Recent advances in detoxification strategies for zearalenone contamination in food and feed
2021-05-19NaWuWenOuZhidongZhangYuwenWangQingXuHeHuang
Na Wu,Wen Ou,Zhidong Zhang,Yuwen Wang,Qing Xu,*,He Huang,2,*
1 School of Food Science and Pharmaceutical Engineering,Nanjing Normal University,Nanjing 210046,China
2 School of Pharmaceutical Sciences,Nanjing Tech University,Nanjing 211816,China
3 Xinjiang Laboratory of Special Environmental Microbiology,Urumqi 830091,China
ABSTRACT Zearalenone(ZEN)is a widely distributed mycotoxin that frequently contaminates crops and animal feed.ZEN can cause serious health problems in livestock and humans alike,leading to great economic losses in the food industry and livestock farming.Therefore,approaches for efficient ZEN decontamination in food and feed are urgently needed.Traditional physical and chemical methods may decrease the nutritional quality of food and palatability of feed,or leading to residues and safety concerns.By contrast,biological methods for the removal or degradation of ZEN overcome these problems,especially for biological degradation by microorganisms and specific enzymes extracted from strains that can convert ZEN to less toxic or even completely harmless products.In this review,we comprehensively describe methods for ZEN degradation,focusing especially on biological strategies.Finally,emerging strategies and advice on remaining challenges in biodegradation research are also briefly discussed.
Keywords:Mycotoxin Removal Degradation Zearalenone Food safety
1.Introduction
Zearalenone (ZEN) is a nonsteroidal estrogenic mycotoxin produced by Fusarium species,notably F.cerealis,F.crookwellense,F.culmorum,F.equiseti,F.graminearum and F.semitectum [1,2].Six major types of ZEN and its derivatives have been found to date(Fig.1),including zearalenone (ZEN),zearalanone (ZAN),αzearalanol (α-ZAL),α-zearalenol (α-ZEL),β-zearalanol (β-ZAL),and β-zearalenol (β-ZEL).The chemical structure of ZEN (6-[10-h ydroxy-6-oxo-trans-1-undecenyl]-β-resorcylic acid lactone) and its derivatives are similar to natural estrogen,so it can competitively bind to the mammalian estrogen acceptor,causing infertility and reproductive disorders in livestock [3].It has been reported that ZEN can also bind to estrogenic receptors in human mammary glands,potentially causing breast cancer [4,5].ZEN has also been found to be hepatotoxic,hematotoxic,immunotoxic,and genotoxic[6,7].Therefore,oral intake of ZEN-contaminated products is a serious threat to the health of humans and livestock.Moreover,ZEN can accumulate after entering the food chain,causing large economic losses to the agricultural industry [8,9].These problems underline the urgent need to eliminate ZEN contamination from animal feed and food products for human consumption.Accordingly,most countries have strict guidelines for the maximal content of ZEN in grains,feed and food products.For instance,the European Union proscribes a limit of 100 μg·kg-1in cereals,while in China and Australia it is 60 μg·kg-1[10].ZEN is very heat-stable and shows great resistance to conventional detoxification methods,such as heating,sterilization and other thermal applications[11,12].In addition,several environmental factors,such as temperature,humidity,foggy weather,or insect damage,along with failure in the application of good agricultural practices,may exacerbate ZEN contamination,resulting in the necessity for detoxification of polluted food and animal feed [13].Accordingly,numerous strategies have been developed to eliminate ZEN contamination from agricultural products,which can be classified into physical,chemical and biological approaches.
Most reviews to date only included ZEN in the discussion of detoxification strategies for several different mycotoxin types,and lacked an in-depth summary and specific discussion.However,as ZEN contamination of food and feed has become increasingly severe in recent years,people have paid increasing attention to ZEN decontamination,and a large number of new detoxification methods were studied in detail.In this paper,nonbiological and biological methods for ZEN decontamination developed in recent years are discussed in detail (Fig.2).We analyze the different advantages and disadvantages of these detoxification methods,and provide a theoretical basis for potential and practical applications of ZEN detoxification technology.

Fig.1.Molecular structure of ZEN and its metabolites.

Fig.2.Summary of the different approaches used in published strategies for ZEN decontamination.
2.Methods for the Inactivation and Detoxification of Zearalenone
2.1.Physical methods
Physical strategies for detoxification of ZEN can be divided into two main types according to the different mechanism of action,aiming either at the physical removal or physically-induced degradation of the toxin,respectively.
2.1.1.Removal of ZEN
Physical removal of ZEN relies on separating the contaminated outer coat and pericarp of the kernels,via procedures usually used in cereal processing,such as separation,solvent extraction and adsorption.
Separation:In the early phase,the separation of grain from the husks contaminated with ZEN is accomplished using a dehuller and sieving.ZEN is very unevenly distributed in crops,and most of it is found on the epidermis and germ.Thus,ZEN can be removed by mechanical peeling and degerming to achieve the purpose of detoxification.Zhenget al.[14]utilized the grinding method to remove ZEN from Japanese wheat-based flour with an efficiency of greater than 50%.Cetinet al.[15]reported that 81%of ZEN could be removed from contaminated corn grit by extrusion processing.Similar results were demonstrated by Bullermanet al.[16]who found that extrusion processing at temperatures over 150 °C was appropriate for removing or greatly reducing the ZEN content of cereal-based foods.Both dehulling and sieving has an excellent effect on reducing ZEN levels in contaminated grain near the surface.However,sieving is effective only when there is a small amount of fungal dissemination into the grains,because of the relatively large loss of the crop mass during removal [17].
Solvent extraction:ZEN is a lipophilic toxin that is highly soluble in organic solvents,and thus can be extracted,separated,and detoxified using a mixture of water and organic solvents.Common solvents for extraction reported in the literature include 90% acetone,80% isopropanol,methanol,and acetonitrile [18].Methanolacetonitrile was used as extraction solvent to leach the ZEN from wheat and corn [19].Nevertheless,the extraction technique has many limitations,such as high cost,the need for large-scale processing equipment,and environmental pollution.In addition,the nutritional value and quality of cereals is decreased during the extraction process.
Adsorption:ZEN removal can be achieved by adsorbing the toxins onto foreign substances to achieve the purpose of detoxification.The mainstream methods use diverse adsorbent materials(Table 1),such as clay,activated carbon,montmorillonite (Mt)and nanomaterials,which can immobilize and adsorb the toxins.Denil et al.demonstrated that the addition of activated diatomaceous clay(ADC)to feed contaminated with ZEN(0.8 mg·kg-1feed)could decrease the ZEN contamination by 79.8% [20].Kalagaturet al.investigated the ability of activated carbon(AC)derived from seed shells of Jatropha curcas (ACJC) to adsorb ZEN,and subsequent desorption experiments confirmed that binding of ZEN by ACJC was stable [2].Zwitterionic surfactant-modified montmorillonites (ZMts) can simultaneously adsorb aflatoxin B1 (AFB1) and ZEN,and nearly no desorption occurred in the simulated gastrointestinal tract[21].Sunet al.[22]also investigated organomontmorillonites modified by binary surfactant mixtures (NZMts),combining polyoxyethylene ether with lauramidopropyl betaine,and the adsorption rate of ZEN was greater than 70%.Graphene oxide (GO) and its functionalized forms (FGO) were also used for decontamination of ZEN,and the maximum adsorption rate reached 95% [23,24].ZEN was extracted and enriched by magnetic-surface pseudo molecularly imprinted polymers(SPMIPs) combined with Fe3O4.When modified halloysite nanotubes were used to adsorb ZEN,the adsorption rate in maize ranged from 75%to 88%[25].At present,mycotoxin adsorbents are the most direct,operable and feasible way to alleviate ZEN contamination in feed and food,and there are many types of mycotoxin adsorbents commonly used in the market.For example,adding a few percent of antifungal agents such as calcium propionate,propionic acid,sorbic acid,benzoic acid,etc.can greatly decrease the contamination.However,the quality of these adsorbents varies,and there is no uniform national standard.Thus,there is an urgent need to establish a reliable evaluation method for the detoxification effect of mycotoxin adsorbents [11].
2.1.2.Degradation of ZEN
Physical methods for ZEN degradation rely on destroying the chemical structure of ZEN and forming new non-toxic or less toxic compounds,mainly by heating,irradiation,or low-pressure cold plasma (Table 2).
Heating:Thermal treatments are capable of decontaminating mycotoxins produced by fungi growing on cereals [16].However,due to the heat-stability of ZEN and its analogues,the efficacy of thermal processing systems used for mycotoxin decontamination is known to depend on a number of factors,such as time of exposure,temperature,moisture content,and type of food[33,34].Gbashiet al.[26]found that the optimal decontamination conditions for ZEN are 216.57 °C per 63.28 min and 210.85 °C per 54.71 min for pure standards and ZEN spiked into maize flour.The average thermal degradation of ZEN ranged from 48%-50%for pure standards,and 36%–41%for spiked maize[26].According to Numanogluet al.,baking maize bread at 100 °C for 6 h did not degrade ZEN,but 250 °C for 70 min afforded optimal ZEN degradation[27].Another study investigated the effects of microwave heating on ZEN contaminated wheat grains,and the results showed that microwave drying of washed wheat samples could decrease the ZEN content by 89.0% [35].
Irradiation:Irradiation of food has been tested using gamma,x-rays or electron beams [36].The mechanism of radiation-based detoxification relies on breaking the chemical bonds in macromolecules,which are thereby degraded into small molecules to achieve the purpose of detoxification.Klopfensteinet al.[37]treated ZEN contamination in corn with 17% moisture using gamma irradiation at doses of 5,7.5,10 or 20 kGy,and the results showed that ZEN concentrations were significantly decreased byirradiation at 20 kGy.Sebaeiet al.[28]also evaluated the influence of gamma irradiation(5,10,15 and 20 kGy)on ZEN,and found that the ZEN concentration was effectively decreased with the increase of radiation dose.Ultraviolet(UV)irradiation has also been applied to the degradation of mycotoxins in contaminated agricultural products and feeds.Furthermore,UV irradiation can decrease contamination with pathogenic molds distributed on the surface of grains,but its effectiveness depends on factors such as the intensity of radiation and time of exposure[38].According to H.Murata,both mild(0.1 mW·cm-2)and strong(24 mW·cm-2)UV treatments effectively decreased the levels of ZEN.30 mg·kg-1of ZEN was completely decreased by mild irradiation at 60 min and strong irradiation at 15 min,respectively [39].The ZEN from maize kernels was decreased by 62%–79% after exposure to the UV-C light at dosage of 15 J·cm-2[29].

Table 1 Recent studies on physical methods for ZEN removal
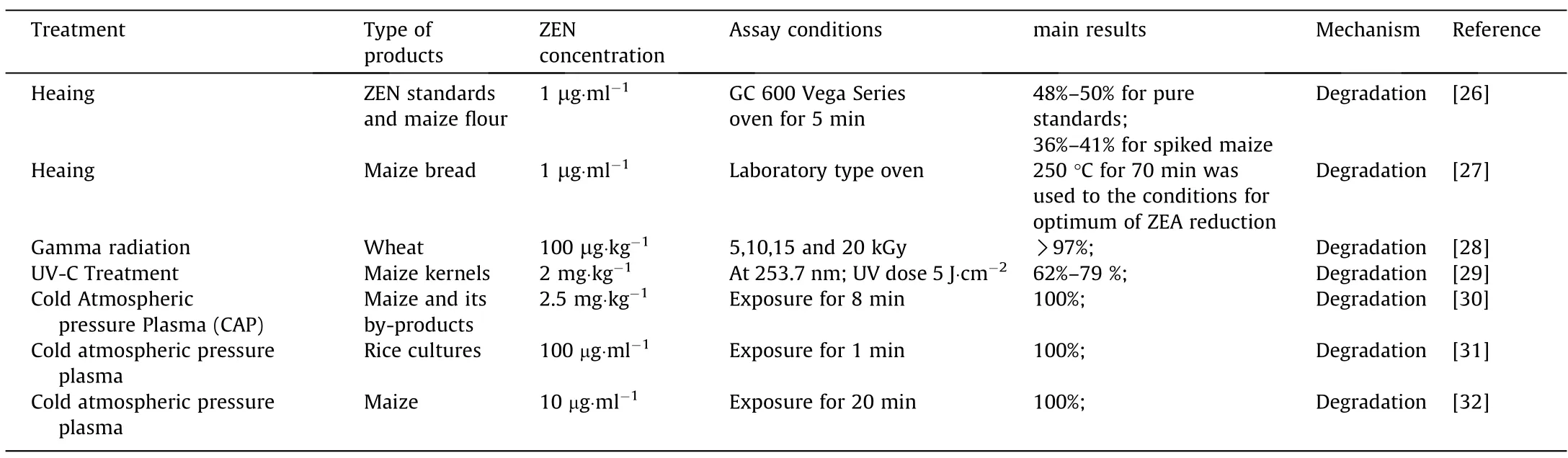
Table 2 Recent studies on physical methods for ZEN degradation
Low-pressure cold plasma:A series of plasma detoxification technologies can decontaminate mycotoxins with a slight effect on the quality and flavor of food products,with advantages including having a low cost and being environmentally friendly [40,41].Hojniket al.[30].evaluated the effect of cold atmospheric pressure plasma(CAP) on ZEN contaminated food (2.5 mg·kg-1),and found that 100% of the ZEN was removed after 8 min of exposure.However,CAP treatment can generate a large number of nitrogencontaining molecular species.Pfohlet al.[31]also exposed ZEN to cold atmospheric pressure plasma(CAPP)in ambient air,and found that 100 mg·L-1of ZEN were completely degraded within 60 s.Wielogorskaet al.[32]exposed ZEN to CAPP in helium with 0.75%O2and a few hundred ppm of humid air in the gas flow,and the ZEN was almost completely degraded with 20 minutes[41].
2.1.3.Disadvantages of physical methods
Physical detoxification methods often lead to a loss of nutrients in feed and food.For instance,solvent extraction can remove ZEN from oilseed flours,but at the expense of reducing the nutritional value and the quality of the cereal at the same time [18].Adsorbents may increase the costs and cause loss of nutrients in the raw materials while adsorbing ZEN,which has made the development of efficient and specific ZEN adsorbents into the focus of future research [42].Finally,irradiation generally modifies the chemical composition of products during the process of ZEN degradation treatments [37].
2.2.Chemical methods
2.2.1.Chemical compounds
Chemical detoxification relies on the use of strong oxidants(ozone gas) or specific chemicals (sodium sulfite,β-cyclodextrin polymers) to destroy the chemical structure of mycotoxins,or to neutralize their active groups.
Ozonization:Ozone (O3) is a gas with high oxidizing potential,capable of decomposing ZEN and producing oxygen at the same time,as presented in Table 3.Allanaet al.[43,44]studied the removal of ZEN from wheat bran under ozone treatment,and found that the optimum decontamination of ZEN reached 62.3%at 60 min.Reinholdset al.[45]investigated the use of ozone gas for ZEN detoxification,and found that ozonization could decrease the ZEN content in wheat grains by 58.6%.In another study,ozone treatment was able to degrade 95.1% of ZEN in corn flour [46].Studies have also shown that the degradation of ZEN is affected by the moisture content of the contaminated material.Qiet al.[47]observed that high degradation efficiency (90.7%) of ZEN can be achieved after treating corn with 19.6% of moisture contents,but the quality of ozonized corn was slightly affected at the same time.However,ZEN degradation by ozone gas have been limited due to low O3production capabilities of conventional systems and the high associated costs.
Sodium sulfite and cyclodextrin:When piglets were exposed to contaminated maize with added sodium sulfite (SoS),there was a remarkable reduction of the ZEN concentration by approximately 75%[48].ZEN-contaminated ground maize was treated with different concentrations of sodium metabisulfite (SBS) by Rempeet al.,[49]and the results indicated that hydrothermal processing using SBS combined with a strongly alkaline chemical (calcium hydroxide) seems to be a promising way to decontaminate high doses of ZEN.Beta-cyclodextrin can form stable complexes with ZEN and ZELs,which may be suitable tools for ZEN detoxification in the future.Poór reported that even small amounts of βcyclodextrin bead polymer (BBP) can completely remove ZEN.Moreover,BBP could be entirely reactivated through the removal of ZEN from the β-cyclodextrin polymers using a 50% (v/v) ethanol–water mixture [50].
2.2.2.Plant extracts
Numerous plant extracts for ZEN detoxification have been studied in recent years (Table 4).The compounds in the plant extracts are environmentally friendly,often have a low cost,and are mostly safe and effective.Grape pomace was investigated as a potential food fiber that can be used to remove ZEN.Grecoet al.[51]investigated grape pomace,artichoke waste,and almond hulls as materials for ZEN decontamination,and found that maximum ZEN adsorption on some plant extracts reached 74% at pH 7.Avantaggiatoet al.[52]extracted a grape pomace from a red grape variety(pulp and skin),and found that maximum ZEN adsorption reached 70% at 37 °C and pH 7.0.Gambacortaet al.[53]found that whitegrape pomace could remove 69%of ZEN.Grape pomace can therefore be utilized in the food and feed industries as a natural,cheap,and effective binder for multiple mycotoxins.In addition to grape pomace,other components of grape were also found to effectively remove ZEN.Fernandeset al.[54]found that 10 mg·ml-1of micronized grape stems could effectively bind at least 90%of ZEN.Althaliet al.[55]carried out experiments with the grape seed extract(GSE) and assessed its protective effects against ZEN-induced toxicity.GSE had no negative effects on pregnant mice,and efficiently eliminated the harmful effects of ZEN in dams and fetuses[55,56].
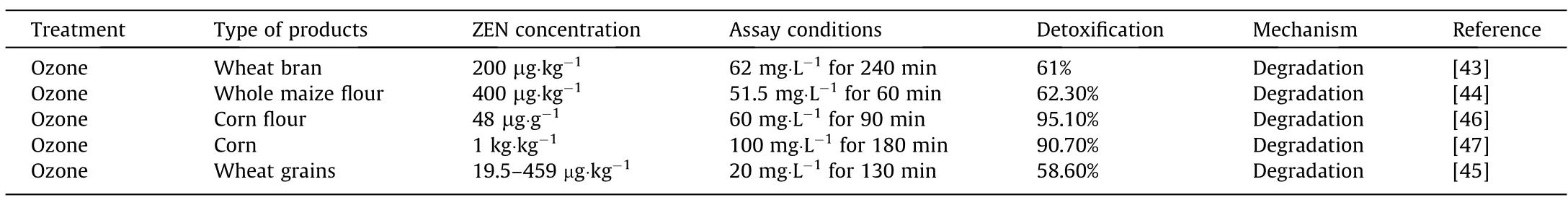
Table 3 Recent studies on the utilization of chemical compounds for ZEN degradation

Table 4 Recent studies on the utilization of plant extracts for ZEN decontamination
2.2.3.Disadvantages of chemical methods
Chemical degradation can effectively detoxify and remove ZEN from contaminated products.However,chemical treatment often influences the palatability and nutritional composition of feeds and foods,and is not suitable for large-scale detoxification [57].Although chemical methods have enable rapid ZEN degradation,their use can also easily cause secondary pollution.
2.3.Biological methods
Biological methods mainly include the adsorption of ZEN onto the walls of living cells,or degradation of ZEN by secreted microbial enzymes,which have attracted much attention because of their specificity,mild reaction conditions and minimal nutritional damage.Therefore,biological strategies for the removal or degradation of ZEN are widely recognized as the most preferable methods for practical applications in the future.
2.3.1.Adsorption of ZEN by cell walls
Cell walls can adsorb ZEN via their proteins,lipids and carbohydrates (such as mannose,peptidoglycan,glucan),which contain various adsorption centers,and present different adsorption mechanisms including ionic interactions,hydrogen bonding,and hydrophobic interactions [58](see Table 5).
In addition,S.cerevisiaehas been reported as one of the most effective yeast species for ZEN adsorption.Shanget al.[59]found that when 0.2% yeast cell wall material was added to ZENcontaminated corn,it could effectively alleviate the reproductive toxicity in piglets.Hiramet al.[60]investigated 15 strains ofS.cerevisiaeand found that the ZEN content of beer wort was decreased by between 31% and 72% depending on the strain.Similarly,Campagnolloet al.[61]estimated the ability of the beer wort residue to bind ZEN at different of pH,and found that 75.1% and 77.5% of ZEN were removed at pH 3 and 6.5,respectively.In addition,the ability ofS.cerevisiaeto decrease the ZEN content can be influenced by physical or chemical pre-treatment.For instance,the maximum adsorption capacity ofS.cerevisiaeincubated under stress-free conditions was 36.5%,while incubation under stress conditions (induced by silver nanoparticles and lyophilization),increased the maximum sorption value to 57.0% [62].The cell diameter⁄cell wall thickness relation showed a correlation between the cell wall amount and ZEN removal ability,and there was a significant increase in ZEN binding after exposure to gastrointestinal conditions [66].It has been reported that the yeast cell wall may bind mycotoxins due to it contained functional carbohydrates(glucomannan polymers).Yiannikouriset al.[67]demonstrated that the cell wall ofS.cerevisiaecould bind ZEN,and found that β-D glucans were the major compounds responsible for this process.
Lactic acid bacteria(LAB)strains are a group of microorganisms such asLactobacillusspp.,Streptococcusspp.,Leuconostocspp.orLactococcusspp.,which are widely utilized to produce lactic acid and are a main component of the intestinal microflora.In addition,LAB strains are also one of the most promising organisms for ZEN decontamination,as the cell walls of LAB can effectively bind mycotoxins [68].For instance,Sangsilaet al.[63]investigated the effects of eight strains of Lactobacillus pentosus on ZEN,and showed that the highest adsorption was as high as 83%.Lactobacillus rhamnosus strains GG and LC705 possess the same binding sites on the cell wall that could bind ZEN and α-ZOL[69].Lactococcus lactisandBifidobacteriumsp.possess the deprotonated carboxyl groups(mainly from asparagine and glutamine)from LAB proteins and peptidoglycan,which could absorb ZEN effectively[64].Lactobacillus rhamnosusand other species could absorb from 40% to 68.2%of ZEN,and these strains could form a permanent bond with ZEN,which inspired the design of a new freeze-dried powder based on LAB stains for ZEN detoxification [65].In addition,LAB strains were also found to adsorb multiple mycotoxins.Kazachstania servazziicould bind 82% to 100% of aflatoxin B1,ZEN and ochratoxin A[70].Juodeikieneet al.[71]showed thatLactobacillus sakeicould effectively bind 23% of ZEN,34% of deoxynivalenol (DON),58% of T-2 and 73% of HT-2 toxins.
2.3.2.Degradation of ZEN by microorganisms
A large number of microorganisms can be utilized for the degradation of ZEN,as shown in Table 6.For instance,Bacillus licheniformis,Bacillus subtilisandBacillus nattowere found to degrade ZEN in feed and alleviate the adverse effect of ZEN in piglets[72,73].Bacillus cereusstrain isolated from moldy feed could degrade 100% and 89.31% of ZEN in LB medium and GSF,respectively[74].Pereyra and colleagues evaluated the effects of 11Bacillusstrains on ZEN,and reported that all strains were able to degrade 58%–96.9% of ZEN within 72 h.They also proposed that the cleavage of the lactone ring might be the main degradationmechanism[75].One reportedBacillusstrain showed strong esterase activity and exhibited the highest detoxification capability in maize contaminated with 5 mg ZEN per kg [76].Additionally,LAB strains can not only adsorb ZEN well,but also degrade it.In LAB strains with high esterase activity,it was found that ZEN degradation by the supernatant fraction was dependent on esterase activity [77].Zlochet al.[78]investigated LAB strains that could transform ZEN into α-and β-ZOL.It should be noted that the external environment also has some influence on the degradation of ZEN.For instance,when response surface methodology was used to optimize the ZEN degradation conditions,it was found the optimal conditions were pH of 7.6 and a temperature of 40.1 °C,which increased the degradation ratio byBacillus pumilusto 95.7%.The degradation product was identified as 1-(3,5-dihydrox yphenyl)-6′-hydroxy-l′-undecen-l0′-one [79].The addition of cycloheximide toS.cerevisiaecombined with ZEN significantly slowed down degradation compared to 38.7% when exposing viable yeast cells alone,which showed that intracellular enzymes of the microorganism are involved in this process [80].In addition to adsorption,S.cerevisiaestrains could also degrade more than 90%of ZEN,and the observed elimination was mainly caused by the biotransformation of ZEN into β-ZOL (53%) and α-ZOL (8%) [81].

Table 5 Recent studies on the use of microorganisms for ZEN adsorption

Table 6 Recent studies on the use of microorganisms for ZEN degradation

Fig.3.(A) Attack sites of enzymes for the bio-catalytic detoxification of ZEN;(B) Biodegradation mechanisms of ZEN.
In addition to the discussed yeasts and LAB,some filamentous fungi were also found to have the ability to degrade ZEN.It was reported thatRhizopusspp.could catalyze the glycosylation of ZEN at the phenolic hydroxyl groups to produce a new metabolite called zearalenone-4-beta-D-glucopyranoside [83].Fusariumspp.combined withRhizopus arrhizusalso catalyzed the sulfation of ZEN at the phenolic hydroxyl groups to form zearalenone-4-sulfate [84,85].It was also found thatAspergillus oryzaecould transform ZEN to ZEN-4-sulfate,whileRhizopus oryzaeandRhizopus oligosporuscould transform ZEN to ZEN-4-glucosides and ZEN-2-glucosides,respectively [86].Aspergillus nigerwas tested for its ability to degrade ZEN by Sunet al.[87]and the results showed that the sulfation of zearalenone at the C-4 hydroxyl group could be enzymatically hydrolyzed byA.niger.
In recent years,the application of microbial consortia to degrade ZEN has also attracted the interest of many researchers.Benefiting from the mixed flora,microbial consortia are adaptable to environmental changes and can perform functions that are impossible for single strains.Moreover,the detoxification capability of microbial consortia depends on highly complex microbial communities and their synergistic interactions [88].Probiotics such asBacillus subtilisandCandida utilis,combined with cell-free extracts fromAspergillus oryzae,could degrade ZEN effectively,and those mixed strains could alleviate the toxic effects of ZEN in female pigs.Wanget al.[89]screened a novel microbial consortium (NZDC-6) to detoxify ZEN and found that NZDC-6 could degrade>90%of ZEN,α-ZAL and β-ZAL at an optimal temperature of 60 °C.
2.3.3.Degradation of ZEN by enzymes
According to the difference of three groups on the molecule,three main types of biological enzymes that can degrade ZEN were distinguished,that is laccase,lactone hydrolase and peroxidase(Fig.3).The first position is the ester bond of the lactone ring,and if the lactone ring can be opened,the resulting molecule loses its estrogen-mimicking properties.The second reaction site is located on the long lactone ring,especially the C-C bond near the carbonyl group.In addition,opening up the aromatic ring is also a way to degrade ZEN [90].
Lactone hydrolases have attracted the most attention as enzymes for ZEN degradation.Takahashi-Andoet al.[91]extracted the lactone hydrolase ZHD101 fromClonostachys rosea,and found that it could degrade ZEN into less toxic products.Gliocladium roseumwas found to hydrolyze the lactone bond of ZEN to form non-toxic 1-(3,5-dihydroxyphenyl)-100-hydroxy-1-undecen-6-on e,and the lactone bond in the ZEN structure was found to be broken by a lactone hydrolase [92,93].Biet al.[94]reported that the ZEN lactonase fromNeurospora crassais encoded by the zenc gene and over-expressed it inPichia pastoris.Subsequently,the purified ZENC enzyme was added to distiller’s dried grains with solubles(DDGS),maize byproducts and corn bran,and the concentration of ZEN was decreased by 71%,89% and 95%,respectively.Penget al.[95]analyzed the crystal structure of the lactone hydrolase ZHD101 fromGliocladium rosea,and found that ZHD101 belongs to the α/β-hydrolase family.Its molecular structure is composed of the catalytic core domain and an α-helix cap domain,with thesubstrate bound between the two.The catalytic triad was found to be composed of Ser102,His242 and Glu126.In addition,the interaction between the enzyme and the substrate proceeds through hydrogen bonds and non-polar bonds.In order to improve the degradation efficiency of of α-zearalenol by ZHD101,Xuet al.[96]studied the interactions between ZHD101 and the substrates ZEN and α-zearalenol.A series of mutation sites were designed to modify the structure of the substrate-binding site,and the catalytic activity of the V153H mutant on α-zearalenol was 3.7 times higher than that of the wild type,while maintaining the catalytic activity on ZEN [96].

Table 7 Recent studies on enzymes for ZEN degradation
Laccase are considered to be a very effective ZEN antidote.Banuet al.[97]investigated a laccase isolated fromTrametes versicolorand decreased the ZEN content in liquid medium by 81.7 % after 4 h at its optimum temperature.Wanget al.[98]reported that the CotA laccase fromBacillus subtilis(BsCotA) combined with chemical compounds could degrade aflatoxin B1 and ZEN.The addition of methyl syringate enabled BsCotA to degrade 98% of Aflatoxin B1 and 100%of ZEN.In a recent study,the ability of a laccase fromPleurotus eryngiito degrade multiple mycotoxins was evaluated,including aflatoxin B1,fomonisin,ochratoxin A,ZEN and T-2 toxin.The degradation of all mycotoxins was found to be significantly increased by the tested laccase-mediator systems,and ZEN was completely removed in this experiment [99].
Studies have shown that a peroxidase fromAcinetobactersp.SM04 could degrade ZEN.Yuet al.[100]found that anAcinetobactersp.could degrade approximately 90% of ZEN when incubated in ZEN-contaminated corn samples.Subsequently,they extracted enzymes fromAcinetobactersp.,and ZEN could be degraded into smaller non-estrogenic products.In addition,the enzyme was identified as peroxiredoxin,and hydrogen peroxide continues to oxidize ZEN and its products by the catalysis of peroxiredoxin[101].Wanget al.[102]found that,eight manganese peroxidases from different lignocellulose-degrading fungi could degrade 90.2%–94.3%of ZEN in the presence of malonate,whereby free radicals play an important role (see Table 7).
Enzymatic detoxification technology possesses excellent repeatability,uniformity,simple operation and high safety,as well as a number of other interesting properties.Consequently,enzymatic degradation is considered to be the most commercially valuable and useful technology for future applications.However,research on the enzymatic degradation of ZEN is still in its preliminary stages,and there is still a long way ahead in the discovery and optimization of new degrading enzymes.Since natural enzymes are often easily inactivated in industrial environments,such as strong acid,strong alkali,high temperatures,etc.,their large-scale applications are often limited.Currently,only ZHD101 with its homologues with more than 95% homology,BsCotA and peroxidase were investigated as ZEN degrading enzymes [58].Thus,cloning new ZEN degrading enzyme genes is a promising future development direction.In recent years,researchers have been working on the identification and development of new enzymes.The peroxidase gene responsible for ZEN degradation was successfully cloned and expressed inE.coli[104].In addition,multiple mycotoxins can be degraded by constructing recombinant enzymes.ZEN hydrolase and carboxypeptidase (CP) were combined to generate a recombinant fusion enzyme,and the results showed that ZEN was completely degraded in 2 h at an optimum pH of 7.0 and 35 °C by the fusion enzyme [103].
3.Conclusions
Due to its significant toxicity,ZEN can cause enormous economic losses in agriculture and animal husbandry,as well as being a potential threat to human health.Compared with traditional physical,chemical,biological method has incomparable advantages,but for microorganisms,there are still some problems need to be solved:(1)The degradation mechanism of many microorganisms is still unclear,and the toxicity of the degradation products is also unknown,which limits its further application;(2) The cost of microbial degradation is high,mainly due to the low degradation efficiency and long degradation time.In comparison,the efficiency of enzymatic degradation is higher,but this method is still in primary stage.In the past 10 years,only 3 new ZEN-degrading enzymes have been reported,and the native enzymes usually do not meet the industrial requirements,further in-depth exploration and research is needed.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We acknowledge the financial support from National Key ResearchandDevelopmentProgramofChina(No.2018YFC1604100).
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Molecular design,synthesis strategies and recent advances of hydrogels for wound dressing applications
- Recent advances in microbial production of phenolic compounds
- The production of biobased diamines from renewable carbon sources:Current advances and perspectives
- Efficient production of chemicals from microorganism by metabolic engineering and synthetic biology
- Food synthetic biology-driven protein supply transition:From animal-derived production to microbial fermentation
- A comparative analysis of China and other countries in metabolic engineering:Output,impact and collaboration
