Protein A-based ligands for affinity chromatography of antibodies
2021-05-19QinghongShiYanSun
Qinghong Shi,Yan Sun*
Department of Biochemical Engineering,School of Chemical Engineering and Technology,Tianjin University,Tianjin 300350,China
Key Laboratory of Systems Bioengineering and Frontiers Science Center for Synthetic Biology (Ministry of Education),Tianjin University,Tianjin 300350,China
ABSTRACT Protein A chromatography is a key technology in the industrial production of antibodies,and a variety of commercial protein A adsorbents are available in shelf.High stability and binding capacity of a protein A adsorbent are two key issues for successful practice of protein A chromatography.Earlier versions of protein A adsorbents ever exhibited serious fragility to typical cleaning-in-place protocols(e.g.washing with sodium hydroxide solution),and suffered from low binding capacity,harsh elution,ligand leakage and other problems involved in industrial applications.During the last three decades,various techniques and approaches have been applied in the improvement of chemical stability and enhancement of binding capacity of protein A-based ligands and adsorbents for antibody purifications.This mini-review focuses on the technical explorations in protein A-based affinity adsorbents,especially protein A-based ligands,including the efforts to increase the chemical stability by site-directed mutations and to improve the binding capacity by ligand polymerization and site-directed immobilization.Moreover,the efforts to develop short peptide ligands based on the structure of protein A,including the biomimetic design strategies and the synthesis of peptide-mixed mode hybrid ligands are discussed.These peptide and peptidebased hybrid ligands exhibit high affinity and selectivity to antibodies,but noteworthy differences in the binding mechanism of antibody from protein A.As a result,bound antibody to the ligands could be effectively eluted under mild conditions.Perspectives for the development of the protein A-based peptide ligands have been extensively discussed,suggesting that the ligands represent a direction for technological development of antibody purification.
Keywords:Protein A Antibody Peptide ligand Rational design Protein stability Binding capacity
1.Introduction
Therapeutic antibodies have grown as a very imposing and powerful bio-pharmaceutics since Muromonab-CD3,the first therapeutic monoclonal antibody (mAb),was approved by Food and Drug Administration of USA (FDA) in 1986 [1,2].By a survey,FDA approved 68 mAbs,including Fc fusion proteins,in the period of the four and a half years from 2012 [3].The number of mAbs authorized by the regulatory agency decreased from 11 in 2018 to 5 in 2019[4],but mAbs still reigned supreme among ten biologics approved in 2019,and occupied over half of the top ten drugs by sales worldwide[3,5].What’s more,it is predicted that the global therapeutic mAb market will generate revenue of 300 billion USD by 2025[6].Therapeutic antibodies and those in development are all produced by mammalian cell culture developed by a series of steps including gene transfection and cell line selection,optimization of media and culture condition,and the scaling-up to industrial production[7–9].Historically,mammalian cell was ever difficult to work with due to medium complexity,serum supplement,long cycles for cell culture and shear sensitivity [8,10,11].More importantly,antibody titer,cell density and volumetric productivity,and other issues related to capacity for commercial antibody manufacture have to be considered to meet the large doses demands for clinical mAb therapeutics[9,12].In the last three decades,the improvement of expression technology,generation and selection of highly productive cell lines,development of metabolic engineering and bioreactor system have enhanced the upstream processes significantly,leading to the exponential increase of manufacturing capacity [11,13].
In contrast to the intensified progress in upstream process for antibody production,however,downstream processing technology did not keep pace with the increased capacity and yield of mAb in upstream processing,and resultantly it became the serious process bottleneck in manufacturing scheme [14–16].A typical downstream process of mAb production from mammalian cell cultures consists of protein A chromatography followed by cation exchange chromatography and anion exchange chromatography.The latter two chromatographic techniques were used mainly in polishing stages for the removal of host cell proteins,residual genomic DNA,leached protein A ligands,mAb aggregates,and other impurities in the flow through fractions [17–19].Protein A chromatography is adopted as the preferred technique for direct capture of antibodies [20,21].As an affinity ligand,protein A is a component on theStaphylococcus aureussurface,comprising five highly homologous antibody binding domains [22–24],and the domains exhibit specific interactions with all the subclasses of immunoglobulin G (IgG) except IgG3.These IgG molecules have a high degree of homology on their Fc fragments,and strongly bind with protein A at their CH2 and CH3 interfaces of the Fc fragments.Thus,it allows protein A chromatography to establish a highly efficient platform for the purification of this types of proteins.The first commercial protein A-based affinity adsorbent was introduced in 1978 [25].Although protein A chromatography experienced many years for overcoming the obstacles on ligand stability and leakage,the technical innovation has brought about striking improvement in alkali and chemical stability of protein A ligands as well as enhanced capacity for antibody binding in the last two decades[16,26–28].Currently,protein A chromatography has become the golden standard in the antibody production pipelines [14,20],and dozens of protein A adsorbents suitable for commercial capture of antibody has put into the market [20,29,30].At the same time,many reviews on protein A chromatography were published[14,20,21].Among them,most of earlier reviews focused on the application of protein A chromatography in antibody production,and generally merged with mammalian cell cultures for mAb production and other purification techniques for antibody[9,14,18,21,31].Until recently,a key review was published and focused on the performance of protein A chromatography as well as a brief history of its development [20].
The purpose of this mini-review is to provide an overview of the technical exploration of protein A ligands and its chromatographic performances.In protein A adsorbents,chemical stability and binding capacity are two key issues of a successful protein A adsorbent for commercial applications,which depend on several important factors summarized in Fig.1.We focus on seeking the technical implementation and mechanistic interpretations of the design of protein A-based ligands and the performance of related chromatographic materials.To this end,we give complete interpretation of experimentally observed behavior and discuss the present challenges in protein A chromatography for more sophisticated design of high-performance protein A ligands and adsorbents.
2.Recombinant Protein A Ligands and Related Techniques
Staphylococcusprotein A is an important protein on the cell surface ofStaphylococcus aureusand exhibits high affinities to the Fc fragments of animal and human antibodies.The phenomenon was first observed in 1950s at serological typing ofStaphylococcusby the well-known immunoprecipitation reaction in gels[32].Protein A is composed of five tandem antibody-binding domains with highly homology (E,D,A,B and C) as well as an anchoring region for its binding to cell surface.The first commercial application of protein A adsorbents was reported in 1978 for the isolation of mouse IgG1,IgG2aand IgG2bin serum fromN.dubiusimmune mice[25].Since the approval of Muromomab (OKT3),the first mAb pharmaceutical,protein A chromatography has become a key tool adopted for antibody capture in antibody production[20,21].With the technical development of protein A chromatography,an increasing number of protein A adsorbents with high dynamic binding capacity and productivity have been put into commercial uses,representative by MabSelect PrismA that has a binding capacity over 150 mg·ml-1[20,30].The key techniques used to improve protein A performance are discussed in the following sections.
2.1.Protein mutation for alkali resistance
It is definite that a successful adsorbent for protein A chromatography relies on protein A ligand,besides the good properties of base matrices,including the pore structure and distribution,that suit the proteinaceous ligand.Earlier versions of protein A adsorbents suffered from serious limitations such as weak alkaline tolerance,low binding capacity,serious ligand leakage and other problems involved in industrial applications.Non-enzymatic deamidation of asparaginyl residues at neutral pH often causes conformational changes and the charge properties of proteins,and even biological alterations of protein molecules in extreme circumstances [33–35].In the antibody-binding domains in native protein A,the deamidation,especially in the asparagine-glycine dipeptides at position 28–29 of all the domains,has been considered as a key factor for ligand stability in buffers during chromatographic operation.This problem kept until an engineered variant of domain B from protein A(called as domain Z)was reported in 1987[26].By the G29A mutation of domain B in native protein A,domain Z with the G29A mutation has improved resistance against deamidation of the asparaginyl residue at neutral pH,and maintains an identical binding affinity for antibody.The recombinant domain Z consists of an antiparallel three-helix structure with two interhelical loops as depicted in Fig.2.The application of the representative protein A ligand derived from domain Z and its multi-meric forms has brought about great increase in the performance and availability of protein A chromatography for long-term operations [16,21,29].

Fig.1.Important factors influencing binding capacity and chemical stability of protein A-based affinity adsorbents.
Like most affinity chromatography with proteinaceous ligands,protein A chromatography exhibited serious fragility to typical cleaning-in-place(CIP)protocols in industrial application.In a regular CIP protocol,sodium hydroxide solution is applied for the removal of contaminants and denatured proteins in chromatographic column after a regeneration step [36,37].Proteinaceous ligands are sensitive to such an extremely harsh environment,and the CIP can result in the irreversible denaturation of protein A ligands.It was considered as a serious drawback that protein A chromatography had to face in the commercial applications [37].In the last two decades,significant efforts have been made to improve the stability of protein A ligands at extreme alkaline conditions[38–42],or to explore small-molecular alternatives instead of protein A [43–47].
In addition,the stability of protein A ligands is sensitive to hydroxylamine and cyanogen bromide especially at extreme alkaline pHs.Therefore,this was the reason for amino acid mutation as the starting point in earlier researches to improve alkaline tolerance of protein A ligand.As mentioned above,domain Z with G29A mutation improved the alkali resistance against deamidation at neutral pH [26].Further investigation to analyze the contribution of G29 to alkaline tolerance revealed that the mutation by several different amino acids at G29 could also significantly improve the alkaline tolerance of domain C [42].Among the mutants,G29W kept over 50% IgG binding capacity even after treatment in 0.5 mol·L-1NaOH for 25 h at 30 °C.Moreover,Gülichet al.[48]addressed this problem by mutation of all the asparagine residues withinStreptococcusprotein G,a cell-surface protein analogous to protein A,and the mutants showed enhanced stability in 0.5 mol·L-1NaOH solution commonly used for a CIP protocol.However,the asparaginyl residues within protein G had different contributions to alkaline tolerance,and the most sensitive residue was located at position 36.The finding was also observed by Linhultet al.[39]in the analysis of the positional contribution of asparaginyl residue in mutated domain Z (F30A) to alkaline tolerance,and the most stable mutant was obtained by F30A/N23T mutation.The results demonstrated the position-dependent behavior of the contribution of asparaginyl residues to alkaline tolerance.
The stability of protein A ligand is not only sensitive to hydroxylamine,but also depends on the local and confined interactions(e.g.,electrostatic interactions,hydrogen bonding and hydrophobic interactions).Based on the calculation of change in electrostatic free energy with pH,Palmeret al.found that the side-chain deprotonation of Tyr,Lys and Arg would likely unstabilize protein G at alkaline conditions [40].To address this issue,a triple mutation of Y3F/T16I/T18I was introduced to construct a recombinant protein G for the improvement of thermodynamic stability.Compared with native protein G,the mutant was more stable at alkaline pH and protein unfolding occurred at higher pHs.Shi and coworkers indicated that a combined mutation strategy (N11T/A12W/N23T/Q32T/K35R mutation) in domain Z improved the thermodynamic stability and the unfolding occurred at higher heat transition temperature than native domain Z [41].Moreover,by introducing a more hydrophobic residue,isoleucine,at position 12 within domain Z,the heat transition temperature increased by 2.3 °C without obvious influence on the binding capacity of antibody.The replacement of non-asparaginyl residues in protein A can bring about other promising variations in the performance in protein A chromatography besides enhanced ligand stability.For example,the introduction of additional S33E and D36R mutations in G29A mutant of domain C eliminated the affinity of the ligand for the variable region of antibody without compromising its alkaline resistance [49].Moreover,milder elution condition for the recovery of purified antibody product could be exploited by replacing the second loop of domain Z with more residues and different loop forming residues [50].In this case,antibody could be effectively eluted at pH 4.5 rather than pH 3.3 commonly used in protein A chromatography.However,a drawback of the mutation is the thermal stability decrease.Combining the strategies reported in literatures[39,50],Xiaet al.[51]recently synthesized a protein A ligand used in protein A adsorbents for antibody purification.It resulted in an increase of elution pH from 3.0 to 4.0–5.0 and increased alkali tolerance in 2 mol·L-1NaOH.Table 1 summarized the mutation sites and the changes of ligand properties that result from the mutations.
2.2.Ligand polymerization for improved capacity
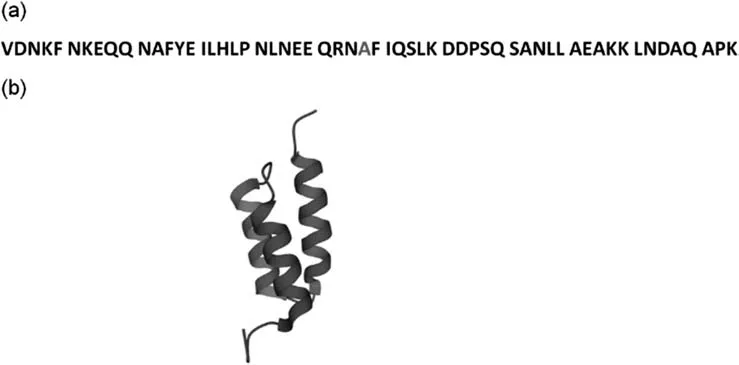
Fig.2.Amino acid sequence and crystal structure of recombinant domain B(domain Z)from protein A.(a)Amino acid sequence of domain Z with a G29A mutant highlighted in red;(b) The three-dimensional (3D) structure of domain Z (PDB ID:1Q2N).
Besides increasing the stability of protein A-based ligands,extensive efforts have also been made for improving the binding capacity and productivity of protein A adsorbents.Binding capacity of protein A adsorbent are highly dependent on available surface of the matrices,the number of ligands coupled on the surface as well as their accessibility for antibody binding.It has been revealed that the binding capacities of general protein A-based affinity adsorbents are quite low due to the limited availability of matrix surfaces for the proteinaceous ligand,which leads to small number of coupled ligands for specifically binding antibodies [55,56].It has been widely recognized that polymer-grafted ion-exchange resins generally exhibited enhancement in both protein adsorption and mass transfer as compared to non-grafted resins,and the increase in adsorption capacity is considered due to the fact that extended grafting layer provides 3D adsorption space for proteins[57,58].Therefore,protein A ligand was coupled onto dextrangrafted agarose gels to obtain a 3D ligand distribution [55].This coupling to surface polymer exceeded the physical limitation of the matrix surface.Zhaoet al.[55]found that dextran-grafted protein A adsorbent increased binding capacity by 24% as compared with the non-grafted protein A counterparts.
More generally,polymerization of antibody binding domains can extend the ligands along the surface of the base media.The technique was hatched from the synthesis of polymerized protein A ligands comprised of different numbers of antibody binding domains using recombinant domain Z in 1987 [26].Ghoseet al.[59]found that binding stoichiometries for antibody and Fcfusion proteins in solution were in the range of 2.4–3.1 when a pentameric domain was introduced for protein A ligand.The result indicate that the binding domain polymerization could effectively improve the binding capacity of antibody,but did not guarantee a full utilization of each domain in protein A ligands(50%–60%availability for the five binding sites in the pentameric ligand) due to steric hindrance effect of the intra-protein A ligand.Our previous study by isothermal calorimetric measurement indicated that the site availability of protein A ligands for antibody binding greatly decreased with the polymerization of domain Z and only onethird of binding sites was available for antibody binding [56].The role of polymerization of protein A ligand on adsorption capacity was further investigated by von Romanet al.using polymerized domain B[60].At similar molar densities,increase in the polymerization degree of domain B led to increased adsorption capacity of antibody until octamer of domain B was applied.Moreover,the ligand availability of polymerized protein A ligand is highly dependent on ligand density.For example,the availability of protein A ligand on Sepharose FF gel decreased greatly as ligand density was higher than 20 mg·ml-1[56].By optimizing the polymerization degree and density of protein A ligand,maximum adsorption capacity of antibody,118 mg·ml-1,was obtained on protein ASepharose FF using tetrameric domain Z as the ligand[56].At present,polymerization of antibody binding domain is a widely adopted strategy in the fabrication of commercial protein A adsorbents.
2.3.Site-directed immobilization for improved binding efficiency
High-efficient covalent attachment of protein A ligands onto matrices is a prerequisite for fabricating protein A adsorbents for high-efficiency antibody binding and purification.Fig.3 presents two attachment techniquesviaEDC/NHS chemistry extensively applied in protein immobilization onto solid matrices.It includes random immobilizationviaamino groups and oriented immobilizationviathiol coupling.In the earlier stage,protein A adsorbents were fabricated by randomly immobilizing protein A ligands onto base matrices through the reaction of amino groups in the ligand molecules to the carboxyl groups on the solid phase (Fig.3) or the reverse [28,56].These coupling approaches result in random orientation of the immobilized ligands due to the wide distributions of the amino or carboxyl groups on the ligand [61],leading to poor binding site availability and decreased binding efficiency(capacity).To address this problem,a variety of site-directed immobilization have been exploited to obtain an oriented ligand alignment on matrices [60,62–64].In fact,site-directed immobilization is a key issue not only in protein chromatography using proteinaceous ligands,but also for biocatalysis and biosensors involving macromolecules as host molecules [61,65–67].Our research indicated that oriented immobilization of domain Z markedly increased the adsorption capacity of antibody by~50%as compared with a random immobilized counterpart [56].However,the difference in adsorption capacity between the two types of protein A adsorbents decreased with an increase of polymerization degree of the domain.For example,the adsorption capacity increase decreased to~36%for dimeric ligand and further to~24%for tetrameric ligand.The finding was also reported in other protein A adsorbent using polymerized domain Z and B as ligand[56,60,62].von Romanet al.[60]reported that protein A adsorbents had similar capacities for octameric ligands of domain Bviaoriented and random immobilizations.

Fig.3.Random and oriented immobilization of protein A ligands (domain Z) via EDC/NHS chemistry.EDC,1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride;NHS,N-hydroxysuccinimide;PDEA,2-(2-pyridinyldithio) ethaneamine.
More importantly,the application of site-directed immobilization of protein A ligand determines the orientation of bound antibody on protein A adsorbents,which is also critical for the improvement of antibody adsorption efficiency.It is well known that antibodies adopt a distinct three-lobed structure,including two identical lobes corresponding to the Fab regions [68].As shown in Fig.4,antibody may have at least four typical orientations at a solid–liquid interface,namely those referred to as“end-on”,“head-on”,“side-on”and “lying-on”.Yanget al.[69]attempted to deduce the molecular orientation of bound antibody based on the hydrodynamic dimension of antibody,and found that adsorption capacity of antibody on commercial Protein A Sepharose CL-4B (2.9 mg·m-2) was approximate to adsorption amount of antibody in “side-on”orientation (2.4 mg·m-2).For a packed monolayer with “end-on”orientation,however,the surface concentration of antibody on the adsorbents increased by~ 400%based on the theoretical calculation from the same group.The result suggests the possibility to improve the adsorption capacity of antibody by optimizing the orientation of protein A ligand and resultantly the bound antibody.Tijimaet al.[63]provided a good example for this strategy.With the help of tyrosinase-catalyzed site-specific coupling technique,they attempted to control the orientation of protein A molecules on an substrate to enhance the immunological activities of antibody.The results revealed that the antibodies bound to protein A coupled by the enzymatic catalysis captured 1.8 antigens on average,in contrast to 1.4 antigens captured for physisorbed protein A.It indicated an“end-on”orientation of bound antibody on the substrate [63].Recently,Fiedleret al.reported a novel Fc binding protein for antibody purification,in which at least one amino acid in helix III of domain C was replaced by cysteine [54].The example showed that mutation of G46C could improve the alkaline tolerance over 72 h.Currently,the control of protein A ligand orientation and resultantly the bound antibody pattern is still a challenge in the development of high-efficient protein A chromatography due to the lack of a direct tool for the investigation of antibody–protein A ligand interaction and the complex.

Fig.4.Presentation of antibody orientations on solid surface:(a) head-on,(b) end-on,(c) side-on and (d) lying-on orientations.
3.Peptide Ligand Design Based on Protein A Molecules
3.1.Rational design of protein A-mimicking ligands
The popularity in the industrial application of protein A chromatography inspired scientists and engineers to exploit novel alternatives to overcome the technical disadvantages of protein A ligands discussed above.Among the alternatives to protein A ligands,the most common molecules used for antibody purification are mixed-mode and peptide affinity ligands[47,70,71].These ligands are often screened from combinational libraries of all or most the possible candidate compounds including several specific moieties.In general,these candidates are firstly screened using antibody or its Fc fragment as the template based on computation-based virtual screening by flexible docking,mRNA and phage display,or the combination of these screenings.Then,a limited number of potential candidates are evaluated by chromatographic techniques [72,73].Based on these strategies for ligand screening,several novel mixed-mode ligands (MML)[47,69,74,75],peptide affinity ligands [69,76,77],and MMLpeptide hybrid ligands [78]have been obtained for antibody chromatography.
In this process,the establishment of compound library is the foremost issue for ligand screening.The library could be built using chemical databases(e.g.MDL Availability Chemical Directory)[79],phage display technology,and mRNA display technology[73].Sun and his co-workers developed a biomimetic design strategy to construct peptide library for ligand screening for antibody chromatography [80,81].The biomimetic strategy is outlined in Fig.5.Based on energetic analysis of the interactions in the Fc fragment-domain B complex using the molecular mechanics/Poisson–Boltzmann surface area (MM-PBSA) method (Fig.5(a)),the key residues of domain B in the binding was determined (Fig.5(b)) [80].These residues in protein A referred to as “hot spots”.Based on these hot spots and their spatial distribution(Fig.5(c)),several peptides were designed (Fig.5(d)) for constructing a peptide library (Fig.5(e))[81].In the library design procedure,different types and number of amino acid residues (represented by X in Fig.5(e)) were inserted between neighboring hot spots to fill the gaps between them,and one of the central amino acid residue was defined by cysteine residue for the ease of ligand immobilization to solid matrices by thiol chemistry.In order to reduce library size,the amino acid residues which had sufficient binding affinity for the Fc fragment were selected as X[81,82].As a result,the size of peptide library reduced by~70%,and an octapeptide library including 2173 peptides was built for ligand screening by dockings and molecular dynamics (MD) simulations (Fig.5(f)).Docking of each peptide in the library to Fc fragment using Autodock Vina program led to the determination of top 150 peptides,and flexible docking using Rosetta FlexPepDock program further reduced the candidates to 15 peptides.Then,MD simulation was used to confirm the binding affinities of the 15 candidates and exclude negative ones.Finally,the 15 candidates were immobilized to Sepharose gel to evaluate by affinity chromatography (Fig.5(g)).The procedure identified five high affinity peptide ligands (FYWHCLDE,FYCHWQDE,FYCHNQDE,FYCHTIDE and FYCHWALE) [83].The affinity peptide-based adsorbents showed very high adsorption capacities of hIgG (104–176 mg·ml-1) [83],and the binding sites of the peptide ligands on hIgG were identified to be similar to those for Protein A [77].Chromatographic experiments further demonstrated that the affinity peptide adsorbents had a high selectivity for hIgG because the impurities in serum and culture broth were barely bound [81].The research demonstrated that biomimetic design strategy was successful to achieve high affinity ligands for antibody purification.This strategy was proven effective in the design of peptide affinity ligands for the capture of capsomere of virus-like particles [84]as well as platelet adhesion inhibitor of thrombosis [85].High stability of the peptide ligands was demonstrated by 20 breakthrough cycles [83].The successful application of biomimetic design strategy in the development of peptide ligands and platelet adhesion inhibitor fully demonstrated the importance in deep analysis of molecular orientation and structure of proteins,and it was more conductive to the rational design of novel ligands for protein chromatography and potential drugs for critical diseases.
3.2.Performance of peptide affinity chromatography
In a typical protein A chromatography,IgG binding was carried out at acidic pHs ranging from 2.5 to 4.0 for antibody elution[37],and so serious aggregation of IgG becomes a great challenge for antibody production [17,86,87].Moreover,protein A ligand often experiences strong alkaline conditions in the CIP operation as mentioned above,which results in a decrease of the ligand stability and adsorption capacity.In the last decades,several peptide-based ligands including hexapeptide,octapeptide and peptide hybrid ligands have been explored for the antibody purification to overcome these limitations in protein A chromatography [76–78,81,88–90].In 1990 s,for example,Fassinaet al.[43]designed a peptide dendrimer,(RTY)4K2KG,as the ligand for antibody capture and the affinity adsorbent had a capacity of 25 mg·g-1adsorbent [91].As reported by Naiket al.[90],the hexapeptide affinity chromatography could completely recover the adsorption capacity in either 2 mol·L-1guanidine–HCl or a combination of 0.85%phosphoric acid followed by 2 mol·L-1urea whilst key impurities,DNA and host cell proteins,reduced by 4 and 2 lg (reduction values)during chromatographic process.Zhaoet al.[81]found that FYWHCLDE-based peptide affinity adsorbents had different pH range for hIgG binding(5.0–6.0)and elution condition(0.5 mol·L-1NaCl) for bound hIgG as compared with protein A adsorbents.It revealed that hIgG binding on the peptide affinity adsorbents had a different mechanism from IgG binding to protein A adsorbents.Namely,hIgG binding was dominantly driven by electrostatic interactions in the peptide affinity chromatography.In antibody purification,therefore,the peptide affinity chromatography provided a real possibility to overcome the antibody aggregation in acidic condition during the antibody elution.On the other hand,the peptide affinity adsorbents had much higher adsorption capacities than those of commercial protein A adsorbents [29,92].A combined comparison of the three affinity octapeptides(FYWHCLDE,FYCHWALE and FYCHTIDE) obtained by the biomimetic design strategy described above found that all the three ligands bound hIgG and Fc fragment specifically,but barely bound Fab fragment [77].Moreover,ligand binding competition revealed that the binding sites on hIgG for the three octapeptides were the same as protein A.Based on the three octapeptides,Zhaoet al.further developed a dual-ligand affinity chromatography by coupling two of these octapeptides onto Sepharose gels [88].The novel affinity adsorbents exhibited a synergistic effect on hIgG binding,leading to higher affinity for hIgG than those adsorbents synthesized by coupling a single octapeptide ligand.Among these dualligand affinity adsorbents,the FYWHCLDE/FYCHTIDE dual-ligand affinity adsorbent had the highest affinity,corresponding to a dissociation constant of 0.69 μmol·L-1at a molar ratio of 2:1 and total ligand density of 31.1 μmol·ml-1.In this dual-ligand affinity adsorbent,the synergistic effect of the two ligands increased with increasing the total ligand density of the two peptides.Similarly,Zouet al.[78]developed peptide-MML hybrid ligand by coupling hot spots of protein A ligand to MML,and the resultant adsorbent with peptide-MML hybrid ligand exhibited high affinity (dissociation constant,1.6 μmol·L-1) and adsorption capacity (81 mg·g-1adsorbent).Dual-ligand affinity chromatography exhibits great potential in the purification of hIgG and mAb because over 95%pure hIgG and mAb at recovery yield over 90% could be achieved[88].The dual-ligand strategy also offers an effective approach for the refinement of ligand design and development of novel protein A-based affinity adsorbents.

Fig.5.Biomimetic design strategy of peptide affinity ligands for antibody purification.(a)Complex of Fc fragment of hIgG and domain B from protein A.(b)Key residues in binding with Fc fragment determined by energetic analysis.(c) Hot spots and their spatial distribution.The two helices are colored in pink.The hot spots in helix I(i.e.,Phe132,Tyr133,and His137) are colored in silver;the hot spots in helix II (i.e.,Glu143,Arg146,and Lys154) are colored in red.(d) Library design and representative peptides are colored in transparent red,green and blue.The distances between neighboring hot spots are presented in dotted lines using the visual molecular dynamics(VMD) software (http://www.ks.uiuc.edu/Research/vmd/).(e) Construction of peptide library for ligand mining,and colors in the table correspond to the representative peptides in(d).(f)The screening steps for peptide ligands by docking with Fc fragment using Autodock Vina and Rosetta FlexPepDock programs,and finally by MD simulation using GROMACS package.(g) Experimental evaluation by chromatography.
4.Perspectives
Since 1970s,a series of crystal structures of antibody binding domains,IgG Fc fragment and their complexes have been reported with X-ray crystallography and NMR spectroscopy[23,93–95].The analysis of molecular structures of antibody binding domains and the complexes with Fc fragment provide the original ideas for the development of high-performance and stable ligands for antibody purification.In this case,the ligand development was started with native antibody binding domains,and IgG molecule and its Fc fragment are often applied as the targets for the ligand design and screening [26,39,42,43].The structural insight of Fc fragment and antibody binding domains further have driven the evolution of rational design of chromatographic ligands for antibody capture.With the rapid development of antibody-based biopharmaceutics,antibody biosimilars and Fab fragments (e.g.Certolizumab Pegol)have begun clinical applications [96–98],and lots of them have entered pre-clinical and clinical trials.A couple of adsorbents such as Lambda FabSelect have been commercialized for the purification of antibody fragments [71,99].In order to adapt the diverse situations encountered in antibody purifications,an envisioned direction is platform development for the rational design of ligands used in the capture of biosimilar and Fab fragments.Biomimetic design of peptide affinity ligands was such an attempt of platform technique development[81].However,design strategy and screening methods should be further improved to implement highthroughput and effective mining of ligand candidates.
On the other hand,the improvement in the performance of protein A adsorbents has been mostly related to the innovation of materials.As mentioned above,antibody binding capacity of protein A adsorbents was limited by the limited surface availability of conventional matrices.With the advance of polymer-grafted ion-exchange adsorbents[58,100,101],the utilization of pore space in matrices has provided a new way to the development of highcapacity protein A adsorbents.Recently,dextran-grafted agarose gel was also applied in the antibody purification by coupling protein A and tetrapeptide ligands [55,102].Compared with corresponding non-grafted protein A adsorbents,dynamic binding capacity of antibody increased by~24% in dextran-grafted protein A agarose gel [55].Moreover,it is well-known that protein-matrix interactions are particularly important for the protection of protein structures on support materials[103].At liquid–solid interface,the coupled proteins (e.g.,protein A ligands) on various matrices via chemical reactions often experience conformational changes due to protein-surface interactions [103].However,this issue was rarely discussed,especially in protein A chromatography.So,a complete understanding of the structural evolution of protein A ligands at liquid–solid interfaces is still lacking for researchers and engineers working in the related fields.Recently,Yanget al.[56]investigated the binding kinetics of antibody to protein A ligand in different immobilization modes by using surface plasma resonance measurement,and found that oriented ligand coupled through carboxyl terminus of protein A was more preferred than those through amino terminus and randomly immobilized ligands,demonstrating that the interfacial reactions have significant influence on the affinity of IgG to protein A ligands.In the following research,the spectral results of the immobilized protein A ligand further showed that the molecular structure of the ligands on carboxymethyl dextran(CMD)coated magnetic nanoparticle(NP)was dominated by ligand-matrix interactions[104].In CMD-coated NP,the molecular structure of immobilized ligands relied on the electrostatic interactions between surface and ligands.At pH 4.5,the immobilized ligands were disrupted by electrostatic attractions between the negative-charged surface and ligand (pI,5.53).At pH 7.0 and over,electrostatic attractions between the negativecharged surface and specific positive-charged residues scattered at amino terminus (K4),Helix I (K6),Helix II (R27 and K35),Helix III(K49 and K50)and carboxyl terminus(K58)of the ligands likely caused the molecular structural changes of the ligands [104].
The molecular structure and orientation of protein A ligand on matrices bring about different antibody binding behaviors and then the orientations of bound antibody at liquid–solid interface.It is equally critical for the development of high-performance protein A chromatography.For example,Yanget al.[69]showed that the surface concentrations of IgG on protein A adsorbent,Toyopearl AF Amino 650 M,approached to that in closely packed“side-on”monolayer(2.4 mg·m-2),and was much lower than that in closely packed “end-on”monolayer (13 mg·m-2).It demonstrated a real possibility for improving the adsorption capacity by adjusting the orientation of bound antibody in protein A chromatography.Previously,Tajimaet al.[63]reported enzymemediated coupling techniques to immobilize protein A ligand on phospholipid polymer-grafted surface,and the result showed that those antibody bound to protein A could capture 1.8 antigens on average,implying a “end-on”orientation for antibody,in which both active antigen binding sites (Fab fragment) were exposed completely in bulk solution.Recently,atomic force microscopy(AFM) has become a powerful tool to investigate molecular orientation of antibody at solid–liquid interfaces[105,106].As reported by Vilhenaet al.[106],for example,many of the antibodies adopt vertical orientations on a hydrophobic surface modeled with a three-layer graphene slab even at very small coverages.In this case,at least one Fab binding site was exposed for recognition events of antigens.Therefore,further investigations to improve protein A ligand,to design new peptide ligands and to modulate the ligand orientation on solid surface are essential for the design and development of high-performance protein A chromatography.
5.Concluding Remarks
In the last decades,the rapid development in the related techniques of upstreaming processing shifted the bottleneck to downstream processing,especially in protein A chromatography.The development of high-performance and stable protein A chromatography is thus particularly important for antibody production,and many efforts have focused on the design and application of protein A–based ligands.In this review,an overview of the technical explorations of protein A–based ligands and their chromatographic performance are discussed.In order to obtain stable and high affinity protein A adsorbents,a variety of techniques and approaches including site mutation,polymerization and sitedirected immobilization have been applied in the exploration and successful applications of the ligands.As a result,protein A adsorbents have received greatly improved performance in alkaline tolerance and binding capacity.Moreover,novel short peptide ligands alternative to protein A have been developed based on different strategies.The short peptides exhibited high affinity to antibody with a different mechanism from protein A ligands,and thus antibody could be eluted under mild conditions.It is expected to exploit these peptide ligands for commercial applications to investigate their long-term stability in practical processes.On the other hand,existing literatures suggest that there are still large rooms for the performance improvement in protein A chromatography.To this purpose,it is crucial to improve the ligand properties by engineering the molecular structures of protein A and the short peptide ligands and to understand and control their surface behaviors as well as interactions with antibodies at solid–liquid interfaces in a molecular level.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Nos.21476166 and 21878221),and the Foundation for Innovative Research Groups of the National Natural Science Foundation of China (No.21621004).
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Molecular design,synthesis strategies and recent advances of hydrogels for wound dressing applications
- Recent advances in microbial production of phenolic compounds
- The production of biobased diamines from renewable carbon sources:Current advances and perspectives
- Efficient production of chemicals from microorganism by metabolic engineering and synthetic biology
- Food synthetic biology-driven protein supply transition:From animal-derived production to microbial fermentation
- A comparative analysis of China and other countries in metabolic engineering:Output,impact and collaboration
