effect of ethanol extract of Chondracanthus tenellus in RAW 264.7 macrophages in vitro
