The potential of ionic liquids in biopharmaceutical engineering
2021-05-19XuanLinZhiguoSuYanliYangSongpingZhang
Xuan Lin,Zhiguo Su,Yanli Yang*,Songping Zhang*
State Key Laboratory of Biochemical Engineering,Institute of Process Engineering,Chinese Academy of Sciences,Beijing 100190,China
ABSTRACT Biopharmaceuticals,such as proteins,peptides,nucleic acids and vaccines,bring about great hopes for the prevention and treatment of various diseases,but the industrialization of these products still faces challenges such as structural instability,inefficient bioactivity and low bioavailability.Ionic liquids(ILs),the marvelous solvent media with inimitable and tunable properties,may provide alternative solutions to overcome the above problems of biopharmaceutical industry.Progress has gradually been made through studies by combination of ILs with biomacromolecules.The applications involved the stabilization,protection,and delivery of biopharmaceuticals.Recent trends are being forwarded to using ILs in vaccines and nucleic acid drugs.However,challenges remain on the toxicity and safety issues.Besides,the cost of adding ILs to the benefits of biopharmaceuticals need to be considered.
Keywords:Ionic liquids Biopharmaceutical engineering Biomacromolecules Stabilization Vaccine Delivery
1.Introduction
Biopharmaceuticals including peptides,cytokines,antibodies,vaccines,nucleic acids,etc.,are of great importance in prevention and curing of diseases.However,the development of biopharmaceuticals from laboratory to industrial application is still facing challenges.The unstable nature of complex structures of biomacromolecules makes them vulnerable to denaturation that causes subsequent loss in bioactivity [1].Therefore,the biopharmaceuticals usually require expensive cold chains for transportation and storage,as well as addition of stabilizers to prolong their shelf-life.The low bioavailability of biomacromoleculesin vivois another facing challenge due to their hydrophilicity,large size and vulnerable to enzymatic degradation [2,3].Therefore,the design of appropriate drug delivery systems (DDS) is also critical for efficient therapeutic effects.
ILs are a class of liquid salts comprising organic cations and anions with melting points below 100°C[4].Their unique and tunable properties have made them attractive for a wide range of applications in energy,chemical engineering and materials,etc.[5,6].By tailoring the ions of ILs,they have found broad applications during last decade in pharmaceuticals such as enhancing activities,solubility,permeability,and stability of small molecular drugs.By combining a pair of pharmaceutical active ions,ILs-based active pharmaceutical ingredients (IL-APIs) with physicochemical properties and activities which are different from their precursor APIs have been constructed [7].For example,compared with the precursor APIs lidocaine hydrochloride,the lidocaine docusate([Lid][Doc]) exhibits modified solubility,increased thermal stability,and a significantly enhanced efficacy for topical analgesia [7].By constructing and employing ILs-based DDS,the bioavailability of poorly water-soluble and poorly organic-solvent soluble small molecular drugs,such as acyclovir,5-fluorouracil,amphotericin B,danazol and coumarin,etc,was enhanced [8,9].More applications of ILs in the research of small molecular drugs have been reviewed [6,8].
Compared to small molecule drugs,the application of ILs in biopharmaceutical engineering is just beginning,but it has already exhibited extraordinary potential in solving the above mentioned problems of biopharmaceutical industry [8,10,11]by acting as excipients to improve the stability of protein-and nucleic acidbased drugs[12–15],or as novel DDS to enhance the bioavailability of biomacromolecule during administration [2,3,16,17].
In order to provide reference for the design of ILs suitable for biopharmaceutical engineering,this review will first give a brief summary on the structures of ILs used in biopharmaceuticals,then their applications in the stabilization and delivery of biomacromolecules will be focused on.Potential applications in the design of vaccine adjuvants will be prospected and challenges of ILs in biopharmaceutical engineering will be discussed at last.
2.Structures of Presentative ILs for Biopharmaceuticals Use
Both the cations and anions determine the properties and functions of ILs.The cations of ILs usually are large volume organic structures with low symmetry,that contribute to the low melting point of ILs;while the anions usually endow the ILs with some specific physicochemical properties and even biological functions[18,19].Different from other fields,the safety issues including biocompatibility and cytotoxicity of ILs should be given priority when applied in pharmaceutical fields [20–25].Therefore,the cations and anions of ILs are incline to choose ions that are found naturally in the body or have already been proved safe[20,21].Table 1 summarized the structures and functions of some presentative ILs that have been used in biopharmaceuticals.We can find that choline is the most commonly used cation because of its good biocompatibility.Choline is an important constituent of lecithin that is present in both plants and animals and required for various physiological functions,therefore choline is recognized by Food and Drug Administration as safe ingredient[3].When combined with proper anions,the choline-based ILs can exhibit various functions such as stabilizing proteins,nucleic acids,virus particles [13,15,26–29],and enhancing the permeation of biomacromolecules in noninvasive administration [2,3,30–33].In addition,the ILs with tetraalkyl-ammonium or 1,3-Dialkyl-imidazolium as cations are also used to stabilize and deliver biomacromolecules,see Table 1.
3.Stabilization Roles of ILs in Biopharmaceuticals
3.1.General criterions for ILs as drug stabilizers
The potential of ILs in pharmaceutical applications has been well demonstrated in solving some critical challenges facing in small molecular drugs.However,the development of biopharmaceuticals faces problems different from those for the small molecule drugs.The stabilization of three-dimensional structures of biomacromolecules during manufacture and storage is the priority for biopharmaceutical engineering since the structures determine their functions and biological activities [20].
ILs stabilized proteins including enzymes have been summarized in [4,36–38].ILs have been proven to significantly enhance the thermal stability of proteins [39–42],protect threedimensional structure of proteins [43,44],inhibit protein aggregation or unfolding [34,45–47],prevent proteins from contacting with denaturants [48],and maintain the activity of enzymes[20,49].However,looking at the biopharmaceutical applications,the safety of ILs is prior to stabilization effects.Some ILs which can excellently stabilize model proteins are not suitable formedical applications because of their potential toxicity.Therefore,the cations and anions of ILs are prefer to choose from components that have been proved safe such as choline [20,21].
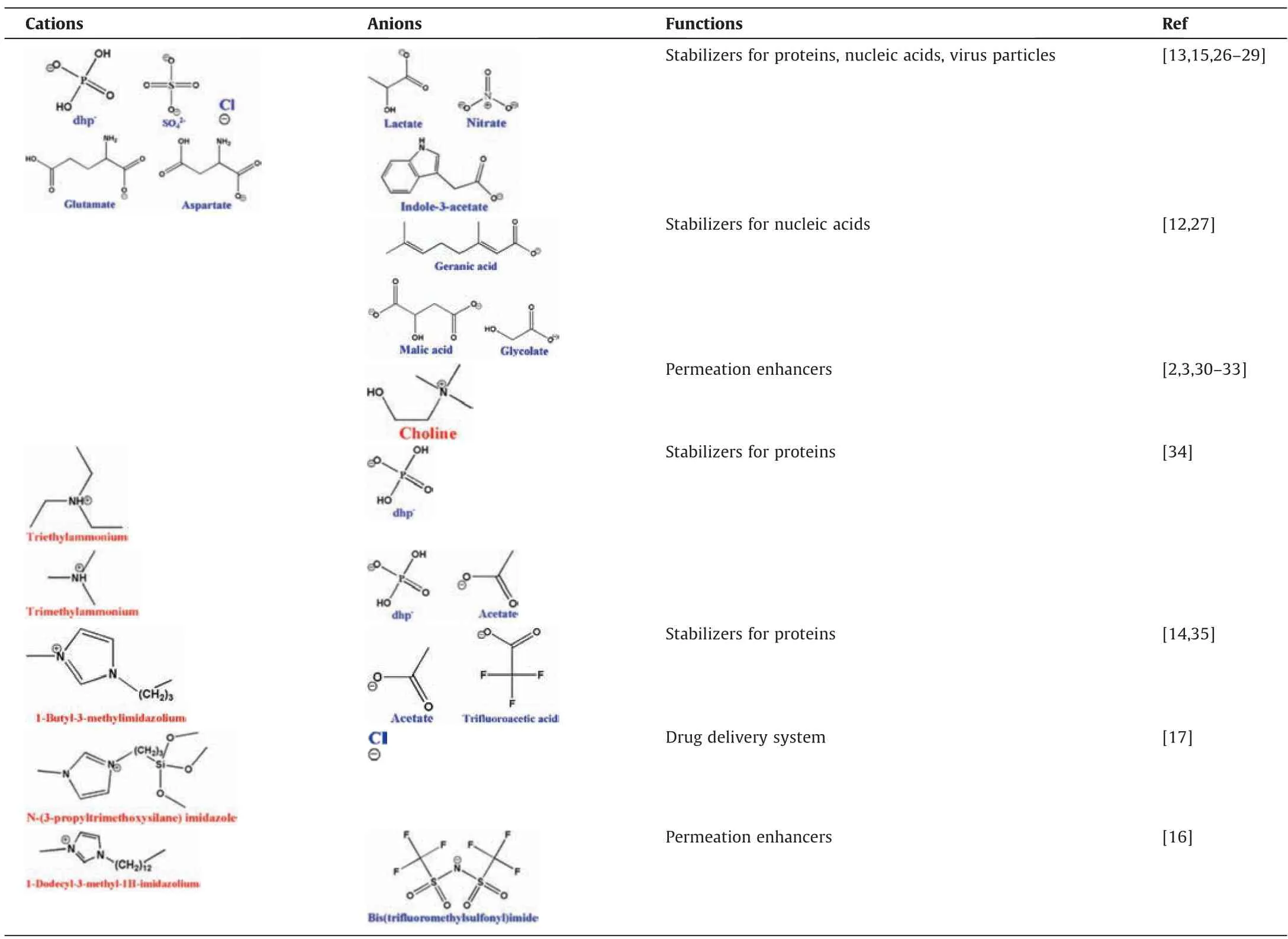
Table 1 Structures and functions of the presentative ion sources of ILs used in biopharmaceuticals
Some studies have reported the stabilization effects of ILs for various kinds of biopharmaceuticals including polypeptide drugs,cytokines,antibodies,nucleic acids,and even vaccine antigens with complex molecular structures through somewhat different stabilizing mechanism,as will be discussed below.
3.2.Stabilization roles of ILs on peptide and cytokine drugs
For an important peptide drug insulin,a variety of ILs were reported to have excellent stabilizing effects.Insulin is a globular protein containing two chains linked with two disulfide bridges,which easily become unfolded and subsequently form inactive fibrillar aggregates under processing conditions such as low pH,agitation,filtration,etc.[34,35].Ammonium-basedILs,triethylammonium dihydrogen phosphate (TEAP) was reported to prevent insulin from associating into an inactive form and also stabilize insulin against thermal influence [34].Imidazole-based ILs,1-butyl-3-methylimidazolium acetate ([Bmim][CH3COO]) could increase the transition temperature (Tm) of insulin from 75.4 °C to 86.9°C at a ILs concentration of 0.3 M by preserving or enhancing helical structure of insulin [35].Gunchevaet al.synthesized a series of choline amino acid ILs and revealed that theTmof insulin shifted to higher values by 9.7°C and 4.5°C in presence of choline L-glutaminate ([Cho][Glu]) and choline L-asparaginate ([Cho]2[-Asp]),respectively [26].
Recombinant human interleukin-2 (rhIL-2) is one of most important cytokines produced by the body during immune processes.The rhIL-2 formulated in the presence of 680 mM choline dihydrogen phosphate ([Cho][dhp]) showed aTmvalue 12.5 °C higher than that in basic buffer formulation [13,50].Excluding water from the protein surface and shielding the proteins from denaturation of bulk water were considered the main mechanisms for stability enhancement by [Cho][dhp].In addition,low concentration of [Cho][dhp]exhibited no cytotoxicity toward primary splenocytes or B16-F10 cells in culture.
3.3.Stabilizing roles of ILs on antibody and nucleic acid drugs
Monoclonal antibodies (mAbs) have dominated the list of top 10 best-selling drugs worldwide in the last decade because of their wide applications in diagnostics and in the treatment of a variety of ailments including cancer and auto-immune diseases [15,51].However,unfolding and aggregation of mAbs will not only result in loss of activity but also cause undesired immunogenic reactions to patients[15,52].[Cho][dhp]has been found to be the most suitable ILs for the stabilization of mAbs owing to its overall stabilization effect and biocompatibility [15].Stability of Trastuzumab,a marketed therapeutic mAb used for the treatment of HER2+breast cancer,against unfolding and irreversible aggregation in high concentrations was significantly improved in combination solution of[Cho][dhp]with some other excipients[15].In the presence of 53%[Cho][dhp],theTmand onset temperature of aggregation (Tagg) of 20 mg·ml-1trastuzumab were increased by 5.6 °C and 10.4 °C,respectively.Cortisol antibody and IL-6 antibody were also stabilized by [Cho][dhp]in concentrations higher than 60% (v/v),while these antibodies tend to form aggregates in aqueous buffer [28].Forcing protein into more compact conformation by inducing water restructuring and forming strong interactions with positively charged residues were regarded as the main mechanisms for [Cho][dhp]to stabilize antibodies [15,53,54].
Besides proteins,ILs were also reported to stabilize nucleic acids,another kind of important biomolecules that have been applied to biomedicine [12,27].Vijayaraghavanet al.showed that choline lactate ([Cho][Lac]) could retain the double-helical structure of DNA (from salmon testes) during storage at room temperature for six months.A similar storage stability was observed in[Cho][dhp]and choline nitrate ([Cho][Nit]) solutions.By comparison,the DNA dissolved in water lost the double-helical structure in one month after stored at room temperature[27].Similar results were observed by Mukeshet al.that choline-indole-3-acetate[Cho][IAA]could dissolve the same kind of DNA up to 3.5% (w/w) with long term structural and chemical stability of the molecule [12].
3.4.ILs as stabilizers for virus particles
The stabilization of complex biomacromolecules such as virus particles is more challenging than proteins mentioned above,as these bioparticles with size from tens to 100 nm are composed of multi subunits by non-covalent bonds.The intact assembly structures of these particles must be guaranteed to provide ideal immunogenicity or efficacy when used as vaccine antigens or vectors for drug delivery [55–57].However,most of these biomacromolecules are easily disassociated or aggregated into inactive states[58].To address this issue,sugars,salts,polyols,surfactants,and other nonreactive components are usually used as excipients,but sometimes was found insufficient to provide adequate stabilization effects for viral particles [29,59].
In 2012,Byrneet al.[43]reported the earliest attempts on stabilization oftobacco mosaic virus(TMV) by ILs.The TMV is rod shaped virus with approximately a length of 300 nm and a diameter of 18 nm.The shelf life of the TMV in ethylammonium mesylate(EaMs) was prolonged to 4 months without significant lose in tertiary structure,but it was completely degraded after three weeks in buffer solution.Recently,our lab found choline-based ILs,choline chloride ([Cho][Cl]) and choline sulfate ([Cho][SO4]) could improve the thermo-and long-term stability of inactivated footand-mouth disease virus (iFMDV) (Fig.1a and b),an important FMD vaccine antigen which is vulnerable to dissociation.The ILs were superior to the existing stabilizers of iFMDV in preventing the virus dissociation (Fig.1c) [29].
3.5.Stabilization mechanisms of ILs on different biopharmaceuticals
Till now,there is no definite mechanism for the stabilization effects of ILs for biomolecules.Laws that are similar to Hofmeister series are often used to explain the effects of different anions of ILs on their stabilization capacity.This rule was found applicable for insulin,which was stabilized by Br-and Cl-,while denatured by CN–,CH3COO–,and I-[14].However,a lot of ILs are difficult to be classified into Hofmeister series like conventional salts.For example,[Cho][dhp]are the most promising stabilizers for many kinds of proteins.The stabilization effect of[Cho][dhp]on antibody attributes to the dhp-anion of[Cho][dhp]inducing water restructuring(the water molecules may heavily interact with the proteins,thus causing aggregates) around the proteins,and forming strong interactions with many positively charged residues on the antibody surface forcing it into a more compact conformation[15,53,54].Nucleic acids were stabilized by choline-based ILs as they can provide a mild H-bonding environment of relatively low water activity,hence slowing the rate of hydrolytic reactions that would otherwise slowly depolymerize or degrade the molecules[12,27].The ammonium-based ILs stabilizing insulin by interacting unfavourably with the surface of insulin and enhancing more ordered secondary structure [34],while imidazole-based ILs can preserve or enhance the helical structure of insulin [14,35].

Fig.1.Choline-based ILs on the stability of iFMDV.(a)The Tmvalues of iFMDV in ILs.(b)The long-term stability of iFMDV in ILs during storage at 4°C.(c)The comparison of ILs with other stabilizers on the stability of iFMDV at 37 °C.(d) Microenvironmental pH of iFMDV in different ILs at varying concentrations.The ΔpH was defined as pH difference between pH surrounding iFMDV in ILs and that of bulk ILs.(e) Schematic depiction of stabilization mechanism of choline-based ILs for iFMDV from Ref.[29],Copyright (2019) The Royal Society of Chemistry.
For the stabilization of virus particles with complex assembly structures,the mechanisms are different from those for proteins was followed.Specifically,[Cho][dhp]was found to destabilize iFMDV although it can stabilize many proteins.The proton intensity around the iFMDV has been proven to be the most crucial role in determining the stability of the assembly[29,60].By monitoring the microenvironmental pH of the virus particles in different ILs,a relatively lower proton intensity was observed in [Cho][Cl]and[Cho][SO4]than in buffers and[Cho][dhp](Fig.1d)[29].Therefore,the stabilization mechanism was supposed to be mainly due to suppression of protonation of histidine residues in the interpentameric interface of virus particles in choline-based ILs with anions of Cl-or(Fig.1e),which is distinct from the mechanisms reported for other proteins.In contrast,the weakly acidic dhp-would release the proton into the microenvironments around the virus and therefore destabilize iFMDV (Fig.1e).
Therefore,in addition to safety consideration of ILs stabilizers,full considerations on the structural characteristics and stabilization mechanisms of specific biomacromolecules should also be given.Furthermore,the effects of ILs on the biological activities of biomacromolecules should also be regarded.For example,[Cho][Cl]and [Cho][SO4]can not only stabilize iFMDV,but also improve the immunogenicity of iFMDV [29].However,Foureauet al.observed a 10-fold loss in biological activity of rhIL-2 in 30 mM [Cho][dhp],although it can stabilize rhIL-2 [13].
4.Ionic Liquids for Delivery of Biopharmaceuticals
Delivery of biomacromolecules through efficient but noninvasive routes is another hot topic.Non-invasive administration routes,such as transdermal and oral drug delivery,have been appreciated because of their safety and patient compliance.However,the major challenge associated with transdermal drug delivery is the almost impermeable barrier created by the stratum corneum(SC)measuring 10 μm in thickness with variances in different parts of the body [6].For the oral routes,gastrointestinal barriers have to be overcome[3].Therefore,enhancing the permeation of drug molecules against these barriers are crucial for designing non-invasive delivery systems.ILs and ILs-based drug delivery systems have been proved to facilitate delivery of small molecular drugs by enhancing the solubility and permeation of these drugs [61–64].For biomacromolecular drugs,ILs can act as permeation enhancers and stabilizers in the transdermal and oral delivery process [2,16].The types of ILs-based drug delivery systems depend on the physicochemical properties of ILs and the delivery routes.This part will focus on the applications of ILs in the delivery of biomacromolecules and the methods of preparing corresponding delivery systems.
4.1.As permeation enhancers for transdermal delivery of biopharmaceuticals
Though the permeation enhancement mechanisms of ILs are diverse depending on the properties of different ILs,the interaction between ILs and bio-membranes largely determines the permeation enhancement ability of most ILs.During permeation of ILs,the skin barrier function can be reduced by disturbance of the regularly ordered arrangement of corneocytes and modification of the surface properties.Hydrophilic ILs can open the tight junctions within the SC to promote the paracellular transport of drug molecules,whereas hydrophobic ILs improve partition of drugs into the epithelial membrane by providing channels,thus promote transcellular transport of drugs in the lipid regions [6,65].Imidazolebase ILs with long alkyl side chains can disrupt structural integrity of membrane by inserting into the membrane.While the hydrophilic imidazolium-based ILs could even fluidize the cell membranes by extraction the lipids in the SC (Fig.2a) [8,66,67].Hydrophobic ILs with long alkyl side chain,1-dodecyl-3-methyl imidazolium bis(trifluoromethyl sulfonyl) amide ([C12mim][Tf2N]) was introduced as a permeation enhancer in solid-in-oil (S/O) nanodispersion for transdermal delivery of ovalbumin (OVA).To prepare this DDS,an aqueous solution of OVA and a cyclohexane solution of the surfactant were mixed to form a water-in-oil emulsion by homogenizer.Then,the emulsion was frozen rapidly and lyophilized to get viscous materials employed as surfactant-OVA complexes.Finally,isopropyl myristate containing[C12mim][Tf2N]was added to yield the S/O nanoemulsion with particle diameter about 110–180 nm (Fig.2b) [16,68].In vitroandin vivoexperiments showed that the introduction of [C12mim][Tf2N]in the nanoemulsion significantly enhanced the skin permeability of OVA and produced higher OVA specific IgG titers [16].

Fig.2.(a) A model of the interaction between imidazolium-based ILs and bio-membranes where a gold-coated sensor surface (orange) has a chemisorbed self-assembled monolayer (brown) tethered by biotin linkers (green) to streptavidin which also tethers liposomes (blue) from Ref.[67],Copyright (2017) American Chemical Society.(b)Formulation of solid-in-oil nano-dispersions in isopropyl myristate with [C12mim][Tf2N]for transcutaneous delivery of ovalbumin showed a significantly enhanced skin permeability of OVA and specific IgG titers from Ref.[16],Copyright (2015) The Royal Society of Chemistry.

Fig.3.(a)Confocal images of skin permeation of BSA,OVA and insulin prepared in PBS,CAGE,50:50(v/v)PBS:diethylene glycol monoethyl ether(DGME),or 50:50(v/v)PBS:ethanol showed FITC–protein penetration into the dermis layer for the CAGE samples but not in the PBS or DGME controls.Scale bar:300 μm.(b) In vivo evaluation of the efficacy of CAGE as transdermal insulin delivery material.Percent change in blood glucose level with time after administration of various formulations in nondiabetic rats from Ref.[2],Copyright (2017) WILEY-VCH Verlag GmbH &Co.KGaA.
Mitragotri’s group reported a marvelous Choline-based IL,choline and geranate (CAGE) that exhibited excellent capability in enhancing permeability of bovine serum albumin (BSA),OVA and insulin into and across the dermis (Fig.3a) [2,69].The CAGE-based delivery system was prepared by simply dissolving the drugs in CAGE and was applied by directly placing on the skin.In vivostudies in rats showed that topical application of 10 U insulin dispersed in CAGE (25 U·kg-1insulin dose) led to a highly significant 40% drop in blood glucose levels in 4 h that is relatively sustained for 12 h (Fig.3b) [2].Recently,the CAGE was also used for topical delivery of small interfering RNA(siRNA)[33].The complexation of the siRNA with CAGE led to a significantly enhanced epidermal and dermal permeation.In vivoapplication of siRNA formulated with CAGE to SKH-1E hairless mice significantly suppressed glyceraldehyde 3-phosphate dehydrogenase (GAPDH)expression with no clinical evidence of toxicity [33].Wuet al.reported a choline and malic based IL,[Cho][Mal]that could enhance the transdermal delivery of hydrophilic macromolecules dextran into deep skin.The amount of dextran delivered to epidermis and dermis by[Cho][Mal]is approximately two folds that of by dextran solution [30].
4.2.As permeation enhancers and stabilizers for oral delivery of biopharmaceuticals
For oral delivery of biopharmaceuticals,besides the gastrointestinal barriers [3],easy denaturation of biomacromolecules in gastric fluids and intestine caused by low pH environment and enzymatic degradation is another big challenge has to face.Several of ILs-based delivery systems have exhibited potentials in solving the problems.The star molecule,CAGE was found to significantly enhance the paracellular transport of insulin,insul protect insulin from enzymatic degradation and interacting with the mucus layer resulting in its thinning[3].The CAGE-based DDS was prepared by encapsulating CAGE-insulin mixture in elongated size 9 capsules to evade gastric degradation of insulin and enhance its intestinal permeability.In vivoexperiments showed that oral delivery of 10 U·kg-1insulin formulated in CAGE and enterically coated capsules achieved a sustained decrease in blood glucose of up to 45% [3].CAGE was recently encapsulated into a polyvinyl alcohol (PVA)gel to form a mucoadhesive ionogel patch (CAGE-patch) designed to adhere to the intestine.The CAGE-patches formedviarepeated freeze–thaw cycles demonstrated mucoadhesive strength,swelling,and controlled release of both CAGE and insulin (Fig.4) [32].In vitrotransport studies revealed a more than 30% increase in insulin transport when compared to controls across Caco-2 and HT29-MTX-E12 coculture layers.This design provides a novel approach to localize ionic liquids and therapeutics in the GI tract for enhanced oral delivery [32].

Fig.4.Fabrication of CAGE-patches for oral delivery.CAGE-patch ionogels were fabricated by combining CAGE,PVA,and insulin in a mixture.Mixtures were poured into cell culture plates and either left to dry for 48 h to make D-CAGE-patches or subjected to repeated freeze–thaw cycles for FT-CAGE-patches.In the potential clinical setting,patches would be loaded into enteric-coated pills that dissolve and release patches into the small intestine [32],Copyright (2020) American Chemical Society.
Mahkamet al.reported imidazole-based ILs functioned silica nanoparticles for oral delivery of insulin[17].By entrapping insulin in the nanoparticles connected through an ionic liquid-like network,this system exhibits a protective effect on insulin and the biological activity remained after the treatment with gastric fluid[17].Mitragotri’s group reported a new ionic liquid and deep eutectic solvent,choline and glycolate (CGLY) for gastrointestinal administration of TNFα antibody.CGLY maintains the stability and structure of antibody,enhances paracellular transport of antibody and reduces the viscosity of the intestinal mucus,which make CGLY ideal for oral delivery of therapeutic antibodies [31].
5.Potential Application of ILs in the Design of Vaccine Adjuvants
Adjuvants are defined as substances that are deliberately added to vaccines to enhance adaptive immune responses to antigens[70,71].Adjuvants in current clinical use including aluminum hydroxide,emulsion adjuvant and particulate adjuvant are essentially a kind of DDS.Different from the traditional DDS,adjuvants not only deliver the antigens to the target sites,but also help to improve the immune response of the vaccinated body to the antigens.The complimentary roles of ILs in the biopharmaceutical engineering discussed above are expected to endow them great potentials in the applications of vaccine adjuvants,yet which have not been explored,in the following aspects.
Firstly,the structural integrity of the antigens should be kept in the presence of adjuvants and stabilizing the antigen by adjuvants is desired.The excellent stabilization effects of ILs on the antigenic protein like OVA[16]and the virus antigens like iFMDV[29],have been fully demonstrated,although the study against other antigens is rarely reported by now.The stabilization effects of ILs on biomacromolecular antigens will provide solutions to the easy denaturation problem of antigens not only in storing solution,but also in emulsion-based adjuvants,which usually make the antigens more unstable because of the destructive effect of the oil–water interface [59].Different types of emulsions can be constructed to stabilize the antigens by choosing ILs with proper polarity.Moreover,some previous studies indicated that ILsbased emulsions are more stable and easier to be constructed[62,72].
Secondly,mucosal and transdermal delivery routes hold great promise as non-invasive immunization strategies,since they do not require trained personnel,avoids the use of needles and improves overall patient compliance and acceptance [73].However,it necessitates the implementation of effect delivery systems to improve their efficacy.In this regard,the successful mucosal and transdermal delivery of a variety of biopharmaceuticals through ILs-based delivery systems gave us great confidence to broaden their applications in vaccine delivery.The permeation enhancement function of ILs-based DDS can be further tailored as needed.For example,a [C12mim][Tf2N]-based solid-in-oil (S/O) nanodispersion have showed effectiveness in enhancing the skin permeability of OVA and in inducing higher levels of specific IgG as consequence,compared to the nano-dispersion without ILs [16].The abilities of ILs to topical deliver nucleic acids [33,74]and polysaccharides [30]would also make it possible to construct novel ILs-based delivery systems for nucleic acid-based and polysaccharide based vaccines.The successful application of ILsbased emulsion in transdermal delivery for sparingly soluble drugs[63,72]also makes it possible for transdermal or even mucosal delivery of antigens.Combining the advantages of ILs in stabilizing and enhancing permeability of a variety of vaccine antigens,the application of ILs in vaccine adjuvants will be a prospective direction.
6.Challenges of ILs in Biopharmaceutical Engineering
Safety is the most concerned issue in the application of ILs in biopharmaceutical engineering.To ensure the biocompatibility of ILs,the cation and anion sources of ILs have been selected from the substances that have been proved to be safe or exist in bodies.However,comprehensive evaluations are needed when used in human such as acute toxicity,long-term toxicity.The researchers have begun to pay attention to the safety evaluations of ILs[20,21,25,75–77],and have assessed the toxicity of ILs at cellular and animal levels[78,79].Nevertheless,some toxicity of ILs is still inevitable at present.For example,ILs with long side alkyl side chain are ideal permeation enhancers and antibacterial agents,but they also have strong cytotoxicity [80].Another limitation is there is still lack of pharmaceutical grade ILs,therefore it is difficult to determine whether the toxicity comes from the impurities in the synthesis process of ILs or from ILs themselves.Moreover,whether the combination of the two low toxic ions will induce unexpected toxic reactions also needs to be fully evaluated.Etodolac-Lidocaine Topical Patch (MRX-7EAT) is the first and only IL-API that has reached clinical trials.However,the increased skin absorption and mild to moderate adverse effects were disclosed in the Phase I trial,and the trial was finally terminated because of the unsatisfactory results of Phase II/III trials [8].
ILs exhibit ideal stabilization effects,but it often requires high concentration to obtain satisfying protective effects.On the other hand,the price of ILs are usually more expensive than conventional excipients as stabilizers at present.Therefore,the cost and largescale supplying are two other important issues to be considered in the application of ILs in biopharmaceutical engineering.
7.Conclusions and Prospects
ILs have shown great potential in pharmaceutical researches from small molecule drugs to biopharmaceuticals in recent years due to their designable properties.So far,a number of studies have reported the stabilization effects of ILs on almost all kinds of biopharmaceuticals including proteins,nucleic acids and even virus particles,by protecting them from dissociation,aggregation or enzymatic degradation through diverse mechanisms.Combining these stabilization effects with unique interactions between some ILs and bio-membranes,ILs used as effective permeation enhancers are playing outstanding roles in constructing novel non-invasive DDS including transdermal or oral delivery of biopharmaceuticals.When ILs were applied in vaccine antigen formulations,transformation of ILs-based DDS into vaccine adjuvants might become a perspective direction of ILs used in biopharmaceutical engineering,though we should keep in mind that a critical difference between adjuvant and DDS is that enhancement in immune responses is demanded for adjuvant.In this regard,the unique “designable”properties of ILs will provide almost infinite opportunities by choosing and designing their ions sources derive from immunostimulators such as TLR agonists to induce stronger and specific immune responses.
Although the ILs hold great promise in biopharmaceutical engineering,we have to admit that the research on the pharmaceutical applications of ILs is still in its infancy and faces challenges.The selection of ILs was empirical and mainly depends on massive experimental screening at present.To select and design ILs in a more rational manner,molecular dynamics simulation (MSD)would be helpful,which is recently explored to identify the ILssiRNA interactions for enhanced solvation and stability,as well as the lipid bilayer interactions and translocation mechanisms of the IL-drug combination [81].In the future,the ions source of ILs stabilizers can be designed based on MSD of the ILsbiopharmaceutical interactions.Besides,challenges also remain on cost and safety of ILs.How to prepare pharmaceutical grade ILs on a large scale and to make comprehensive evaluation on the toxicity of ILs are important for their pharmaceutical application.In addition,regulatory agencies need to issue specific guidance to accelerate the development of ILs in pharmaceutical engineering.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors are thankful for the financial support from the National Natural Science Foundation of China (Nos.21808226,31970872,and 21821005).
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Molecular design,synthesis strategies and recent advances of hydrogels for wound dressing applications
- Recent advances in microbial production of phenolic compounds
- The production of biobased diamines from renewable carbon sources:Current advances and perspectives
- Efficient production of chemicals from microorganism by metabolic engineering and synthetic biology
- Food synthetic biology-driven protein supply transition:From animal-derived production to microbial fermentation
- A comparative analysis of China and other countries in metabolic engineering:Output,impact and collaboration
