Molecular design,synthesis strategies and recent advances of hydrogels for wound dressing applications
2021-05-19DanZengShihongShenDaidiFan
Dan Zeng,Shihong Shen,Daidi Fan*
Shaanxi Key Laboratory of Degradable Biomedical Materials,Shaanxi R&D Center of Biomaterials and Fermentation Engineering,School of Chemical Engineering,Northwest University,Xi’an 710069,China
ABSTRACT With the changes in the modern disease spectrum,pressure ulcers,diabetic feet,and vascular-derived diseases caused refractory wounds is increasing rapidly.The development of wound dressings has partly improved the effect of wound management.However,traditional wound dressings can only cover the wound and block bacteria,but are generally powerless to recurrent wound infection and tissue healing.There is an urgent need to develop a new type of wound dressing with comprehensive performance to achieve multiple effects such as protecting the wound site from the external environment,absorbing wound exudate,anti-inflammatory,antibacterial,and accelerating wound healing process.Hydrogel wound dressings have the aforementioned characteristics,and can keep the wound in a moist environment because of the high water content,which is an ideal choice for wound treatment.This review introduces the wound healing process and the development and performance advantages of hydrogel wound dressings.The choice of different preparation materials gives the particularities of different hydrogel wound dressings.It also systematically explains the main physical and chemical crosslinking methods for hydrogel synthesis.Besides,in-depth discussion of four typical hydrogel wound dressings including double network hydrogels,nanocomposite hydrogels,drug-loaded hydrogels and smart hydrogels fully demonstrates the feasibility of developing hydrogels as wound dressing products and their future development trends.
Keywords:Hydrogels Wound dressing Molecular design Crosslinked networks Biomedical applications
1.Introduction
Hydrogel wound dressing is a type of synthetic dressing that is particularly good for keeping wounds moist,such as burns or necrotic wounds [1].Since the 1980s,the skin healing potential of hydrogels has been investigated along with its clinical application since the effectiveness and importance of moist-based wound care for skin regeneration was proven[2,3].Initially,the hydrogels were able to absorb and retain wound exudates.Afterwards,fibroblast proliferation and keratinocyte migration were included in order to complete the epithelialization of the wound or its healing [4].It is usually used as a flat dressing in the form of a sheet with a variety of shapes,or as a free-flowing gel that can be fit for different types of wounds.
Hydrogel wound dressing is designed to hold sufficient moisture in the superficial layer of the wound,providing an ideal environment for proper and easier wound cleaning as well as pain management [5].As a result,the granulation and formation of necrotic tissues are reduced,simplifying the entire healing period.Since hydrogel wound dressing is 90%water-based,it gives a comfortable soothing effect to the wound site,which is essential in severe wounds or in the presence of comorbidities like diabetes.Moreover,the high moisture content is beneficial in providing a barrier against infections in order to inhibit bacteria and oxygen from reaching the wound to minimize bacterial infestation.In addition,hydrogel wound dressing is non-adhesive to the wound or tissues,which minimizes pain during dressing changes and does not disrupt wound healing[6,7].The unique and tuneable mechanical properties of hydrogels increase their suitability towards elasticity and flexibility to adapt with wounds of different body sites.Hydrogels bring immediate relief to patients in distress compared to traditional bandages,pads or gauze-based dressings.They are easy to handle and have great absorptive ability and are affordable.However,secondary injury could occur when peeling off the gauze.Such characteristics of hydrogels are particularly useful for burns and other types of open or chronic wounds [8].The classification of hydrogel wound dressings is shown in Fig.1.
The classic stages of wound repair consist of three phases:(a)inflammation;(b) new tissue formation;and (c) remodeling,(Fig.2) [9].The inflammation stage lasts until about 48 h after injury.The characteristics of the wound encompass a fibrin clot that forms in a hypoxic (ischaemic) environment with abundant bacteria,neutrophils and platelets.The actions of the coagulation cascade,inflammatory pathways and immune system are necessary in order to prevent blood and fluid losses,remove dead and devitalized (dying) tissues and prevent infection.The new tissue formation stage occurs about 2–10 days after injury and is characterized by cellular proliferation and migration of different cell types.An eschar is then formed gradually,where most cells from the previous stage of healing have migrated from the wound and new blood vessels populate the area.The sprouts of capillaries associated with fibroblasts and macrophages replace the fibrin matrix with granulation tissue,forming a new substrate for keratinocyte migration during later stages of healing.The keratinocytes that are behind the leading edge proliferate and mature,finally restoring the barrier function of the epithelium.The migration of epithelial cells can be observed under the eschar.The remodeling stage lasts for a year or longer,in which most of the endothelial cells,macrophages and myofibroblasts undergo apoptosis or exit from the wound,leaving a mass consisting mostly of collagen and other extracellular-matrix proteins.Disorganized collagen are laid down by fibroblasts that have migrated into the wound [10].The wound contracts near its surface,and the reepithelialized wound becomes slightly higher than the surrounding surface.
The wound healing process is regulated by complex molecular mechanisms and is susceptible to interruption by many factors,such as size,depth,origin,and level of contamination [11].The types of wounds that may heal faster using hydrogel wound dressing include:
1.Minor burns.Hydrogel wound dressing provides a soothing effect to the wound site in order to alleviate excruciating pain and tenderness of the skin.
2.Wounds that are very dry or necrotic,such as superficial abrasions,severe scrapes,and scratches.Hydrogel provides sufficient hydration to help with skin moisture retention and provide effective healing.
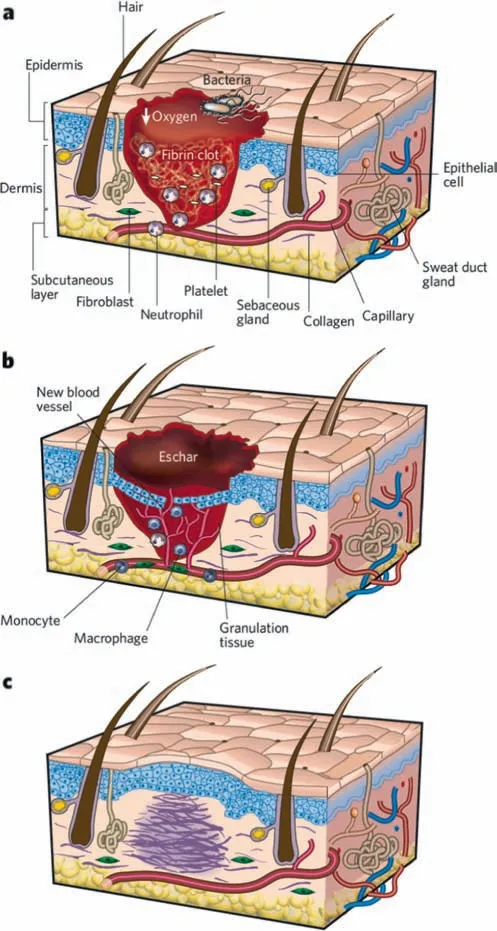
Fig.2.Classic stages of wound repair.(Adapted from Gurtner et al. [9]).
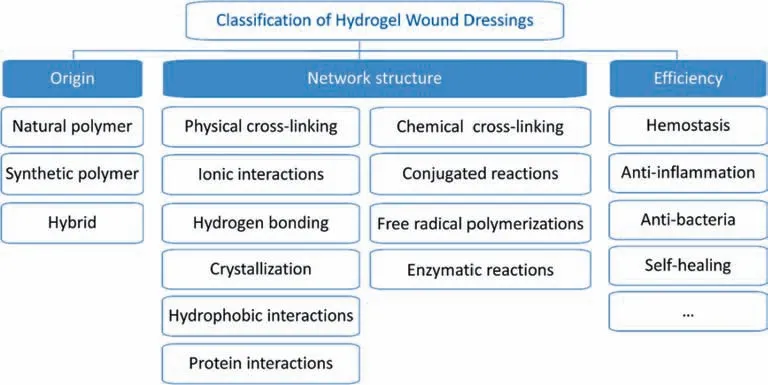
Fig.1.Classification of hydrogel wound dressings.
3.Wounds that create cavities or depressions in the skin.Applying hydrogel wound dressing for depressed wounds will likely improve tissue growth and minimize the risk of having a healed but dented wound site.
4.Partial or full-thickness skin wounds.Hydrogel wound dressing promotes faster healing since it does not stick or allow necrotic tissue to slough off during healing.
A wide variety of hydrogels for wound dressings are commercially available in the market for the treatment of minor burns to full-thickness skin wounds.They are available in numerous forms like amorphous gels,gel-impregnated gauzes,sheets or plasters[12].Amorphous gels are generally prescribed for superficial burns like cavity wounds,sheets and gel-impregnated gauzes [13].Plaster-like hydrogel dressings (e.g.MySkin®) are very userfriendly and attractive,which may be appropriately positioned on the wound without using adhesives and bandages.The development of hydrogel wound dressing has been brought into a new stage(Table 1)in order to address different aspects of wound healing and management.
In this review,the development,recent advances in design and application of hydrogel wound dressings are systematically reviewed.At the end of the paper,future improvements are discussed.In the next section,the choice of materials used in the synthesis of hydrogel wound dressings will be discussed before conducting a more extensive discussion considering potential factors for the optimization of hydrogels for specific wound healing purposes.
2.Material Categories Used in the Synthesis of Hydrogel Wound Dressings
The technical features of hydrogel wound dressings are [20–22]:
– Proper mechanical strength (preferred particle size and porosity)
– Nontoxicity
– Excellent water absorption capacity (provides a warm,moist environment for a fast and effective healing process)
– Infection control
– Biocompatibility
– Breathability (gas transformation)
– Biological activity (such as hemostatic activity,antibacterial activity and non-immunogenicity)
– Maximum biodegradability without formation of toxic groups
– Biodegradability
Although certain properties can be attained,it is not possible to produce a perfect hydrogel with all of the above features.The properties of the hydrogel are mostly determined by the chosen polymer.When hydrogels are prepared for wound dressings,ensuring the proper mechanical strength is necessary in conjunction with the biocompatibility and biological activity for clinical application.Table 2 summarizes the commonly used natural and synthetic polymers as well as nano-composites in fabricating hydrogel for wound dressing.
The choices of raw materials highly define the generative properties of a hydrogel wound dressing.Natural polysaccharide/protein-based hydrogels have both the performance advantages of polysaccharides and proteins,including:(1) The structure and properties are similar to the extracellular matrix;(2)Excellent biocompatibility,no rejection reactions with human tissues;(3)Good biological activity with antibacterial and cholesterol-lowering effects;(4) Promotes the growth of epithelial cells and accelerates wound healing;(5) Can be degraded into glucosamine under the action of lysozymes and directly enters the metabolic pathway,which is gentle and safe;(6)There is no active site and tissue adhesion [71].However,current polysaccharide/protein-based hydrogels still have certain disadvantages,such as poor mechanical properties,single function,poor re-swelling of xerogels,and complex construction,which greatly limit the application of natural bio-based hydrogels.In contrast,synthetic hydrogels can better control the matrix structure and have strong mechanical properties,however,their biological activity,biocompatibility and degradation properties are relatively poor[72].Accordingly,the current method to solve such problems is combining artificial synthetic materials (such as polyvinyl alcohol and polyethylene glycol) and natural biopolymers (such as collagen,chitosan,and alginate)through different crosslinking processes [73].The composite hydrogel is built for a defect for which a single type of material hydrogel may be unable to meet the multi-functional requirements.As a result,the combination of natural materials and polymer materials has greatly promoted the innovative development of hydrogel wound dressings.
3.Network Structure of Hydrogels
Crosslinking is a critical step in the preparation of hydrogel wound dressings.The main techniques are divided into physical crosslinking due to ionic interactions,hydrogen bonding,crystallization,hydrophobic interactions,and protein interactions,and chemical crosslinkingviaconjugated reactions,free radical polymerizations and enzymatic reactions.The choice of raw materials influences crosslinking,and the nature of crosslinkage determines the physicochemical properties of the hydrogel as well as the target application [4].Some of the typical physical and chemical crosslinking strategies of hydrogel wound dressings are summarized in Fig.3.
3.1.Physical crosslinking
Physical crosslinking refers to the formation of supramolecular hydrogels with no crosslinking agent or added external force.Here,a three-dimensional network structure between molecules may be formed through supramolecular interactions such as ionic bonds,hydrogen bonds,van der Waals force or electrostatic attraction and intermolecular entanglement under certain conditions.Depending on the type and nature of the raw material,the physical crosslinking methods used by various polymers may vary.

Table 1 List of various hydrogels wound dressings

Table 2 Summary of commonly used source materials for hydrogel wound dressings
3.1.1.Ionic interactions
Ionic interactions are effective in hydrogel synthesis through the dynamic interactions that exist between oppositely charged groups or metal–ligand interactions [75].Polyanionic polymers and polycationic polymers are complexed to form hydrogelviapolyelectroyte complexation,which is also termed complex coacervation [76].These hydrogels have advantages in fast environmental response and self-healing [77,78].For example,alginate can carry out ion exchange with calcium ions in calcium chloride solution for crosslinking,which may then be used to embed microorganisms in order to carry drugs for sustained release [79].
3.1.2.Hydrogen bonding
Hydrogen bonded hydrogels can be obtained by lowering the pH of an aqueous polymer solution when carboxylic functional groups are present on the chains.This pH-responsive property is very popular in tissue engineering.At an acidic pH,the reduction of aqueous polymer solubility promotes hydrogen bonding to form hydrogels[80].However,the high gel concentration and low crosslink density make H-bonded hydrogels difficult to use in injections and have weak mechanical strength [81].

Fig.3.Schematic illustration of hydrogels produced by typical physical and chemical crosslinking methods.(The example of enzymatic reaction is reproduced with permission from Kuo et al. [74]).
3.1.3.Crystallization
Repeated freezing and thawing is the most common method of performing physical crosslinking,and the key of this process is the formation of crystalline.An aqueous solution of PVA can form hydrogen bond crosslinking through repeated freeze-thawing,where the degree of crosslinking is related to the molecular weight of PVA,freeze-thawing time and number of freeze-thawing cycles[82,83].The viscoelastic behavior of physically crosslinked PVA hydrogels formed by repeated freezing and thawing methods was reported to be equivalent to that of joints and meniscal cartilage [84,85].By finely adjusting the parameters in preparing hydrogels by repeated freezing and thawing or physical crosslinking,such as the degree of crosslinking between molecular chains or the crystallinity of crystals formed during repeated freezing and thawing,the obtained hydrogel can be controlled,especially in regard to its mechanical properties [86,87].
3.1.4.Hydrophobic interactions
Hydrophobic interactions is one of the hydrogel crosslinking methods existing in water-soluble polymers with hydrophobic end groups,side chains or monomers.Both thermal induction and ultrasonic treatment create hydrophobic interaction [88].For phase transition by thermal induction based on LCST(Lower Critical Solution Temperature) or UCST (Upper Critical Solution Temperature),hydrogels can be synthesized by promoting sol-to-gel transition at a critical temperature.Denget al.[89]prepared akcarrageenan/polyacrylamide based hydrogelviastrong hydrophobic interactions between sodium dodecyl sulfate micelles and the hydrophobic alkyl groups of stearyl methacrylate.This formation is due to helix-formation,association of helices,and forming junction zones.In some cases,hydrogels have been obtainedviablock copolymerization by simply warming the polymer solutions [90].
3.1.5.Protein interactions
In addition to natural polymers-polysaccharides,proteins can be used to make hydrogels,such as gelatin,silk protein,collagen and fibrin [91–94].This type of hydrogel is mainly assembled by the interaction of non-covalent bonds between proteins or polypeptidesviaphase transformation.Generally,threedimensional space arrangement will occur due to the series of behavioral changes in the quaternary structure of the protein[75].With the development of protein engineering,performing hydrogel synthesis with recombinant proteins has garnered particular interest[95].Human-like collagen(HLC),a novel recombinant human collagen,can be engineered and serves as a promising material for hydrogel synthesis in wound management due to its good biocompatibility,low immunogenicity,reduced risk from hidden virus,stability as well as its capabilities in mass production[47].
3.2.Chemical crosslinking
Hydrogels formed by chemical crosslinking adopt certain crosslinking techniques such as photo-polymerization and irradiation (gamma ray or electron beam),or through the addition of a crosslinking agent in order to combine the chemical or covalent bonds between the macromolecular chains to create a crosslinked network.Compared with physical crosslinking,hydrogels with different mechanical properties and crosslinking densities may be more easily prepared by controlling certain conditions.
3.2.1.Conjugated reactions
Hydrogels crosslinked by conjugated or coupling reactions have become popular in research,mainly including Michael addition,Schiff base reaction,and Diels-Alder addition reaction [96–100].This form of crosslinking can be carried out in mild conditions,and the entire gelation process does require the addition of chemical additives that are not conducive to cell growth,which is highly beneficial in tissue engineering.In contrast to a physical crosslinked hydrogel,a conjugated reaction crosslinked hydrogel will not undergo a solution-gel phase transition,hence,there will be no obvious volume changes,which is beneficial in filling organ defects.
Among them,the Schiff base reaction (condensation of aldehyde groups and amino groups to form amide bonds) is the simplest and most feasible method for crosslinking hydrogels.Many polysaccharides contain adjacent hydroxyl groups in the molecule,such as alginic acid,chondroitin sulfate,hyaluronic acid and cellulose,which may be oxidized by periodate to form this kind of hydrogel [101].For example,an injectable gelatin/hyaluronic acid composite hydrogel scaffold crosslinked by Schiff base reaction has slow degradability and is composed of a carbohydrazidemodified gelatin (Gel-CDH) and hyaluronic acid mono-aldehyde(HA-mCHO).Due to the stable Schiff’s base formation between the aldehyde and carbohydrazide groups,as well as the suppression of ring-opening oxidation by mono-aldehyde modification,the prolonged degradability of Gel-CDH/HA-mCHO hydrogel is suitable for inducing angiogenesis [102].
3.2.2.Free radical polymerizations
Free radical polymerization crosslinking makes the precursors with unsaturated or photosensitive functional groups undergo free radical polymerization or crosslinking under the action of heat or light to form a covalent bond crosslinked hydrogel.The generation of free radicals mainly takes place through the addition of thermal initiators or photoinitiators in order to initiate the polymerization and crosslinking of functional groups on the macromonomers[103].The most commonly used types of initiation include ultraviolet radiation(UV)and thermal initiation.Generally,the thickness and depth of the material seriously affects the light intensity,hence,UV polymerization has great limitations forin vivoapplications.As a result,it is mainly used for the preparation of skin surface repair materials,such as hydrogel dressings and eye contact lens.Thermal initiation is more suitable for in-situ injection molding and can be used for tissue engineeringin vivo,such as cartilage repair [104].
In thermally initiated polymerization,the functional group is generally a C=C double bond,for which a redox initiation system is mainly utilized.This redox initiator has good water solubility and high activity,however,utilization of such a redox system inevitably causes pH changes in the entire hydrogel system.Accordingly,its by-products may affect the survival and growth of cells,thus,the choice of initiating system is vital.
In photo-initiated polymerization,precursors containing photosensitive functional groups (such as azide) can be polymerized directly under UV irradiation,whereas precursors containing double bond functional groups can be polymerized under UV irradiation by adding a photoinitiator [105].The photo-initiated polymerization rate is relatively fast and contains few byproducts,however,the UV irradiation intensity and irradiation time,photoinitiator concentration and temperature changes during the reaction must be strictly controlled.Compared with thermally-initiated polymerization,photo-initiated polymerization has a faster rate of polymerization and has less of an impact on cells[106].However,due to limited UV transmittance,it cannot be used in repairing deeper tissues,unlike thermally initiated polymerization.The gel formed by free radical crosslinking is covalent bond crosslinking.Here,the gel structure is relatively stable,strong,and has better reaction controllability,making it easy to be used in surgery.However,it requires the participation of other substances and is accompanied by chemical reactions,hence,it affects cells more.By optimizing various parameters of the initiation system and strictly controlling other operating conditions,the influence of the system on cells can be reduced or eliminated to meet the needs of tissue engineering[107].By employing different types and concentrations of initiators as well as different ratios of crosslinking agents,crosslinked polymers have significantly different characteristics,among which the ratio of crosslinking agents affects the crosslinking density and,thus,the final mechanical properties.
The preparation of hydrogels by free radical polymerization usually involves graft modification of the matrix polymer[108,109].Natural polymer materials have abundant functional groups,which facilitate the introduction of polymerizable functional groups or water-soluble groups.Polymerizable functional groups generally refer to groups or small molecules with unsaturated C=C double bonds,of which methacrylic acid is the most widely reported application source.There are two main methods in regard to graft modification.One technique is using watersoluble carbodiimide EDAC (or EDC) as a condensation agent to condense polymers that have amino groups with methacrylic acid.The other method is utilizing polymers with hydroxyl or amino groups directly esterified with methacrylic anhydride.At present,a variety of natural polymers have been prepared into hydrogel materials by free radical polymerization,including gelatin,chitosan,sodium alginate,heparin,hyaluronic acid,and chondroitin sulfate.Among them,chitosan has good biocompatibility and specific antibacterial properties,which is widely used in drug carriers and tissue engineering.Due to the strong hydrogen bonding force between molecules,chitosan can only be dissolved following protonation under acidic conditions.Moreover,it can be crosslinked with covalent bonds or ionic bonds to form hydrogels by altering the pH.However,acid solubility and method of gel formation limit the application of chitosan as an injectable hydrogel for tissue repair in vivo.Therefore,it is important to modify the molecular structure of chitosan in order to promote its water solubility under neutral conditions and establish a more feasible gel method.
Crosslinking via high energy radiation is a cost effective technique in polymerizing unsaturated compounds for hydrogel synthesis by gamma ray or electron beam radiation as there is no need for separate sterilization of the hydrogel and use of toxic chemical crosslinking agents [110].Free radical polymerization is initiated between the radicals formed on polymer chains by high energy radiation.With the recombination of these radicals,covalent crosslinked hydrogels are subsequently formed [111].Silver nanoparticles loaded AMPS-Na+hydrogel [15],CS/gelatin/PVA hydrogel [112]and moxifloxacin loaded tragacanth-PVA-alginate hydrogel[113]are synthesized by using a gamma irradiation technique,and PVP/PEG/agar [114],BC/acrylic acid (AA) [115]and polyethylene oxide (PEO)/polyethylene glycol dimetacrylate(PEGDMA) [116]hydrogels are formed by electron beam irradiation,demonstrating promising potential for application as wound dressings.
3.2.3.Enzymatic reactions
Enzymatic methods to catalyze the crosslinking of natural substances (between proteins and other proteins or between proteins and polysaccharides) through enzymes.Commonly used enzymes include transglutaminase (TG) and horseradish peroxidase (HRP)[49,51,117].In organisms,enzymatic reactions mostly occur in at mild conditions with a medium pH,medium temperature and aqueous environment,indicating that the hydrogels are formed in situ [118].Additionally,the substrate specificity of the enzyme may prevent additional reactions and toxicity.Compared with the reactions under harsh chemical conditions,the mildness of the enzymatic reaction brings about another advantage in preventing the loss of biological activity.The enzyme simply acts as a catalyst and does not participate in crosslinking.Hence,it can be washed out after hydrogel formation.Moreover,specific proteins or polypeptide chains containing amino acid residues can be catalyzed by the transamination of TG to form isopeptide bonds,which crosslink to form a protein or polypeptide network structure.Polysaccharide macromolecules with phenolic hydroxyl groups can be oxidized by HRP to catalyze the crosslinking of polysaccharide molecules so as to form a network structure.Therefore,many studies have reported the formation of mussel-inspired hydrogels via HRP enzymatic reactions [117,119].
4.Typical Hydrogel Wound Dressings
Different types of wounds vary in size,shape and thickness portray different clinical manifestations,such as necrotic,sloughy,granulating and epithelializing.According to the “moist wound healing theory”,the ideal wound dressing should have a scaffold to support cell growth,form an anti-infective barrier,promote natural blood coagulation,block nerve endings to relieve pain,absorb wound exudate,and provide proteins needed for wound healing and the strengthening of new tissue.This review listed some typical hydrogel wound dressings for further discussion.Doublenetwork hydrogels andnanocomposite hydrogels are classified by the structure of hydrogel,and drug-loaded hydrogels and smart hydrogels are classified by its functionality.
4.1.Double-network hydrogels
Double-network (DN) hydrogels are a special class of interpenetrating polymer network(IPN)hydrogels that illustrate extremely high mechanical strength and fracture toughness based on an internal fracture mechanism.The structural feature of DN hydrogels is that it contains two independent network structures,one of which is a polyelectrolyte network structure with a higher crosslinking density,while the other is a neutral network structure with low or no crosslinking (Fig.4) [120,121].The polyelectrolyte network structure provides a rigid scaffold for DN hydrogels to maintain their shape,while the flexible neutral polymer fills in the rigid network in order to absorb external stress.DN hydrogels have excellent characteristics,such as viscoelasticity,high water content,high wear resistance and good light transmittance,selfhealing,and thermo-and pH-sensitivity,which are beneficial for wound healing.Meanwhile,DN hydrogels are not being hindered by the low mechanical properties of traditional polymer hydrogels[122–124].
Early in 2003,Gonget al.[125]first succeeded in synthesizing DN hydrogels with extremely high mechanical strength,ushering in a new era of soft and wet materials to be used as substitutes for tissue engineering.Jiaet al.[126]put forward a tuneable DN hydrogel composed of a polyurethane (PU) hydrogel and a stronger,dipole–dipole and H-bonding interaction reinforced (DHIR)hydrogel.Compared to conventional DN hydrogels,this new DN hydrogels exhibited remarkable improvements in mechanical recovery,modulus and yielding,with excellent self-healing and self-gluing properties and outstanding tensile and compression strength,which also possessed properties in shape memory.However,the first network of conventional DN hydrogels was restricted to crosslinked via irreversible covalent bonds,in which damage accumulated onto the network was irrecoverable.Additionally,the mechanical properties of DN hydrogels decrease gradually,constricting the properties and applications of DN hydrogels.With the development of new materials,novel techniques have been devised to expand the DN system.

Fig.4.An illustration a tough DN gel consisting of the first brittle network and the second ductile network.(Reproduced from Ref.[121]).
Sabziet al.[127]created a stiff,tough and self-healing DN hydrogel composed of a strong agar biopolymer gel as the first network,with a tough PVA biopolymer gel as the second network.This DN hydrogel demonstrated multiple-energy dissipating mechanisms with a high modulus up to 2.2 MPa and toughness up to 2.1 MJ·m-3,which was also able to quickly and autonomously self-heal regaining 67% of its original strength after just 10 minutes.Such superior mechanical properties are unmatched by other polymer hydrogels.
Hybrid DN hydrogels composed of a physically crosslinked(reversible) first network and a chemically crosslinked second network have attracted researchers’ interests due to their high mechanical properties and recoverability.Guoet al.[128]fabricated hybrid double-network hydrogels with ionically crosslinked κ-carrageenan as the first network and covalently crosslinked poly(N-acryloyl glycinamide) (PNAGA) as the second network.These hybrid DN hydrogels exhibited excellent mechanical properties with a breaking stress of 1.7 MPa and a breaking strain of 250%,which far outperformed their parent single-network hydrogels.In addition,due to the physical crosslinks among κ-carrageenan and PNAGA,the DN hydrogels possessed satisfactory self-healing abilities.
4.2.Nanocomposite hydrogels

Fig.5.Five main methods used to obtain uniform distribution of NPs in hydrogels.Adapted from Thoniyot et al. [130].(Reproduced with permission from John Wiley and Sons,Copyright 2015.)
Nanocomposite hydrogels,also known as hybrid hydrogels,can be defined as composite material that physically or covalently incorporates nanosized particles or nanostructures into a matrix of crosslinked polymer networks [129].In addition to introducing the secondary polymer into the hydrogel to form DN hydrogels through physical blending or copolymerization,nanocomposite hydrogels have also become an effective strategy for improving the properties of hydrogel wound dressings.NPs have distinct physicochemical properties,nano-sized characteristics,controlled shape and versatile modification possibilities,and well-defined multi-functionalities,which can provide a powerful platform for site-specific and controllable delivery of drugs,gene,proteins and other bioactive agents;some of them exhibited noticeable antibacterial,antiviral and antifungal activities.
Five main methods used to obtain uniform distribution of NPs in hydrogels have summarized as (Fig.5):1) hydrogel formation in a nanoparticle suspension,2) physically embedding the nanoparticles into hydrogel matrix after gelation,3) reactive nanoparticle formation within a preformed gel,4) crosslinking using nanoparticles to form hydrogels,5) gel formation using nanoparticles,polymers,and distinct gelator molecules[130].Variety of NPs have been successfully used for hydrogel wound dressing synthesis,such as polymer-based NPs [131,132],metal and metal oxide NPs(e.g.,Ag[133,134]and zinc oxide[135,136]).Some of them have attractive biological activities,such as antiinflammatory,antimicrobial,and anti-angiogenic properties.These properties are significant for an excellent hydrogel dressing.
Besides NPs,nanostructures like GO [70],and nano-clay [121]are also used for achieving hydrogel wound dressings with multifunctional properties.For example,Hanet al.[137]significantly improved the mechanical properties of hydrogels by incorporating nano-clay with polydopamine and polyacrylamide.The PDA-clay-PAM hydrogel could adhered directly on human skin without causing an inflammatory response and was easily removed without causing damage.The hydrogel also displayed superior toughness,which resulted from nanoreinforcement by clay and PDA-induced cooperative interactions with the hydrogel networks.
4.3.Drug-loaded hydrogels
Drug-loaded hydrogels are the kind of hydrogels as carriers in combination with therapeutic drugs.Both the characteristics of the hydrogels themselves can be retained,and the drug release rate can be controlled,thereby overcoming the shortcomings of traditional drug administration methods.Numerous fundamental and clinical studies have shown that drug-loaded hydrogels play beneficial roles in the repair of complex and difficult-to-heal wounds,such as diabetic wounds and infectious wounds [138].Table 3 depicts the different types of drugs loaded in hydrogel wound dressings and their properties.
Hydrogels have advantages in isolation,moisturizing,drainage,anti-inflammatory and pain relief [7].After the drug is loaded,the special structure of the hydrogel can be used to control the drug release rate,which is beneficial in wound healing.Cells and cytokine drugs can directly participate in wound repair through a slow and controlled release and may also activate multiple cell signal transduction pathways,indirectly regulating inflammation,protein synthesis,capillary angiogenesis,and granulation tissue formation[156].The local slow and controlled release of antibacterial drugs effectively increase the concentration of the local drug on the wound and release the corresponding drug dose according to different wound environments,making the antibacterial effect of the drug more targeted.Accordingly,a beneficial antibacterial effect can be obtained,and adverse reactions of systemic medications can be avoided.
4.4.Smart hydrogels
Currently,wound dressings are considered passive treatments,which may prove difficult in actually treating the wound and meeting the dynamic needs of chronic wounds simultaneously.Due to the realization of wearable health equipment,a new generation of smart wound dressings that contain sensors can solve such problems by detecting the physical and chemical signals related to wound healing.In order to detect the wound status early,physical and chemical markers,such as temperature,pH,inflammatory factors,toxins and enzymes secreted by bacteria,have been employed as indicators.Among them,temperature closely related to the inflammation and infection status of the wound site is considered to be one of the most important and promising indicators.Nowadays,among infrared thermometers,colorimetric sensors,and electronic temperature sensors developed for body temperature measurement,the electronic temperature sensor possesses advantages in high sensitivity,high accuracy,and easy operation in the clinic.Therefore,by designing an integrated flexible temperature sensor,an intelligent wound dressing that possesses real-time wound temperature monitoring functions may be realized.

Table 3 Different types of drugs loaded in hydrogel wound dressings
In this regard,Caoet al.[157]designed switchable antimicrobial and antifouling hydrogels with a smart response.Under acidic conditions,the hydrogels underwent self-cyclization and caught and killed bacteria.Under neutral/basic conditions,however,hydrogels underwent ring-opening and released the killed bacterial cells and resisted protein adsorption and bacterial attachment.Smart hydrogels show a dramatically improved mechanical property,which may be highly desirable in biomedical applications.The MIT team announced the creation of a set of tough hydrogels containing 70–95%water,composed of extraordinary mechanical properties,high stretchability,and transparency,which may sense temperatures at different locations on the skin[158].Additionally,Panget al.[159]designed a smart flexible electronics-integrated wound dressing with a double-layer structure,the upper layer of which contained polydimethylsiloxane-encapsulated flexible electronics integrated with a temperature sensor and UV light-emitting diodes,while the lower layer was a UV-responsive antibacterial hydrogel.This wound dressing continuous monitors wound temperature using integrated sensors;when the wound temperature continues to be higher than a preset threshold (such as 40 °C),an infected wound is diagnosed,and the integrated UV LED is turned,triggering the release of antibiotics in situ.This concept overcomes the wound healing process and provides a new strategy for the implementation of dynamic intervention therapy (Fig.6(a)).Moreover,Qiaoet al.[160]developed a smart hydrogel-based wound dressing capable of monitoring bacterial infection via pH-responsive fluorescence resonance energy transfer (FRET) transition of Cyanine3(Cy3) and Cyanine5 (Cy5) in a bacterial environment,which gave on-demand treatment of infection via near infrared (NIR) lighttriggered antibiotic release.Here,Cy3 and Cy5-modified silica nanoparticles (SNP-Cy3/Cy5) were loaded and acted as a pHresponsive fluorescent probe to detect bacterial infection based on the FRET effect between Cy3 and Cy5.Upon irradiating the hydrogels with NIR light,up-conversion nanoparticles were able to convert NIR light to UV light to trigger the release of GS from the hydrogels for antibacterial treatment (Fig.6(b)).Liuet al.[161]reported a novel smart hydrogel wound patch incorporating modified pH indicator dyes to monitor wound healing using a simple colorimetric display,providing a desirable substrate for printed electronics for smart wound dressing (Fig.6(c)).

Fig.6.Schematics of the structures and working principles of the smart hydrogel wound dressings.(a) a smart flexible electronics-integrated wound dressing with double layer structure[159];(b)a smart multifunctional hydrogel-based wound dressing for infection monitoring and NIR-triggered antibacterial treatment[160];(c)a novel smart hydrogel wound patch incorporating modified pH indicator dyes [161].
5.Conclusion and Future Perspectives
This review introduces the wound healing process and the development and performance advantages of hydrogel wound dressings.Through in-depth discussion of the three aspects of material selection,physical and chemical crosslinking technology,and representative wound dressings,it can be concluded that hydrogel wound dressings are a new type of high-end material with the necessary characteristics of ideal wound dressings,which has great potential in the application of clinical wound management.Nevertheless,the disadvantages of hydrogel wound dressings should not be overlooked,such as (1) excessive moisture of the high water content hydrogel can cause the growth of bacteria and prevent wound healing;(2) hydrogels do not have enough porosity to allow adjacent cells to enter the wound to facilitate repair;(3) Cost.
In recent years,new synthetic methods,especially the introduction of new materials with excellent functions into the design of hydrogels,have solved the above problems to a certain extent and provided innovative ideas for the construction of new hydrogel wound dressings that meet the requirements of biological materials.The innovative development of hydrogel wound dressings needs to be further improved in the following aspects:(1) the types of hydrogel network materials need to be expanded.Due to the limitations of natural polymer materials and the biocompatibility of synthetic polymer materials,the development of hydrogels that combines natural and synthetic polymers will expand the efficacies and extra characters (such as tissue adhesive and mechanical durability) of hydrogel wound dressings.The success depends on the development of new materials,which will be achieved through the synthesis of new polymers or the emergence of modified natural polymers.(2)the user-friendly hydrogels with high-strength and self-healing properties should to be explored.Self-healing hydrogels can be applied to wounds of various types and shapes,and are expected to solve the problems of regional injuries that are difficult to cover with traditional dressings,as well as prolonging the working life of hydrogel wound dressings and improving their safety.Once a traditional hydrogel dressing is damaged,it is difficult to restore the mechanical properties.Introducing non-covalent bonds with self-healing function (hydrophobic interaction,hydrogen bond,electrostatic attraction,π-π stacking,crystallization,etc.)or reversible dynamic covalent bonds(Acylhydrazone bond,imine bond,Diels-Alder reaction,etc.),it will help to prepare the high-strength and self-healing hydrogel system.(3)hydrogel systems with ideal electrical,magnetic and optical properties have yet to be developed.At present,hydrogels are mainly focused on improving mechanical properties and meeting biological performance requirements.The research of hydrogel wound dressings that compatible with physiotherapies such as electrical stimulation,magnetothermal,photothermal,and -nonthermal biological effects of light will be more challenging and promising under the trend of clinical multi-method combined treatment.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Authors acknowledged the funding supports from the National Key R&D Program of China (2019YFA0905200).
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Monoclonal antibody-based cancer therapies
- Recent advances in microbial production of phenolic compounds
- The production of biobased diamines from renewable carbon sources:Current advances and perspectives
- Efficient production of chemicals from microorganism by metabolic engineering and synthetic biology
- Food synthetic biology-driven protein supply transition:From animal-derived production to microbial fermentation
- A comparative analysis of China and other countries in metabolic engineering:Output,impact and collaboration
