Single-molecule biotechnology for protein researches
2021-05-19XiaoyanZhuangQianWuAihuiZhangLangxingLiaoBaishanFang
Xiaoyan Zhuang,Qian Wu,Aihui Zhang,*,Langxing Liao,Baishan Fang,3,*
1 Department of Chemical and Biochemical Engineering,College of Chemistry and Chemical Engineering,Xiamen University,Xiamen 361005,China
2 The Key Lab for Synthetic Biotechnology of Xiamen City,Xiamen University,Xiamen 361005,China
3 The Key Laboratory for Chemical Biology of Fujian Province,Xiamen University,Xiamen 361005,China
ABSTRACT Cells employ proteins to perform metabolic functions and maintain active physiological state through charge transfer and energy conversion.These processes are carried out in a narrow space precisely and rapidly,which,no doubt,bring great difficulty for their detection and dissection.Fortunately,in recent years,the development and expansion of single-molecule technique in protein research make monitoring the dynamical changes of protein at single-molecule level a reality,which also provides a powerful tool for the further exploration of new phenomena and new mechanisms of life activities.This paper aims to summarize the working principle and essential achievements of single-molecule technique in protein research in recent five years.We focus on not only dissecting the difference of nanopores,atomic force microscope,scanning tunneling microscope,and optical tweezers technique,but also discussing the great significance of these single-molecule techniques in investigating intramolecular and intermolecular interactions,electron transport,and conformational changes.Finally,the opportunities and challenges of the single-molecule technique in protein research are discussed,which provide a new door for single-molecule protein research.
Keywords:Single-molecule protein Enzyme Nanopore Atomic force microscope Scanning tunneling microscope Optical tweezers Biotechnology Imaging
1.Introduction
Proteins are crucial biomacromolecules,which also have been widely developed as many critical industrial products to meet the increasing daily demand of people,such as an enzyme,antibody-based drug,and functional peptide [1].More importantly,protein is also a crucial substance,which is not only the component of the cytoskeleton but also plays an essential role in the cell’s life activity [2].Among the process of DNA replication,enzyme catalytic metabolism,and cell proliferation,various forms of protein lay a foundation of normal function for the process of cell activity,which works like machines at the molecular level[3–5].Thus,protein research will provide us a deeper understanding of life activity and pathogenesis and thus be increasingly crucial in various biological research processes.
Single-molecule technique arose in the last century’90s and has focused on the measurement of individual molecules’ movement and change process [6].It is a supersensitive tool that could be used to analyze the characteristics and discrepancies of molecules in individual micro-level instead of group-level [7,8].Thus,compared with many other traditional analysis technologies,its superiority was significant and would bring us many behaviors and property in individual micro-level [9–11].First,single-molecule technique provides a complete distribution of the behavior of individual molecules in a population,rather than just their normal behavior.Second,some rare single-molecule instantaneous state can be captured by this supersensitive technique and obtained from massive amounts of data.Last,the feature of consecutiveness and microsecond makes the measurement step from static to dynamic state that gives us more profound and detailed insight into the quick interaction and reaction process,just like footage in slow motion.Single-molecule technique can be mainly classified into four types based on the power to achieve single-molecule manipulation:electric,force,optics gradient forces,and magnetic field.The classical single-molecule techniques powered by electric include nanopore [12],and scanning tunneling microscope (STM)[13],while representative techniques powered by force and optics gradient forces include atomic force microscope(AFM)[14],optical tweezers [15],and magnetic tweezers [16].
After twenty years of development and innovation,these single-molecule techniques have achieved success in many fields.Thus,this paper will describe the working principle of these crucial and representative single-molecule techniques and review the application example and development of these techniques(nanopore,STM,AFM,optical tweezers) in protein researches in recent five years.We will focus on the detailed discussion of the great significance of single-molecule techniques in analyzing intramolecular and intermolecular interactions,capturing intermediate/transition states,observing the moving behaviors,and conformational changes in a microsecond,and elucidating the mechanism of enzyme-catalyzed reaction in nanoscale.
2.Nanopore
2.1.Working principle
The full application of nanopore technique relies on the invention of the Coulter counter and the measurement technique of single-channel current[17].By controlling the pore size,the nanopore device can achieve single-molecule detection and capture,in which a single nanoscale pore forms in the synthetic or natural material to limit the only pathway for transiting.Ions in the electrolyte solutions are driven to cross the nanopore powered by an external electric field from a pair of electrodes.After applying a constant voltage,ions cross the pore and form the constant and stable current recognized after passing through a low-noise current amplifier,which determines the limit of detection.Once the analyte is added in the device,molecules of interest are driven toward the nanopore,which similar to electrophoresis.Due to the influence of factors of size,electrical charge,and interaction,not only do the amount of time for molecules cross the pore(dwell time)various,but also the molecules inside the pore cause the significant changes of ionic current.Thus,ionic current acts as a sensor to recognize and respond to every single molecule in the solution cross the nanopore one by one [17].
2.2.Solid-state nanopore
In 1976,Neher and Sakmann [18]first reported that they recorded the single-channel ionic currents from frog muscle cells membrane potentials through the tip of glass micropipettes.Among the researches of biological molecules,solid-state nanopore first achieve success in DNA sequencing [19,20].Except for voltage and electrolyte,the material,size,and surface of nanopore also have a significant influence on the properties of nanopores in capture single-molecule protein [21,22].Recently,many scientists have focused on the application of solid-state in protein study through surface modification [23,24].As shown in Fig.1a,Chuahet al.[24]modified the surface of nanopores and magnetic nanoparticles with anti-prostate-specific antigen antibodies to capture the prostate-specific antigen(PSA)molecules.If the nanopores and magnetic nanoparticles“nip”the PSA molecule and form an immuno-sandwich,it cannot escape when the magnetic field is reversed,but the others can be removed.This ingeniously designed nanopore assisted by the interaction between antibody and antigen ensures accurate recognition [24].Solid-state nanopore has also been used to recognize the conformation [25],approximate shape,volume,charge [26],dipole moment [27]changes of the protein.As shown in Fig.1b,Wanget al.[28]employed solidstate nanopore to observe the monomeric,filamentous,and globular actin,and their unfolding drug-binding process.To improve the detection accuracy,Chauet al.[29]reported that macromolecular crowding enhances the detection efficiency of the nanopore,particularly for globular proteins (1000-fold increase in the molecule count) and enable the characterization of the filamentous protein.
These results demonstrate the ability and prospects of solidstate nanopores in single-molecule protein research.However,there is still much room for improvement of detection accuracy to capture the single-protein with a fast translocation rate at millisecond-level [30].Moreover,the application of solid-state nanopore in protein recognition has not been as successful as DNA sequencing due to charge diversity caused by the different arrangements of amino acids[24,31].pH,as we have known,influences the charge and characteristics of the protein,which also has a significant effect on the move rate and interaction with the inner wall of the nanopore.Fortunately,there are many mature techniques related to the shape,size,and surface modification of solid-state nanopore [23,24],which could contribute to the single-protein recognized process.
2.3.Biological nanopore
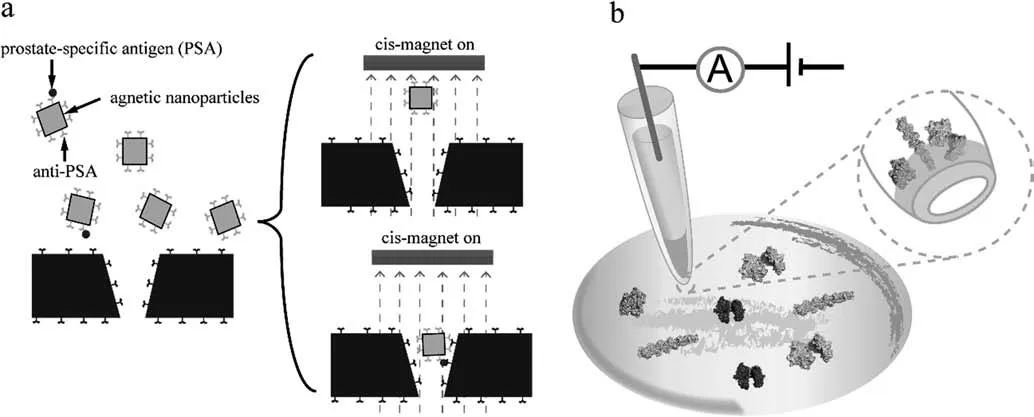
Fig.1.Sketch map of active sensing of single-molecule protein by solid-state nanopore [24,28].Sensing of prostate-specific antigen (a) and actin (b) based on solid-state nanopore.

Table 1 The characteristic of biological nanopore

Fig.2.The structures of four kinds of natural nanopore [32–35].The structures of Fragaceatoxin C (FraC,Fig.2a),Cytolysin A (Cly A,Fig.2b),Aerolysin (AeL,Fig.2c),and Mycobacterium smegmatis porin A (MspA,Fig.2d) biological nanopore,and the sketch map of working principle (Fig.2e).
Unlike synthetic material,some channel proteins located in membranes own a natural nano-size pore structure which has been developed to construct nanopore devices [32–38].The features and structures of many kinds of natural nanopores were shown in Table 1 and Fig.2.Based on the structure of the transmembrane region,this nanopore could be divided into two types of α-helical and β-barrel.Herein,we mainly introduce four kinds of frequently-used natural nano-size pore including Fragaceatoxin C(FraC,Fig.2a) [32],Cytolysin A (Cly A,Fig.2b) [33],Aerolysin(AeL,Fig.2c) [34],and Mycobacterium smegmatis porin A (MspA,Fig.2d) [35]biological nanopores,which have been successfully developed for single-molecule protein recognition.As shown in Fig.2e,in a biological nanopore setup,the cis and trans compartments are filled with electrolyte and connected through the nanopore.Upon applying an electric potential,ions cross the pore,and the changes of the current are detected when the protein of interest interact with the pore and block the current [32–34].
2.3.1.Fragaceatoxin C nanopore
Up to now,compared with the current standard method of mass spectrometry,the advantage of the nanopore technique is mainly concentrated in capture low-concentration proteins that are invisible in the noise of the abundant proteins.Thus,the FraC nanopore device was developed for single-protein recognization.After modified,the engineered FraC (Fig.2a) nanopore was used to identify different angiotensin peptides even in 44-dalton resolution [39].Similar to the solid-state nanopore,the single-molecule proteinrelated information was hide in the data of dwell time,current changes.Restrepo-Perezet al.[40]use FraC nanopore to labelfree study phosphorylation andO-glycosylation of proteins in post-translational modifications,which are closely associated with the pathogenesis of several diseases,such as cancer,diabetes,and Alzheimer’s.They demonstrated that the mixture of unmodified,glycosylated,and phosphorylated peptides could be observably distinguished by the dwell time and their difference in the blocked pore current by using FraC nanopore.
2.3.2.Cytolysin A nanopore
As shown in Table 1 and Fig.2b,cytolysin A was a special nanopore,in which the cis opening (5.5 nm) was more significant than its trans opening (3.3 nm).Thus,the proteins would be blocked inside the nanopore for milliseconds to hours depending on its size,charge,and shape for a longer time[41].Combined with electrostatic simulations,Willemset al.reported that the dwell time of dihydrofolate reductase inside ClyA various from milliseconds to seconds,dependent on its charge distribution [41].ClyA-AS (one mutant of ClyA) nanopore was also applied in quantified the concentration of glucose and asparagine in μM order of magnitude from bodily fluids by electrophoretic trapping the glucose or asparagine-binding protein [42].Wlokaet al.established an engineered ClyA-56W using a tryptophan replace glutamine at position 56,after which the hydrophobicity of the inner surface was increased.This engineered nanopore was used to recognize ubiquitin-conjugating one from the protein and its isomeric ubiquitination conjugates,and even observe the real-time protein ubiquitination based on the parameter of blocked pore current (IB),residual current percent(Ires%),and dwell time[43].ClyA nanopore was also employed to monitor the process of ligand-induced conformational changes of dihydrofolate reductase,in which four ground-state configurations or conformers with different ligands affinities was recognized through current blockades recordings[44].They demonstrated that ligand accelerated the conformer’s conversion and stabilized the transition state,which might be a universal feature in enzymatic catalytic reactions [44].
2.3.3.Aerolysin nanopore
Aerolysin nanopore is a β pore-forming toxin expressed byAeromonas hydrophila,and hydrophobic interactions maintain its structure(Fig.2c)[45].Caoet al.[45]carried out a research to engineered the aerolysin nanopore,which designed several singlepoint mutants to research the effect from pore diameter (replaced by alanine or tryptophan to enlarge or reduce the pore diameter)and electrostatics (positively charged arginine in 220 positions was changed to negative glutamic or neutral glutamine) on the sensing property.By controlling the pore structural and electrostatic interaction,the engineered aerolysin nanopores showed excellent performance in negatively and positively charged peptide recognition[45].The mean relative residual current(Ib/I0)obtained from aerolysin nanopore could be used to recognize twenty amino acids assisted by the short polycationic carrier [46].With the improvement of this technique,they envisioned that the terminal amino acid from the target protein was cleaved and then ligated to carrier peptide,which was captured and analyzed by nanopore subsequently [46].This method might pave the way for nanopore protein sequencing,which mainly relied on approaches to Edman degradation and mass-spectrometry two decades ago.
2.3.4.Mycobacterium smegmatis porin A
Compared with the biological nanopore described above,MspA,a conically shaped nanopore (Fig.2d),is more suitable for probing extremely small analytes,such as short-chain peptides and amino acid.Utilizing molecular dynamics simulations,Zhouet al.[38]found that compared with the smooth change tendency of the long narrow nanopore,electrostatic potentials of MspA show a sharp drop at the constriction state,which triggers the performance difference of nanopore.Wanget al.[35]employed engineered MspA nanopore with histidine,cysteine,or aspartic acid placed at site 91 to sensing different metal ions,which depended on the interaction between metal ions and amino acid residue.Sunet al.[47]identified the different-length short-chain peptides with negative charges by using the MspA nanopore mutant,which provided the potential for concentration measurement of caseinolytic protease P at nanomolar.
Nanopores technique has blazed a new trail for single-molecule protein research.The advantages of high sensitivity,low cost,and high flux offer a better opportunity for low-copy number species.However,the pore size,modification of column surface,and transit time of proteins across the nanopore are the challenges in singlemolecule measurement.With the continuous innovation of nanopores technique,it will yield unusually brilliant results in identify small molecules or folded proteins and monitor enzymatic reactions at the single-molecule level.
3.STM-Expanded Technologies
3.1.Working principle
1981,Gerd K.Binnig and Heinrich Rohrer of Zurich Institute invented the STM and therefore won the Nobel prize five years later [48].As shown in Fig.3a,exerting a bias voltage between the probe tip and the sample surface caused a tunneling effect when the tip moved very close to the sample.The surface morphology of the sample could be successfully characterized by the tunnel current between tip and sample surface with the moving of tip[50].Thus,the STM technique provided access to the electric properties of molecules adsorbed on a conductive substrate by combining the high-resolution imaging with the extension of spatially resolved current sensing spectroscopy (STS) to explore electric phenomena at the molecular scale [51].Initially,as an atomicscale image technique of the surface,the STM has further developed as a single-molecule operational tool in recent years,especially coupling with other techniques,such as break junction(Fig.3b) and electrochemistry (Fig.3c) [50,52].
Controlled by the movement of STM tip,the break junction process was achived by the repeated separation and approaching processes of two gold electrodes.The conductance of gold contact at a single atomic point is 1G0.The conductance changes from tens ofG0(gold atomic contact) to 10-2–10-6G0(gold-molecular-gold junction) and 10-7–10-9G0at last (break of gold-molecular-gold junctions caused by further upward movement of STM tip,also known as background),of which several orders of magnitude was monitored and formed typical conductance-time curve as Fig.3d recorded.The conductance would exponentially decay until reaching the background in aqueous medium (Fig.3d,blue line)only or do not form the gold-molecular-gold junction,while there would form a plateau of molecule (Fig.3d,orange,purple,pink line).One-dimensional conductance-displacement histogram is used to indicate the exact conductance value of molecule which consisted of thousands of individual traces.

Fig.3.The working principle sketch map of STM related technique.The working principle sketch map of STM(a),STM-BJ(scanning tunneling microscope break junction)(b),and EC-STM-BJ (electrochemistry scanning tunneling microscope break junction) (c).Typical individual traces (Fig.3d) and one-dimensional conductance-displacement histograms (Fig.3e) from STM-BJ [9].Conductance distributions derived from many such I-V curves from EC-STM-BJ [49].
3.2.STM-BJ
The STM-BJ (scanning tunneling microscope break junction)technique provides excellent performance in spatial resolution and electrical detection sensitivity,which has been one of the most frequently used techniques in single-molecule electrical detection in recent years[53–58].This technique is a simple and unambiguous measurement of single-molecule,achieved by repeatedly and rapidly forming thousands of molecular junctions by which to capture the changes of single-molecule incisively [9].Thus,the mass data of conductance provide access to resolute the state in the dynamic process by using statistical methods,such as typical individual traces (Fig.3d) and one-dimensional conductancedisplacement histograms (Fig.3e) [9].
The STM-BJ single-molecule measurement technique,originally designed to investigate the charge transport through the small organic compound in 2003 [59],was later extended to the study of intermolecular or intramolecular interaction of biomolecules such as DNA,polypeptide,and protein in the past decade [54,60].In 2004,Xiaoet al.[54]first revealed the pH-induced peptides conductance changes by using the STM-BJ technique,which caused the tunneling barrier of the amino group and a carboxyl group.Zhanget al.[55]reported that the conductivity of single αdomain protein hemoglobin was high than that of the β-domain protein superoxide dismutase enzyme,which was supported by the result of the higher hopping rate in the α-domain by the molecular orbitals simulations.Brisendineet al.[56]measured the conductance of oligoglycine (AAA) and oligohaline (GGG).They found the conductance of peptide was lower than that of alkanes terminated asymmetrically with an amine group on one end and a carboxyl group on the other,which was caused by the charge localization at the peptide bond.Crucially the conductance of peptide decreased as its length increased.They were studying electron transport through immobilized.Aragonèset al.[57]also used the Ni tip STM-BJ technique to succeed in recognizing the single chiral molecule (dextrorotatory and levorotatory) of α-22AA-peptides.
Also,the enzyme is a kind of biomacromolecule with catalytic function mainly consisted of protein,of which the catalytic process was millisecond scale and hard to be analysed.Thus,our group also researched to reveal some dark corners in the enzymatic catalytic process assisted by the STM-BJ technique.Zhuanget al.[58]provide the first demonstration of the STM-BJ technique for investigating charge transport through a single active enzyme junction,in which the binding of NAD+with proteins boosts the charge transport by over 2100% than neutral FDH.Combined with site-specific mutagenesis,we demonstrated the conductance of FDH-NAD+highly correlated with their bioactivities.Furthermore,the flicker noise analysis provided the first experimental evidence of pathway switching to a cofactor-mediated charge transport for NAD+[58].This work offered a fundamental understanding to design and develop biocatalysis and bioelectronics at the essential molecular level.
In general,these reports,dependent on the STM-BJ technique,will lay the foundation for subsequent biophysics studies to understand the electric role of protein in various systems,which are of great interest to biologists,physicists,chemists,and even engineers working on the relevant research area.Thus,STM-BJ is a powerful tool in protein electronic transport researches with single-molecule precision and provides the mass of data that harvests through calculated theoretically in the past.The electronic transport performance researches will also provide relevant proof and pave the way for the proteins dominated life activity.
3.3.EC-STM-BJ
An electrochemical STM was originally an imaging technique developed by Tao and used to obtain spectroscopic information and electron transfer [51].With the development of STM-BJ,the group of professors Tao,Lindsay,and Ulstrup,established ECSTM-BJ (electrochemistry scanning tunneling microscope break junction) successively,which regulated and monitored the molecular electrical transport process through a double layer formed at the electrode [50,61,62].
Biological electron transfer plays a universal and vital role in many essential cellular processes,such as respiration and photosynthesis.Thus,understanding the mechanism behind biological electron transfer is essential to elucidate the charge transport,which ultimately reflects the changes from conformation and functionality [63].It is crucial to construct such a single-molecule nanoscale electronic platform through the protein bioengineering.Ruizet al.[64]reported that charge transport behavior dramatically changed from the classical copper-mediated two-step style transport mode to direct coherent tunneling when introduced a newly Cys41 residue near the cupric ion base on EC-STM-BJ.This amino acid residue alters not the structure of Cu-azurin but charge density of the cupric ion center,which is proofed by the molecular dynamics and density functional calculations.A slight reduction of the Cu originated by the dramatically alters the charge transport behavior of the single-protein junction.This work is direct evidence of control in protein charge transport,although not related to the bio-activity.After that,Zhanget al.[65]further tethered an enzyme (Φ29 polymerase) between the electrodes to sensing and monitor the enzyme,in which the streptavidin contacted enzyme could be captured by the electrodes modified with thiolated biotin.
Proteins are so versatile that integration of them into bioelectronic devices is an exalting,long-term,and challenging goal,owing to their excellent performance in signal transduction,molecular recognition,and selectively catalytic.However,proteins are widely considered to be insulating due to the weakly hopping transport mechanism.Although many reports demonstrated that long-range electronic transport exists in the proteins with redox centers,what about the non-redox-active proteins [66]? Toward this aim,the group of professors Lindsay carried out many types of research and devoted to analyzing the charge transport in non-redox-active protein by using EC-STM-BJ [49,66–68].They reported that the specific bind of ligand to protein forms a reproducible high conductance,in which non-covalent binding of ligand gives significantly higher conductance than direct attachment through covalent modification [67].Compared with one specific and one weak,nonspecific contact,the type in which two ligands bound to a multivalent protein increased the conductance by approximately 10-fold [67].They also found that the size of the electrode gap affected not on the conductance of two fixed points on the antibody but the conductance of one specific attachment to an epitope and a second nonspecific attachment to the surface of the antibody[68].Beyond that,the similar conductance resonance was observed in three non-redox-active proteins (streptavidin,anti-DNP IgE,and Φ29 polymerase),which strongly suggested that the resonance was most likely an intrinsic common feature of the proteins in their recent work[49].The potential of them was about 0.7 V lower than the aromatic amino acid redox potential in solution.The lower potential also indicated the reduction of Marcus reorganization energy when these aromatic amino acid residues were natural optimized and folded inside the protein with an intact structure,and resulting in fluctuations lower barriers to tunneling[49].This finding may be used to solve the puzzle that intact proteins have more excellent electrical conductivity than peptides.
EC-STM-BJ own unique superiority in elucidating electron transfer mechanisms by probing molecular at different electrochemical potentials,which make it more suitable for singleredox-molecule analysis.The electron transport process studied from the redox center of proteins based on EC-STM-BJ could give further insights into the enzymic catalytic process and even the life activity.These results and methods established will lay the fundamental for electrics-based single biomolecules detection and diagnosis.
4.AFM-Expanded Technologies
4.1.Working principle
In 1986,Gerd K.Binning,Calvin Quate,and Christoph Gerber of Zurich Institute further invented the AFM [69].Compared with tunnel current-independent STM,AFM was developed based on the atomic force of between tip and sample,which extended the observed objects from conductors and semiconductors to insulators [70].As shown in Fig.4a,a nanoscale probe was fixed on the elastic cantilever with micron-scale manipulability in the AFM device,which was extremely sensitive to force[70].In the observation and measurement process,cantilever deformation caused by weak interaction force between tip and sample triggered deflection of the laser beam reflected from the back of the cantilever,which was captured by the photoelectric detector [70].After applying a constant interaction force on the sample,the tip was moving along the surface of the sample,and the deflection was recorded and converted into a morphology image indirectly[71].Based on the AFM image system,AFM force spectroscopy is a force-extension(Fig.4b) mode realized by the vertical reciprocating motion of tip in defined distance.Physisorption and chemisorption were common methods to form a bridge between tip and sample,and the interaction is reflected by force changes between the tip and sample,which is recorded by cantilever deflection[72,73].As shown in Fig.4c,upon stretching derived from upward movement of AFM tip,the changes of force would be recorded from contact to rupture.The deformation of sample induced by stretching was also recorded and formed the force-extension curves to study the mechanical strength.The application are described detailedly in Section 4.3.
4.2.AFM imaging
Compared with other similar technologies,AFM is not limited by measurement environmental conditions,which becomes a powerful tool to observe the morphology of biological samples in the physiological buffer to obtain high-resolution images (atomic level) [74].AFM imaging have been widely used to observe behaviours of many molecular motor in the past[75,76].The emergence and development of AFM make a great contribution to promote the precision research progress of protein-receptors and nuclearacidsprotein complex.By using high-resolution AFM imaging technique,Knoops [77]observed that the specific binding of PRDX5 to TLR4 receptors on macrophage-differentiated THP-1 and human TLR4-transfected CHO cells induced a proinflammatory response.From images obtained from high-speed AFM,Sumino et al observed increased affinity of the KcsA channel for Agitoxin-2 (AgTx2)from scorpion during binding process while decreased during dissociation process [78].They also analyzed the binding dynamics of AgTx2 to the KcsA channel and drew a conclusion that the induced-fit pathway was the dominant dynamics model,and accelerated the binding rate by 400 time.Sukhanova et al employed AFM imaging to study the functional differences between poly (ADP-ribose) polymerase-1 (PARP1) and poly (ADPribose)polymerase-2(PARP2)when interacted with different damaged DNA.The image showed that PARP1 slightly preferred nicks to double-strand breaks,while PARP2 was mainly located at a single DNA nick site.Hereby they drew an important conclusion that“PARylated PARP1 and PARP2 retain their interaction with DNA at their specific DNA sites(SSB or DSB)which initiated the reaction of PARP poly(ADPribosyl) ation”[79].

Fig.4.The working principle sketch map of AFM image (a) and AFM force spectroscopy (b,c) technique.L:the contour length of the sample which measured by fitting the force-extension curve.
4.3.AFM force spectroscopy
Besides,the single molecular force spectrum based on the AFM can also manipulate molecules at the single molecular level and measure the weak interaction force at the pN level[80].It is a powerful tool for the study of intramolecular and intermolecular interactions,including protein–protein [81],protein-DNA [82],and protein folding [83].Protein unfolding force could be measured to obtain more information about the structural stability and conformational transition.Xiaet al.[84]reported that the binding of Co2+and Co3+enhanced the mechanical stability of biHis mutant of protein,in which the unfolding force increased from~120 pN(apo-form)~140 pN(Co2+binding form)and~260 pN(Co3+binding form).Bernardiet al.[85]reported that X-module-Dockerin(XDoc)bound to its partner Cohesin (Coh) have more robust mechanical stability based on the force spectroscopy.Molecular dynamics simulations reveal that more robust stability is achieved by higher affinity from binding,which was also validated using singlemolecule force spectroscopy [85].Single-molecule force spectroscopy was also used to study the ADP-dependent glucokinase conformational transition caused by substrate binding through the changes of unfolding force [86].The similar unfolding force demonstrated (apo-enzyme:(54 ± 17) pN,D-glucose added to apo-enzyme:(47 ± 15) pN) that D-glucose did not bind to the enzyme in the absence of the Mg2+·ADP-.They further observed the different intermediates by analyzing the unfolding events[86].
These results showed that single-molecule force spectroscopy based on the AFM technique was a powerful experimental tool to investigate the mechanical properties and conformational changes caused by a ligand binding.Except for capturing and measuring the single-protein in aqueous solution,AFM technique can also observe the subtle changes of proteins in the folding process or enzyme in the catalytic process from the force spectrum precisely,which is of great help in cognizing the relationship between protein structure and function and the understanding structuredependent catalytic mechanism.
5.Optical Tweezers
5.1.Working principle
An optical trap formed by a robust focusing laser to achieve stable capture of a single particle was first reported by Ashkinet al.In 1986,which marked the birth of optical tweezers [87].Soon afterward,the reports using optical tweezers to observe and analyze the motion of single molecules of the motor protein kinesin aroused the high interest and imagination of scientists[88,89].With decades of development,optical tweezers evolved from simply single-cell manipulate devices to achieve the measurement of single-molecule with nanoscale precision [90,91].Optical tweezers technique has been widely used in singlemolecular protein analysis [92,93].In 2018,this proven technique won the Nobel Prize in physics and aroused the attention of more scientists once again.
As shown in Fig.5,the particle molecules in the optical trap,formed by radiation pressure from focusing the laser beam,were clamped by an optical gradient force,and their movement and operation are realized by the moving of laser beam [91].Optical tweezers own many advantages,including non-contact and uniform-force Measurement,and manipulate activated substances without damage.However,a micro ball was indispensably used as a “hand shank”to capture and manipulate the biological samples more stably through the chemical coupling,such as DNA and protein (Fig.5a &b) [95].
5.2.Optical tweezers distance analysis
Ganimet al.[92]employed optical tweezers to reveal the dependence of fluorescence on protein structure in green fluorescent protein unfolding.Fluorescence was lost before the disruption of secondary structure and continues to the end of the unfolding process but was recovered after complete refolding,which suggests the importance of complete structure to fluorescence [92].Optical tweezers were also used to observe the changes of moving distance,which directly exhibit the base length produced by telomerasez (Fig.5c).They also found that the different distance changes reflect the stable substrate DNA binding at an anchor site within telomerase,facilitates the processive synthesis of telomeric repeats[93].The product DNA synthesized by telomerase could be recaptured by the anchor site or fold into G-quadruplex structures[93].This distance-related information obtained from AFM could give us more details in the synthesis of telomeric.
5.3.Optical tweezers force spectroscopy
Optical tweezers were also coupled with single-molecule force spectroscopy to analyze the process of fold/unfold process of protein.The protein (both ends tethered by modified DNA) was held in the optical trap by a pair of micron-sized beads,which are often coated by some material to combined to the end of modified DNA,such as streptavidin [96].Moving the micropipette (at the end of one bead) relative to the optical trap could control the force applied to a molecule,which was determined by measuring the change in light momentum of the laser beams leaving the trap[96].Thus,in the unfolds and refolds process,the sudden changes of force versus distance was recorded and could be used to analyze the explore properties of the protein [97].Based on this method,the conformation and folding mechanism of the HIV-1-protease monomer[96],slipknot protein(Fig.5d)[94,97],and cAMP binding domain A[98]were more in-depth studied through stretching and relaxing manipulate.Pelz employed optical tweezers and singlemolecule force spectroscopy to study the conformational changes of adenylate kinase triggered by binding of the ADP,ATP,and bisubstrate inhibitor diadenosine pentaphosphate [99].Their result more durable suggested that partly closed state was critical in both high-affinity substrate binding and rapid product exchange.Optical tweezers were also used to reveal the efficient translocation process of the single-molecule RTX domain of adenylate cyclase toxin (a kind of bacterial toxin protein) [100].By analyzing the force-distance changes in the dynamic conformational of the RTX domain,their results indicated that Ca2+-triggered folding of holo-RTX in the translocation process generates a stretching force which in line with the secretion mechanism [100].
The advent of optical tweezers has provided single-molecule protein analysis a new approach,allowing us to dissect folding mechanisms,interaction,and even conformational changes.The advantage of high resolution in optical tweezers holds promise for illustrating the trajectories of an individual protein.
6.Disscusion and Perspective
Protein is an essential component of the cell which acts as a crucial biological machine that performs particular function at the molecular scale.Thus,the single-molecule scale protein research could not only reveals the pathogenesis of many diseases,such as cancer,diabetes,and Alzheimer’s,but also provides overarching guidance for protein design,modification,and production.After decades of development,the application of a single-molecule technique has expended from small molecular compounds to activated biomacromolecule,from sensing low-copy number protein to capturing dynamic folding process.Scientists have been focusing on developing many other newer and more precise techniques,such as juncture-DNA tweezers(Fig.6a)[101]and various specific fluorescent probes [102],and applied them in the single-molecule research to dissect undefined mechanisms in unprecedented detail.
Up to now,although many single-molecule technologies have achieved success in protein research,challenges remain.Except the common strengths of high sensitivity resolution,and acuracy,the advantage and limitation of every single-molecule technologies were summarized in Table 2.Molecules’reactions in cells involved charge transfer and energy exchange,which carried out orderly and quickly in minimal and crowded space.The command,such as transfer and modification,executed by individual biomolecules,was controlled precisely and efficiently,which often shows an enhancement characteristic utterly different from the macro situation.However,these single-molecule technologies still cannot realize in situ identification so far and are even impotent to recognize the interest low-copy number protein swamped in a sea of others.Meanwhile,each technique has its weakness in single-molecule detection that could not obtain omni-directional cognition.For example,it is difficult for optical tweezers to identify and classify the component in sample,and the force applied and measured was smaller than that of AFM force spectroscopy.Electric conduction property of the sample is necessary for STM related technique,while the AFM cannot obtain the charge transport information of the sample.Thus,AFM and STM-BJ have been combined in conducting probe AFM break junction(Fig.6b)and applied for reaction observation [103,104],by which we will observe not only the change of electron transport path but also the switch of protein conformation,to realize real-time monitoring of individual protein dynamic behavior in the future work.

Fig.5.The working principle sketch map of optical tweezers related technique.Sketch map of dual-optical tweezers (a) and Mini-tweezer (b),Sketch map of the optical trapping experiment to measure processive telomerase catalysis using dual-optical tweezers (c) [93],and study the folding of the slip knotted protein AFV3-109 using the Mini-tweezer setup (d) [94].
With the development and application of single-molecule technology,high-resolution imaging of proteins has been realized,so as to observe the interaction between the membrane receptor and the protein skeleton under the membrane[105].At the same time,the constant advance of these technology also make a contribution to extend its application in high sensitivity detection field.For example,the binding state of protein and aptamer was captured by the slight change of current in nanopore which realized the construction of the high sensitivity biosensor [106].Besides,singlemolecule technology has also been successfully applied in the recognition and characterization of biomolecules,such as single amino acids,through nanopores and tunnel scanning.Thus,development of single-molecule technique in new methods,devices,and systems could realize the high resolution,in situ,and nondestructive measurement of protein in the future[107].The transient capture of reaction intermediates,reaction pathways,and dynamic behavior of a single molecule protein will provide potential powerful tools for protein-related biochemical reaction research,elucidate the mechanism and dynamics of biomolecular interactions,which also contribute to the early screening of diseases at the single-molecule level.
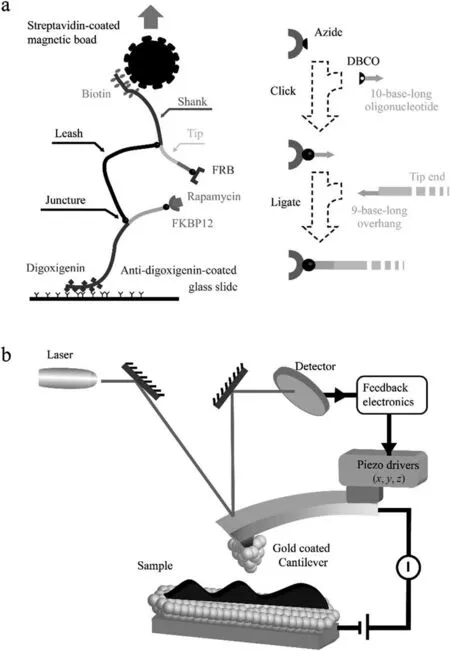
Fig.6.The working principle sketch map of juncture-DNA tweezers (a) [101]and conducting probe AFM break junction (b) [103,104].
7.Conclusions
Proteinresearcheshavelongbeenthefocusofinternationalattention of the public,which plays an essential role in medicine,disease,and health products.Compared with the standard technique of mass spectrum,single-molecule techniques have the advantages of shorteranalysistime,higherprecision,lowerdetectionlimit,andless samplerequirement.Thispaperreviewsthecharacteristicsandwork principle of many single-molecule techniques and significant results obtained in protein research in recent five years.We even discuss the advantages of each technique and its most suitable targeted molecule.Intheend,thesingle-moleculetechniqueisalsoexpectedtofurtherdevelopmentandbenefittoquantitativeandqualitativeanalysis with higher accuracy and repeatability.

Table 2 The advantages and limitations of each single-molecule technologies
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China(No.21978245)and National Postdoctoral Program for Innovative Talents (No.BX20200197).
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Molecular design,synthesis strategies and recent advances of hydrogels for wound dressing applications
- Recent advances in microbial production of phenolic compounds
- The production of biobased diamines from renewable carbon sources:Current advances and perspectives
- Efficient production of chemicals from microorganism by metabolic engineering and synthetic biology
- Food synthetic biology-driven protein supply transition:From animal-derived production to microbial fermentation
- A comparative analysis of China and other countries in metabolic engineering:Output,impact and collaboration
