Insights into constructing a stable and efficient microbial consortium
2021-05-19ChunmengXuHuiminYu
Chunmeng Xu,Huimin Yu,3,*
1 Key Laboratory of Industrial Biocatalysis,Ministry of Education,Beijing 100084,China
2 Department of Chemical Engineering,Tsinghua University,Beijing 100084,China
3 Center for Synthetic and Systems Biology,Tsinghua University,Beijing 100084,China
ABSTRACT Microbial consortia are ubiquitous in nature,in which multiple microbial species cooperate to complete some important tasks such as lignocellulose degradation.Because of the advantages such as reduced metabolic burden and robustness to environment disturbances,developing a microbial consortium is a promising approach for valuable product synthesis,lignocellulose utilization,human health care,bioremediation and sustainable energy, etc. Despite the benefits,however,most artificial microbial consortia confront the problems of instability and low efficiency due to growth competition and metabolite incompatibility.To overcome these challenges,multiple strategies to design efficient synthetic microbial consortia have been reported.In this review,the interactions that determine the stability and performance of microbial consortia were described.Progress of artificial microbial consortia research was summarized,and the key strategies i.e.,spatial or temporal segregation,separated utilization of nutrients,nutrient cross-feeding and division of labor,that will be of great importance for achieving a stable and efficient microbial consortium were highlighted.Two novel advanced tools,signaling molecule systems and computational models,were also introduced and discussed.We believed that combining the universal cell–cell signaling molecule systems with computational models will be promising for synthetic microbial consortia construction in the future.
Keywords:Synthetic microbial consortia Stability Efficiency Interactions Advanced approaches Signaling molecule systems
1.Introduction
Monoculture is the predominant culture way to produce various valuable chemicals [1].However,there are still limitations in monoculture,such as weakness in de novo production of products due to lack of key enzymes or overwhelmed biosynthesis pathway[2].As a result,some chemical products via monoculture only obtained rarely low amount of production or even below detection limit [3].
Microbial consortia are ubiquitous in natural world and an important task such as lignocellulose degradation often needs various microorganisms to cooperate in nature [4,5].Inspired by natural microbial consortia,artificial microbial consortia are extended to solve problems in scientific research and industrial production.Compared to a single strain,microbial consortia have more advantages.Specifically,it can reduce metabolic burden,optimize process more easily,and execute multiple tasks simultaneously via division of labor [1,5–7].Besides,microbial consortia can broaden substrate spectrum[6,8].Additionally,due to limitation of genome size of single strain,coculture with multiple strains is likely to perform unique tasks which monoculture fails [6].Moreover,microbial consortia were reported to be more robust to environment change because of the existence of strains with multiple functions[6,9].
With the mentioned benefits above,microbial consortia have been studied by numerous researchers and regarded as a promising approach for valuable product production,lignocellulose utilization,human health care,bioremediation and sustainable energy[1,7,10–13].However,disability and low efficiency of artificial microbial consortia is still challenging for further applications.We herein summarized the progress of artificial microbial consortia research and particularly discussed the advanced approaches to construct a stable microbial consortium with high-efficiency(stable and efficient microbial consortium:robust,reproducible and profitable,capable of maintaining the synergistic effect of multiple microorganisms for quite a long period).
2.Significance and Progress of Microbial Consortia Research
2.1.Significance and advantages of microbial consortia
In general,production of target products by metabolic engineering approaches involves multiple and perplexing bioprocess.Product production by a single strain is the predominant way in research and industry.However,the titer of target products would be badly hindered due to heavy metabolic burden on a single strain with perplexing biosynthesis pathways [1,14].
Distributing tasks into two or more strains can help to optimize tasks more easily and reduce metabolic load on the strain so that the productivity can be increased[6,7,15,16].Microbial communities are also able to utilize carbon sources simultaneously and efficiently,and even broaden substrate spectrum[8].There have been numerous reports about mixed sugar utilization in two strains(especially inE.colihosts) separately to improve low efficiency of mixed sugar utilization [17–19].Moreover,microbial consortia also expand substrate spectrum while it is difficult to achieve in monoculture [4,20,21].In addition,microbial consortia are more robust to environment disturbances than monoculture [22].The diversity of members in microbial consortia helps to stabilize the system,because of characters of lacking vacant niches of nutrition,different resistance to predators or parasites,or different preference towards abiotic environment[8,23].It was also reported that some silent genes might be activated and new functions thus emerged in microbial consortia because the limitation of monoculture (like incomplete supply of substrates,incompatibility of bioreactions,or toxin of by-products) can be eliminated by division of labor [24,25].The advantages above are illustrated in Fig.1.

Fig.1.The advantages of microbial consortia.
2.2.Progress of artificial microbial consortia research
With the mentioned advantages above,artificial microbial consortia (engineered microbial consortia and non-engineered microbial consortia),especially synthetic microbial consortia(engineered microbial consortia),have been studied by numerous researchers and regarded as a promising approach for producing diverse chemicals in bio-industries (Table 1) [26,27].
Two-step production of vitamin C is a well-known example of microbial consortia application in industry [28].The consortium consisting ofKetogulonicigenium vulgareandBacillus megateriumproduced 100.9 g·L-12-keto-gulonic acid (2-KGA),the precursor of vitamin C.In the consortium,K.vulgareconverted L-sorbose into 2-KGA.B.megateriumhelpedK.vulgareagainst reactive oxygen stress and supplied essential substrates for the growth ofK.vulgare.K.vulgaregrew poorly in monoculture withoutB.megaterium[28].Moreover,Maet al.constructed a novel 3-member microbial consortium to achieve 73.7 g·L-12-KGA by one-step fermentation.In the community,Gluconobacter oxydansandBacillus endophyticussupplied nutrients toK.vulgareto facilitate its growth and 2-KGA production [34].
The long and perplexing biosynthesis pathways hinder the production of valuable products such as polyphenols (with antioxidant and anti-inflammatory effects) from microorganisms [11].The emerging of synthetic microbial consortia improves the possibility to biosynthesize natural products by division of labor [14].Wanget al.constructed twoE.colistrains to accommodate the sakuranetin (a kind of polyphenols) biosynthesis pathway.Upstream strain was responsible for intermediate p-coumaric acid production.Downstream strain was engineered to producesakuranetin from p-coumaric acid.With subsequent optimization of strain inoculation,improvement of intermediate malonyl-CoA production,and enhancement of relevant enzymes CHS and CHI which convert malonyl-CoA to naringenin,the precursor of sakuranetin,the sakuranetin titer was up to 29.7 g·L-1from 5 g·L-1glucose.Significant increase of sakuranetin titer was achieved by twostrain coculture in comparison with monoculture (29.7vs.5.7 mg·L-1) [35].Similar polyphenol production strategy was also reported by other researchers [15,16,36].

Table 1 Some representative and successful applications of artificial microbial consortia
Coculture with two strains are common in the production of polyphenols.However,multiple strains may be more effective sometimes.Liet al.[19]distributed the pathway of rosmarinic acid(RA) biosynthesis into threeE.colistrains.The optimized threestrain coculture system produced 172 mg·L-1RA,with significant increase than that of two-strain coculture (60 mg·L-1).In another instance,Joneset al.[3]expressed 15 pathway enzymes in 4-strainE.colicoculture to accomplish de novo biosynthesis of calistephin.The division of labor allow for reducing the metabolic burden as well as balance and allocation of metabolic resources.
Coculture of bacteria is also a good candidate strategy for biosurfactant production with extended substrate spectrum and inter-species or inter-genera interactions which might enhance biosynthesis of biosurfactant [37,38].Zhiet al.[29]cocultured a strain with high hydrolases activity,Bacillus amyloliquefaciensX82,and a strain with high surfactin production,B.amyloliquefaciensMT45.The coculture system produced 3.4 g·L-1surfactin from 200 g·L-1distillers’ grains,while only 1.04 g·L-1surfactin was produced in a singleB.amyloliquefaciensMT45 culture [29].Furthermore,Ibraret al.found that microbial consortium composing ofLysinibacillus,Paenibacillus,GordoniaandCupriavidus spp.produced two new lipopeptides,which means coculture is not only a good way to improve biosurfactant production,but also a potential strategy to create new biosurfactants [24].
Furthermore,synthetic microbial consortia offer an alternative to utilize lignocellulose effectively in a way of consolidated bioprocessing (CBP),which combines the production of lignocellulose degrading enzymes,lignocellulose degradation and fermentation in one step.It is challenging for a single strain to perform these tasks owing to heavy metabolic burden and low efficiency of mixed sugar utilization[30].CBP can reduce production cost and processing periods by omitting the purification process of enzymes[1,30].
Shahabet al.constructed a stable lactate platform by forming oxygen gradients for each member growth[7].In this consortium,fungusTrichoderma reeseiproduced cellulolytic enzymes.Lactobacillus pentosuswas added to build a niche with a sufficient redox potential for obligate anaerobe which served as a fermenting strain.The platform could directly convert pretreated beechwood into valuable products,including butyric acid,propionic acid,acetic acid,and short-chain fatty acids.As for butyric acid production,the yield of butyric acid was about 100%higher than that of a natural consortium and even equivalent to that of the fermentation from lignocellulosic hydrolysates [7].In Minty’s work,T.reeseRUTC30 with cellulase production andE.coliwith high isobutanol titer were selected as the members of a microbial consortium,which achieved 1.88 g·L-1titer and 62% theoretical yield of iso-butanol directly from corn stover [30].
Artificial microbial consortia also have potential in sustainable energy generation,such as bioelectricity.Microbial consortia showed enhanced bioelectricity generation,as compared to monoculture [31].Specifically,the microbial consortium composed ofShewanella oneidensisandBacillus subtilisexhibited 40.2-fold maximum power density of that of a singleB.subtilis.Besides,it is also promising to produce a kind of typical biofuels ethanol by microbial consortia.The consortium ofClostridium thermocellumandThermoanaerobacterium thermosaccharolyticumproduced up to 2.4 g·L-1ethanol from 8.8 g·L-1corn fiber [32].In addition,Goyalet al.constructed a four-yeast consortium and achieved ethanol titer up to 1.25 g·L-1(87% of the theoretical value) on phosphoric acid swollen cellulose[39].Similarly,a microbial consortium composed of four-yeast members produced 1.80 g·L-1ethanol [40].
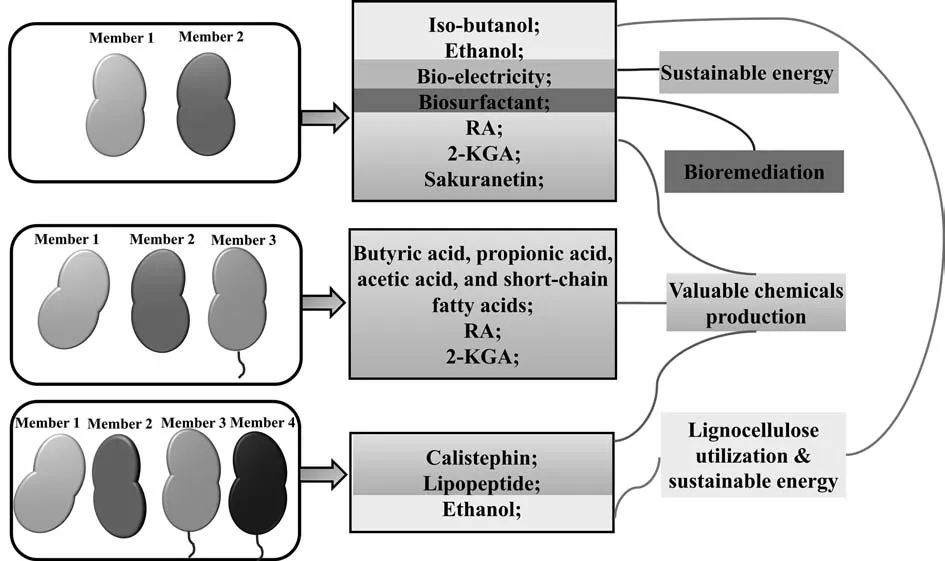
Fig.2.The representative and successful cases of product production by microbial consortia [7,19,28–33,35]
Artificial microbial consortia are good candidates for bioremediation as well.Chenet al.[33]mixed two crude oil-degradation and biosurfactant-production bacteria(Dietziasp.CN-3 andAcinetobactersp.HC8–3S)to construct a consortium,which had higher crude oil and various hydrocarbons degradation efficiencies than that of any single strain.It was also found that microbial consortia performed better than a single strain on petroleum-hydrocarbon degradation in Phulpoto’s work [41].
Illustrating the representative and successful cases of artificial microbial consortia in Fig.2,it can be seen that the members of the artificial microbial consortia have reached 4,and the products have been diversely distributed from small energy molecules to large medicine molecules.
3.Current Common Strategies for Constructing a Stable and Efficient Microbial Consortium
Although microbial consortia have numerous advantages over monoculture,the instability and low-efficiency of many coculture systems hindered their further applications.Strain compatibility is one of the core factors for coculture[4].Strains in microbial consortia may need different growth environmental conditions (such as temperature or pH) or required various nutrients.Additionally,strains in the coculture system interact with each other in different ways.How to design stable artificial microbial consortia(especially synthetic microbial consortia) becomes the key to maximum the production goal of coculture.
Giriet al.reported that a good microbial consortium design has to match ecological and evolutionary principles [8].In the review,five ecological and evolutionary principles were proposed.As for the first one,bacteria exist as a community rather than live independently in nature.A task completion always involves cooperation of multiple bacteria.Also,bacteria share metabolites and signal molecules to maintain a highly efficient community.Moreover,there is always a balance limitation between bacteria growth and production.Authors also claimed that bacteria are prone to live in biofilm because of the structure protection.Finally,it was mentioned that bacteria genome in nature are not immutable but highly dynamic.These principles can serve as guidelines for artificial microbial consortia design.
No matter what kind of tool is used to design microbial consortia,microbial interactions are the major factors for stability and good performance of microbial consortia[42].Positive interactions(such as mutualism,cooperation,and commensalism) could promote the stable co-existence and better production-behavior of a microbial consortium,while negative interactions might ruin the community instead [42,43].
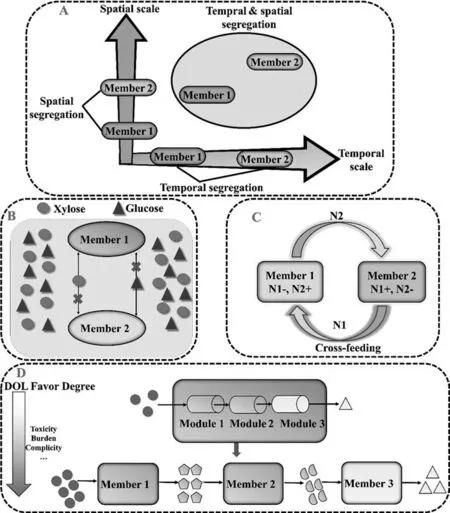
Fig.3.Strategies for construction of stable and efficient artificial microbial consortia.A.Spatial or temporal segregation;B.Separated sugar utilization;C.Mutual crossfeeding of nutrients.N1,N2,nutrient 1 and 2;“-”,“+”,negative and positive;D.Division of labor.
With ecological and revolutionary knowledge,and also guided by the successful cases in literatures,we herein proposed the most important factors to design and achieve the stable and efficient artificial microbial consortia,as summarized in Fig.3.
3.1.Spatial or temporal segregation
For incompatible members in consortia,spatial and/or temporal segregation might fix the incompatibility (Fig.3A) [44].Microfluidic platform is one of the approaches to set up spatial separated environment [45–47].Membrane separation is also an effective way to spatial segregation [48].Combined spatial and temporal segregation,Shahabet al.designed a biofilm reactor for converting lignocellulose to short chain acids by constructing a spatial organization within biofilm,along with sequential inoculation of fungus,lactic acid bacteria and lactic fermenter [7].
3.2.Separated utilization of nutrients
Resource competition is one of the important reasons for instability of microbial consortia[18].Separated utilization of nutrients can avoid resource competition within members and improve the performance of artificial microbial consortia.Separated utilization of carbon substrates has been proved to improve microbial consortia behaviors[18,49].In a 3-member consortium,separated utilization of carbon substrates showed enhanced stability of the microbial consortium and rosmarinic acid titer [19].Liuet al.[18]also adopted the strategy of separated carbon utilization to engineer compatibleE.coli-E.coliconsortium.The strategy not only offered an available approach for the design of artificial microbial consortia,but also showed a feasible way for artificial microbial consortia to utilize renewable lignocellulose.In the mixed sugar strategy above,consortia members were engineered to utilize only one kind of sugar respectively to avoid carbon catabolite repression (Fig.3B).If the instability of consortia is not mainly caused by nutrient competition,the effects of this strategy might be limited.
3.3.Nutrient cross-feeding
Metabolite exchange is ubiquitous for bacteria in nature [50].Inspired by this phenomenon,constructing co-dependent bacteria is an alternative to engineer synthetic microbial consortia(Fig.3C)[6,20,51–54].Cross-feeding interactions might be helpful for the persisting exist of multiple species while competing for a single resource [55].Bi-directional exchange is easier to achieve mutual restriction and system balance by avoiding members to dominate or eliminate others,as compared to single nutrient supply [52].
Amino acid cross-feeding has been widely adopted in designing stable synthetic microbial consortia [23,52,56,57].Meeet al.[58]reported that auxotrophic strains with costly amino acids (methionine,lysine,isoleucine,arginine,and aromatics) are prone to cooperate better than with cheaper ones.It was the first report to clarify how cross-feeding of certain amino acid pairs stabilize synthetic microbial consortia and presented a platform for synthetic microbial consortia rational design.Amino acid crossfeeding can be used to design not only two-member consortia,but also higher dimensional consortia,such as 4 or even 14-member communities [52,58].
However,there is limitation for amino acid cross-feeding strategy.Amino acid or other essential metabolites cross-feeding may bring extra cost to contributing strains and interfere the target production pathway.Moreover,the release delay of essential metabolites may lead to the collapse of synthetic microbial consortia[51].Non-essential metabolite cross-feeding strategy can avoid this problem.McCullyet al.built a stable coexistedE.coli-Rhodopseudomonas palustrissystem which was realized by mutual exchange of a carbon source (organic acid) and a nitrogen source (ammonium) [21].Besides,in Losoi’s work,anE.colistrain was constructed to offer a carbon source (gluconate) to anAcinetobacter baylyiADP1 strain.In return,theA.baylyiADP1 member supplied acetate as a carbon source ofE.colimember.After exchange of carbon sources,the consortium maintained a stable growth despite changes of initial inoculation ratio or substrate concentration[51].Similarly,a three-member consortium consisting ofE.coli,B.subtilisandS.oneidensisutilized glucose to generate electricity for more than 15 days in terms of cross-feeding [59].
However,cross-feeding strategy is not effective all the time for the construction of stable microbial consortia.Vetet al.[60]reported that bi-stability occurred when the mutual cross-feeding nutrients were highly dependent.As for the bi-stability of the system,one state had low growth while the other had balanced mutualism and competition.Sunet al.suggested that the synthesis rates of cross-feeding nutrients determine the stability of synthetic microbial consortia [53].In addition,the existence of cheaters is a big problem in cross-feeding systems [53,61].Cheaters cheat the benefits from cooperative partners but hardly contribute to systems,which threat the stability of microbial consortia.The existence of cheaters might ruin the stable microbial consortia.Strategies like spatial segregation and new environment adaption have been proposed to control the cheaters [61,62].
3.4.Division of labor (DOL)
Besides,proper division of labor can be a powerful approach to design a stable and high-efficiency community [63].With proper distribution of tasks in a community,members might have positive interactions,thereby maintain stable and highly efficient [14,56].Nutrient cross-feeding or separated utilization of nutrients could be regarded as parts of division of labor but we herein refer division of labor as division of target product biosynthesis pathway.
For example,threeE.colistrains were constructed to be responsible for the synthesis of two precursors(caffeic acid-CA;salvianic acid A-SSA)and the product RA,respectively.the titer of RA of the optimized three-strain coculture was 2.9-fold of that of two-strain coculture.In the research,the balance of two precursors (CA and SSA) was hard to achieved.Construction of three-strain coculture system enabled more flexible balance between three modules,as compared to two-strain coculture with CA and SAA+RA modules[19].Product bioproduction usually involves more than one intermediate.The balance of these intermediates determines the yield of product.Therefore,proper division of intermediates production can increase their supply to target product production,thus forms a high-efficient consortium[56].Division of labor is favored under certain situations:intermediate is harmful for the host growth;the original pathway causes great burden on the host;extracellular steps are required;or cell density can increase sufficiently to compensate the intermediate transport inefficiency (Fig.3D) [14].
4.New Development in Constructing Efficient Microbial Consortia
4.1.Quorum sensing system
Quorum sensing process is a kind of cell–cell communication in which bacteria secret signaling molecules associated with cell density and the expression of relative genes alters while a threshold is reached [6,64].

Fig.4.QS systems for synthetic microbial consortia construction.(a) The lux QS system;(b) The agr QS system;(c) The agr system for synthetic microbial consortia construction.
Signaling molecules are different from species to species.Acylhomoserine lactone (AHL) molecule is a kind of small molecules responsible for cell–cell communication in Gram-negative bacteria,which is able to freely diffuse across cell membrane[64].Generally,microorganisms only recognize the AHL molecules produced by themselves [64].
When the young man saw the maiden12 weeping bitterly he said to her, What is the matter, my poor girl? Oh! she answered, I am chained here till a horrible serpent with seven heads comes to eat me
The first and well-known AHL system isluxsystem (Fig.4(a)).There are three important components in the system:LuxI synthase,transcriptional regulator LuxR,and corresponding promotor.Specifically,LuxI synthase synthesizes the AHL molecules which increase as cell density increases.Once the threshold of the AHL molecules is reached,the transcriptional regulator LuxR binds the AHL molecules.The AHL-LuxR complex activates theluxpromoter to initiate the expression of luminescence genes [65].
AHL systems are widely used in synthetic biology due to the well-known knowledge,high compatibility and few required components[6].Many efforts have been made to enhance the effectiveness of AHL response,such as manipulation of regulator LuxR under different strength of promoters or via directed evolution of the regulator.Mathematical approach was also adopted to design sensitivity of QS system [64].
The signaling molecules in Gram-positive bacteria are different from those in Gram-negative bacteria.QS systems in Grampositive bacteria are more perplexing as compared to that in Gram-negative bacteria.Generally,signaling molecules are short peptides named autoinducing peptides (AIPs) in Gram-positive bacteria [66].AIPs are encoded as precursors then processed and delivered via transport mechanism.Mature AIPs bind to membrane-bound histidine kinase,activate the autophosphorylation of histidine kinase receptor and cognate cytoplasmic response regulator,eventually initiate relevant expression of genes [66].
TheagrQS system inStaphylococcus aureusis a typical example in Gram-positive bacteria.In this QS system,AgrD acts as a precursor peptide.The membrane endopeptidase AgrB processes AgrD to mature AIP signaling peptides and transports AIP outside cell membrane.The membrane-bounded histidine kinase ArgC detects AIP then activates transcriptional regulator AgrA through phosphorylation.Activated AgrA activates relevant promotors[66,67](Fig.4(b)).
The furanosyl borate ester autoinducer-2(AI-2)is a kind of universal signaling molecules in Gram-negative and Gram-positive species.Hence,it has been widely used to control interspecies communication [68].
QS systems can be used to control or coordinate specific gene expression,control subpopulation composition,and enable cell–cell communication between certain location in consortia[64,69,70].So far,QS system control has been one of the most effective tools to engineer synthetic microbial consortia,especially for Gramnegative strains [6].For example,theluxandlassystems with toxin/antitoxin system were used to design aE.colipredator–prey ecosystem [71].Extinction,coexistence and oscillatory dynamics of predator and prey cells were achieved under different experimental conditions[71].Similarly,Honjoet al.[72]designed a genetic circuit for AHL based QS-dependent cell lysis.When cell density threshold was reached,the cell lytic genes inE.coliTA4222 were activated and the enzyme β-glucosidase for hydrolyzing cellobiose into glucose was released.ThenE.coliTA4179 detected AHL produced by TA4222 to induce isopropanol production pathway.Theagrsystem fromS.aureusenables communication in Grampositive bacteria or between Gram-positive and Gram-negative organisms[6,73].Theagrsystem was introduced inB.megateriumcells and a QS-based sender cell-receiver cell system in Grampositive host was first constructed(Fig.4(c))[73].
QS system is of great importance in synthetic microbial consortia construction.However,the universality and orthogonality of conventional QS systems limit their further applications [74].Specifically,signaling molecules in conventional QS systems are usually species-specific so that it is challenging to have a wide range of applications.Because some QS signaling molecules such as AHLs and corresponding transcription factors have similar structures,cross-talk exists among the conventional QS systems[75,76].
Duet al.[77]designed a signaling molecule toolbox composing of six cell–cell signaling channels.The precursors of signaling molecules from the toolbox are universal metabolites.With universal precursors and different structures of signaling channels,these signaling channels have more universality and orthogonality than conventional QS systems,which offers another effective approach for synthetic microbial consortia design [77].
4.2.Computational models and other strategies
Interactions in microbial community are variable so that dynamics of microbial consortia is complexing and hard to predict [78].Therefore,comprehensive design guidelines and precise prediction of community dynamics are rather helpful[79].In McCarty’s review,there were three kinds of computational models for designing and predicting behaviors of microbial communities,including dynamic models,stoichiometric models and agent-based models [6].Konget al.[80]constructed a model involving 5 variables(2 for member populations,2 for interactions,and 1 for nutrient in media)from a two-member consortium,which corresponded to the experimental data.The model was successfully extended to well predict dynamic behaviors of three-and four-member communities.
In addition,initial inoculation ratios of members also play important roles in artificial microbial consortia design [81].Optimization of inoculation ratio of strains is a direct and simple way to balance members in microbial consortia to achieve certain production goal.However,it requires numerous trials and sometimes limited if members in the consortium are naturally against each other.Optimization of initial inoculation ratios is usually combined with other strategies to further optimize the performance of artificial microbial consortia.
All mentioned strategies above are not independent but overlapped.In fact,a stable and efficient microbial consortium might need more than one strategy to construct.For example,optimization of inoculation ratio always combines with other strategies like separated utilization of nutrients[82].Before designing a microbial consortium,fundamental knowledge of members (such as growth temperature,pH,growth rate,resistance,major metabolites,and mutual interactions) in the consortium must be mastered.Members with natural compatibility are preferred in artificial microbial consortia;in this case,it will be easier to construct a stable coculture system with simple approaches.
5.Conclusions and Prospects
The key to design a stable and efficient consortium is the control of microbial interactions.Spatial or temporal segregation,separated utilization of nutrients,nutrient cross-feeding,and proper division of labor,are effective strategies to design and construct the required interactions for the consortium.Particularly,the novel tools such as quorum sensing (QS) systems and computational model will be powerful in regulating the microbial members in communities and guiding the stable existence of artificial microbial consortia with efficiently cooperative metabolite-production.
In addition,we highlighted the biosafety issue for the microbial consortia.The engineered microbial consortia should not be used in environmental fields due to the biosafety concerns.We herein focused more on applications of engineered microbial consortia in bio-industries.Engineered microbial consortia will be cultured in the enclosed fermenters and must be sterilized before being discharged into the environment.Therefore,engineered microbial consortia won’t threat the environmental safety.
With more genetic information of microorganisms discovered,more convenient but effective gene-editing tools to engineer microorganisms developed,the construction of a new microbial consortium with desired behaviors will be much simpler.We believed that microbial consortia will be a prospect platform to produce diverse valuable chemicals in the future.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the National Key R&D Program of China(2018YFA0902200)and National Natural Science Foundation of China (No.21 776157;No.22078173).
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Molecular design,synthesis strategies and recent advances of hydrogels for wound dressing applications
- Recent advances in microbial production of phenolic compounds
- The production of biobased diamines from renewable carbon sources:Current advances and perspectives
- Efficient production of chemicals from microorganism by metabolic engineering and synthetic biology
- Food synthetic biology-driven protein supply transition:From animal-derived production to microbial fermentation
- A comparative analysis of China and other countries in metabolic engineering:Output,impact and collaboration
