莫西菌素药效学与药动学研究进展
2021-04-16何广邓小玲戚传勇汤春莲张莉魏青阮祥春
何广 邓小玲 戚传勇 汤春莲 张莉 魏青 阮祥春
摘要 莫西菌素是一种半合成的单一成分的大环内酯类抗寄生虫药,其药代动力学特征与药效密切相关。通常莫西菌素药代动力学会因制剂、动物品种、机体状态以及与其他药物的相互作用而发生改变。综述了莫西菌素药效学和药代动力学,为莫西菌素的临床应用提供参考。
关键词 莫西菌素;药效学;药动学;抗寄生虫药
中图分类号 S859.7文献标识码 A文章编号 0517-6611(2021)05-0005-05
doi:10.3969/j.issn.0517-6611.2021.05.002
开放科学(资源服务)标识码(OSID):
Advances in Pharmacodynamics and Pharmacokinetics of Moxidectin
HE Guang1, DENG Xiao-ling2, QI Chuan-yong3 et al
(1.Hefei Agricultural Administrative Law Enforcement Detachment, Hefei, Anhui 231135;2.College of Animal Science and Technology, Anhui Agricultural University, Hefei, Anhui 230036; 3.Hefei Agricultural Product Quality Test Center, Hefei, Anhui 230092)
Abstract Moxidectin is a semi-synthetic mono-component macrolide antiparasitic drug. The pharmacokinetic characteristics of moxidectin are closely related to its efficacy.In general, the pharmacokinetics of moxidectin are changed by the formulations, breeds, body status and interaction with other drugs.This article reviewed the pharmacodynamics and pharmacokinetic of moxidectin. It was refered to the clinical application of moxidectin.
Key words Moxidectin;Pharmacodynamics;Pharmacokinetics;Antiparasitic drug
莫西菌素(moxidectin,MXD),又稱为莫昔克丁或莫西克汀,是由链霉素发酵产生的半合成单一成分的大环内酯类抗生素。MXD属于米尔贝霉素(milbemyeins)家族,是奈马菌素(nemadectin)的衍生物,属于第三代阿维菌素类(AVMs)药物。MXD与其他AVMs相比,MXD成分单一,具有驱虫谱广,驱虫活性强、长效、安全[1]等特点。与伊维菌素(ivermectin,IVM)相比,MXD能与多种赋型剂组合制成各类制剂,可供开发选择剂型的范围更广。目前,临床上常用的MXD剂型有浇泼剂、注射剂、片剂、透皮剂、口服凝胶等,其被用于牛、羊、马、猪、犬、猫等动物寄生虫病的防治,甚至用于人的盘尾丝虫病的治疗[2-3]。MXD是理想的体内外抗寄生虫药物。该研究拟综述莫西菌素药效学和药代动力学,以期为莫西菌素的临床应用提供参考。
1 MXD理化性质
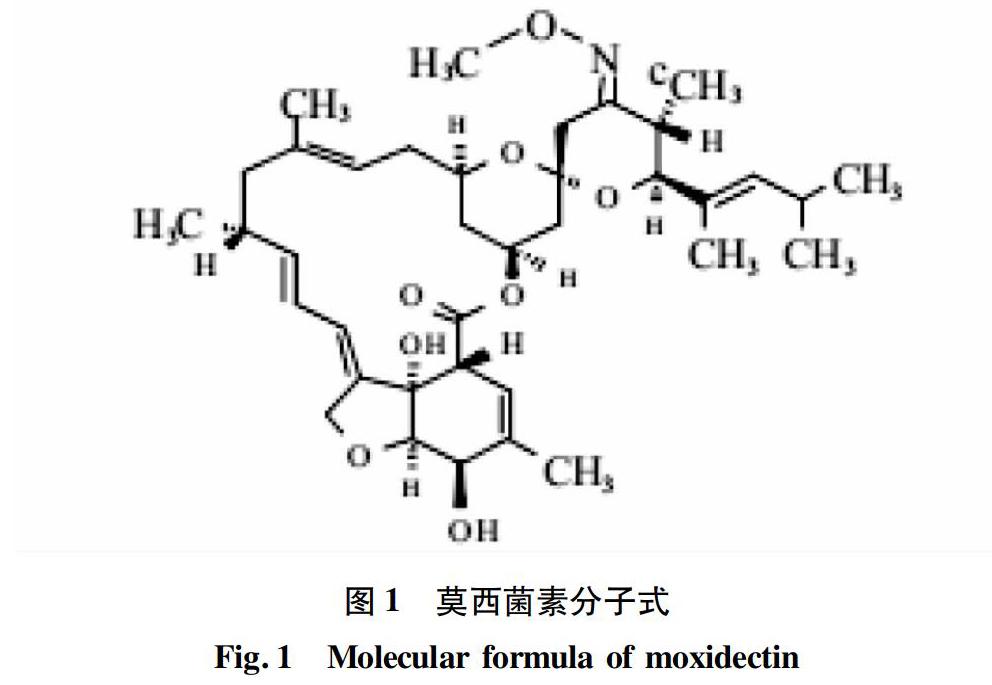
MXD分子式为C 37H 53NO 8,分子量为639.8 g/mol。它的结构类似于IVM的B1,不同处是在C 13上没有双糖,在C 25上有一个含烯烃的链,在C 23上有一个甲氧基(图1)。MXD元素组成C为69.48%、H为8.37%、N为2.15%、O为20%(试验数据)。其性状为白色或淡黄色无定型粉末,熔点为145~154 ℃,酸离解常数(pKa)为12.8±1.0,蒸汽压< 10-7(检测极限)。差示扫描量热法(DSC)测定MXD的最大电热融化温度为274.6 ℃,能量为492.1 J/g。MXD不溶于水,微溶于正己烷,易溶于乙醇(> 96%)、乙腈、乙酸乙酯等有机溶剂。MXD的正辛醇/水分配系数(58 300)显示为亲脂性化合物,其亲脂性为IVM的100倍以上。MXD紫外可见吸收波长在243.8 nm出现最大吸收峰。MXD在酸、碱、光照及氧气存在的条件下均不稳定,在制备过程中需要考虑这些因素对制剂稳定性的影响[4]。MXD在水中溶解度低,无挥发性,不会通过空气迁移,故排出到环境中的MXD与土壤结合较紧密。莫西菌素在环境中发生代谢、吸收以及光降解作用,对环境造成污染的可能性非常小。
2 MXD药效学
2.1 MXD抗虫谱
MXD对多种动物的体内消化道线虫与体表寄生虫均有较强的驱虫作用。对牛羊体内的捻转血矛线虫、古柏线虫、毛圆线虫、仰口线虫、细颈线虫、食道口线虫、网尾线虫等[5-6];对猪体内的蛔虫、毛首线虫、食道口线虫、后圆线虫等;对犬的心丝虫、结膜吸吮线虫、血管圆线虫、毛细线虫等[7];对多种动物体表的蠕形螨、疥螨、虱、蚤、蝇蛆等节肢动物均具有很强的杀灭作用[8]。
2.2 MXD驱虫作用
Fazzio等[9]用MXD治疗自然发病感染捻转血矛线虫(Haemonchus spp.)和古柏线虫(Cooperia spp.)的育肥犊牛,平均粪便卵计数(FECs)降低85%。Rizk等[10]发现MXD对水牛犊牛感染弓形虫的驱虫活性强且持久。用1%的MXD注射液(CYDECTIN-Ford Dodge)治疗患有严重疥螨疾病的山羊,首次给药后皮肤瘙痒症已迅速减轻,治疗8周后,所有羊均治愈[11]。Demeulenaere等[12]报道了MXD对寄生于马的大多数寄生虫表现出比IVM更长的保护时间。
MXD不仅应用于大动物的寄生虫病防治,而且在防治小动物寄生虫病方面也应用广泛。用含MXD成分的爱沃克[Advocate,10%吡虫啉(Imidacloprid)+2.5%MXD]治疗由毛细线虫引起的犬鼻毛细血管病,在给药第(28±2)天的粪便中卵囊数减少了99.14%[13]。用MXD缓释制剂和口服制剂预防临床分离耐IVM的心丝虫人工感染犬,防治效果分别达99.5%和100%[14-15]。爱沃克在连续给药8周后,治疗肾上腺皮质功能亢进继发性全身蠕虫病(蠕形螨)的犬的治愈率为90.1%,并有效维持1年的时间[16]。AdvantageMultifor Cats(10% Imidacloprid+1% MXD)对猫自然感染嗜气毛细线虫(Capillaria aerophila)的治疗效果达100%[17],对猫耳螨(Otodectes cynotis)的治疗效果为100%,并且持续到第50天[18]。
MXD的驱虫作用与其本身具有良好的抗寄生虫活性,临床的合理使用能提高其驱虫效果。MXD的药代动力学受到禁食、动物脂肪沉积厚度等生理状态以及P-gp调节剂的影响(在药代动力学部分具体阐述),使用时依据动物生理状态以及药物的配伍提高其临床疗效。另外,可以结合地域与动物的养殖特点,在特定的周期内使用,提高MXD的抗寄生虫效果。如对高海拔区域放牧的羊群,母羊围产期暂时对肠道线虫感染的抵抗力下降,导致粪便中卵囊数增加[19]。母羊排出的虫卵不仅污染牧草,而且孵出的感染性幼虫感染羔羊[20-21],给羊群寄生虫病防治带来一定困难。围产期使用MXD可以有效防治羊群的寄生虫疾病,也可以减少虫卵对牧场的污染[22]。在临床应用中,MXD的给药方案以及适当时期使用对于寄生虫控制有着非常重要的意义。
3 MXD药代动力学
3.1 MXD药代动力学特征
3.1.1 吸收。
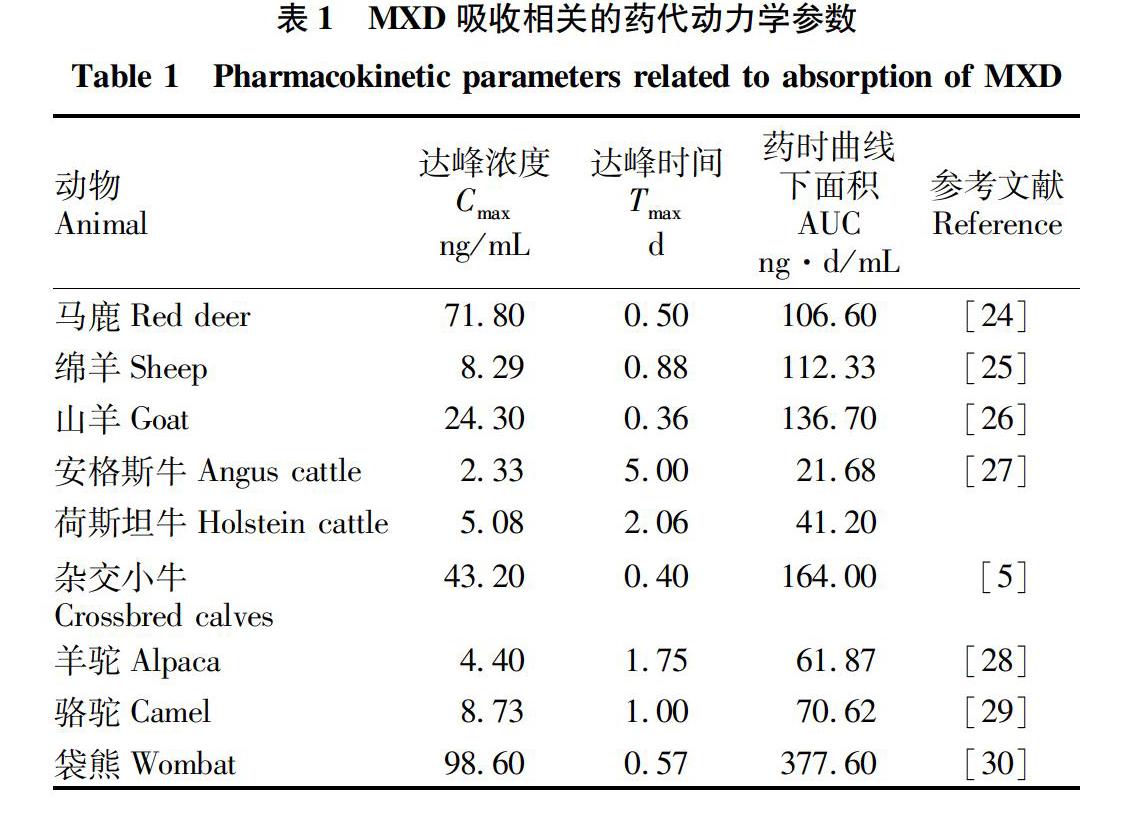
达峰时间(T max)可以反映药物在体内吸收的快慢。MXD在体内的吸收比其他AVMs药物快。Lanusse等[23]报道了MXD、IVM和多拉菌素(doramectin,DRM)在牛体内的T max差异,MXD的T max(8.0 h)要早于IVM(4 d)和DRM(6 d)的T max。MXD皮下给药后在不同动物体内T max先后顺序为安格斯牛>荷斯坦牛>羊驼>骆驼>绵羊>袋熊>马鹿>杂交小牛>山羊(表1)。从表中可以看出MXD在山羊体内T max最快。
3.1.2 分布和代謝。
MXD在体内分布广泛,在脂肪、黏膜、胆汁、血浆、毛皮等组织中均有分布[31]。由于MXD具有高脂溶性和对脂肪组织高亲和力,MXD主要分布在脂肪组织[32]。MXD给药后,在脂肪组织中的浓度最高,依次为肝脏、肾脏和肌肉[33]。
MXD代谢的主要器官为肝脏,肝脏中细胞色素P450主要参与MXD的代谢[34]。MXD的代谢物包含1种羟基化代谢物和至少6种其他的代谢物[35]。MXD在牛体内的主要代谢产物为C 29-30和C 14-羟甲基衍生物[36]。由于动物品种的差异,MXD在肝脏中生物转化的快慢存在一定差异,从而影响MXD在体内的滞留时间。Dupuy等[37]报道使用几种动物的肝微粒体,在体外研究对14C标记的MXD的生物转化率,结果显示,绵羊的生物转化率最高(32.7%),而猪的生物转化率最低(0.8%),其他动物的生物转化率依次为牛(206%)、鹿(15.4%)、山羊(12.7%)、兔子(7.0%)和大鼠(3.0%)。
3.1.3 排泄。
MXD主要是通过粪便排出体外(>95%),仅有少量通过尿液排泄(<1%)。通过检测公牛粪便,在第7、14、28天排出MXD的量分别相当于给药剂量的32.2%、413%和58.1%[35]。但是在哺乳期时,MXD也能通过乳汁排出[38],吮吸乳汁的幼畜体内能检测到MXD[39]。骆驼乳汁中的MXD药代动力学参数C max和AUC是血浆中C max和AUC的3~4倍[40]。MXD通过乳汁排泄,与MXD的高脂溶性特征有关。
3.2 影响MXD药代动力学参数的因素
MXD的药代动力学因机体状态、给药途径、药物剂型的不同而有明显差异,给药前是否禁食和额外补充油脂以及药物的相互作用也能对动力学参数产生明显影响。
3.2.1 机体状态。
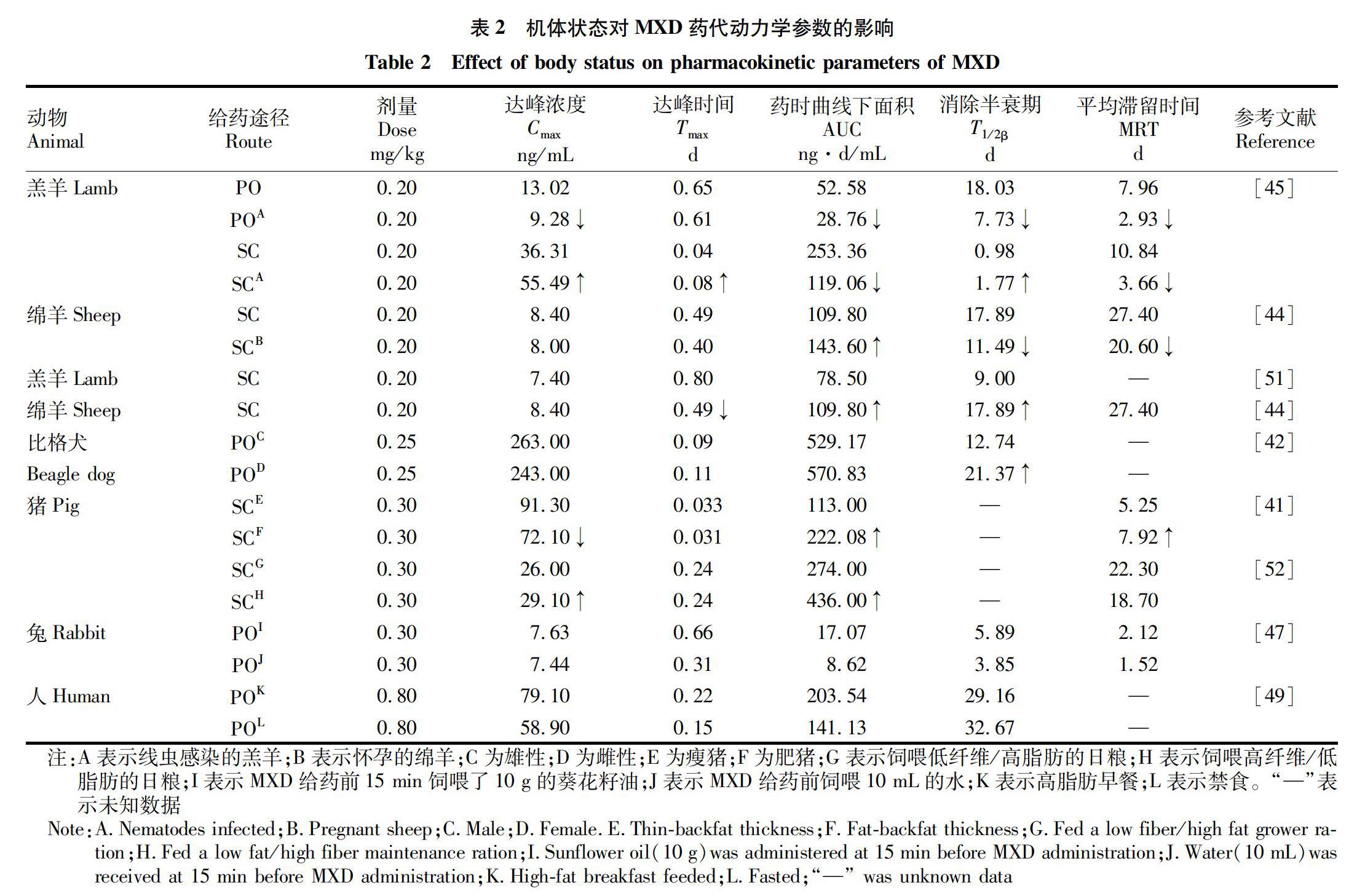
动物不同年龄、性别、生理、病理因素等均会影响MXD的药代动力学特征(表2)。Craven等[41]研究表明肥猪(背膘厚)MXD的C max比瘦猪低,但全身循环的药物总量显著升高;肥猪MXD的体清除率(CL/F)低,平均滞留时间(MRT)长,说明MXD在肥猪体内更长效。雌性比格犬的MXD吸收较雄性比格犬慢,C max低,分布较广,全身循环的药物总量多,清除缓慢[42]。羔羊的MXD吸收更快,C max更高,但到达全身循环的药物总量显著降低[43]。怀孕动物体内MXD的消除速度加快,缩短MXD在体内的MRT[44]。感染寄生虫的羔羊组皮下注射MXD的AUC比健康组降低了2倍,MRT显著缩短。羊患寄生虫病后引起了MXD的CL/F增加,伴随着消除半衰期(T 1/2β)缩短,导致体内MRT缩短,缩短了药物正常的维持时间[45]。
MXD的药代动力学参数除受到上述因素影响外,还受到给药前是否禁食以及额外补充油脂的影响。禁食减少胆汁分泌和肠蠕动[46],增加了MXD在肠道停留时间,延长了MXD的吸收,提高了MXD的AUC。额外饲喂油脂,给药后MXD的AUC会增加。Bassissi等[47]研究了试验前饲喂10 g葵花籽油的新西兰兔体内MXD的药代动力学,对照组MXD的AUC[8.62 (ng·d)/mL]低于饲喂葵花籽油饲组[1707 (ng·d)/mL]。因此,给药前额外补充饲喂油脂可以改善MXD的口服生物利用度,这与Cotreau等[48]的报道相一致。MXD的生物利用度增加可能与MXD在小肠中的吸收增加有关[49-50]。
3.2.2 給药途径。
给药方式不同会造成MXD在体内的药代动力学差异。对牛单次皮下注射MXD时,在给药后的4~6 h达到C max;单次口服给药后C max出现在给药后24 h。与口服组相比,MXD皮下给药的半衰期更长,体内药物循环总量更大,MRT更长,相对生物利用度更高[51]。MXD液体制剂口服后的C max和AUC分别比片剂的高28.6%和28.8%,T max缩短了0.9 h[50],这可能与液体制剂提高MXD的溶解度有关。
3.2.3 剂型。
除了给药途径对MXD药代动力学影响外,MXD的制剂类型也对其药代动力学产生较大的影响。Dupuy等[53]报道了莫西菌素长效制剂(LA)在牛中的药代动力学(1 mg/kg的LA莫西菌素生物利用度相当于皮下给予0.2 mg/kg常规莫西菌素制剂的生物利用度),LA莫西菌素的C max增加了40%,T max延迟了1 062%,MRT增加了198%,AUC增加了450%以上[23]。由于MXD具有较高的脂溶性,适合开发长效制剂,提高其驱虫作用和生物利用度。
3.2.4 药物相互作用。
P-糖蛋白(P-glycoprotein,P-gp)是跨膜蛋白,能够将多种结构化合物泵出细胞外[54]。目前,已有MXD与不同的P-gp调节剂在动物体内相互作用的研究报道[55]。牛皮下注射MXD并联合使用洛哌丁胺(loperamide,LPM),联合用药组牛血浆中MXD的浓度明显高于单独MXD皮下注射组。MXD和LPM合用还导致AUC升高和CL/F降低[56]。LPM提高了MXD在牛体内的生物利用度。与LPM不同的是,MXD和酮康唑联合给药,MXD的血浆浓度与羔羊中单独使用MXD的浓度没有差异[57]。因维拉帕米的半衰期短,发挥的作用时间较短暂,故维拉帕米对绵羊MXD药代动力学没有产生明显的影响[58]。体外研究表明,MXD与AVMs化合物相比,对哺乳动物P-gp亲和力较低[59],这可能是MXD在动物体内维持较长时间的原因之一[60]。
4 展望
由于IVM和阿维菌素(avermectin,AVM)广泛使用,已有寄生虫对IVM和AVM产生耐药性的报道[60-62]。尽管MXD对P-gp的亲和力较低,相对于IVM不容易产生耐药性[55],但是MXD与IVM和AVM有交叉耐药性,近几年也出现了MXD耐药性的报道[63]。MXD缓释制剂是提高防治动物寄生虫病、减少耐药性产生的策略之一。常规制剂由于维持作用的时间较短,需要频繁给药,且体内的血药浓度变化较大。这种频繁给药频率与耐药性线虫的出现之间存在一定的关系[64]。缓释制剂维持的有效时间覆盖在动物整个胃肠道线虫发育的各个阶段,可以减少耐药性的产生。目前,关于缓释制剂的开发有大量的文献报道,如长效注射液、注射用凝胶制剂、微球凝胶(MS-Gel)制剂[65]等。纳米化技术是提高水不溶性MXD生物利用度的一种有前景的制备策略,因为它可以提高MXD的溶解度和吸收。脂质体纳米颗粒载体也可用于透皮制剂,以改善药物的生物利用度[66-68]。MXD的疗效与寄生虫是否产生耐药性直接相关,大多数认为MXD剂量不足可能是导致耐药性的重要因素。如何提高MXD的疗效并延缓耐药性的发展是新制剂设计中要考虑的重要因素。
MXD作为新一代驱虫抗生素,能高效杀灭体内外寄生虫。MXD在用药剂量、剂型开发、耐药性和体内药物分布等方面优于IVM,是一种应用前景广阔的抗寄生虫药。尽管MXD具有很好的驱虫活性及驱虫谱,但也难免存在耐药性。长效缓释制剂的开发、临床合理用药是减少MXD耐药性的有效手段。MXD的药代动力学受多种因素的影响,例如品种、身体状况、给药途径等,临床应充分考虑这些因素,以提高MXD的临床治疗效果。
参考文献
[1] MNEZ CC,SUTRA J F,PRICHARD R,et al.Relative neurotoxicity of ivermectin and moxidectin in mdr1ab(-/-)mice and effects on mammalian GABA(A)channel activity[J].PLoS Negl Trop Dis,2012,6(11):1-10.
[2] TURNER H C,WALKER M,ATTAH S K,et al.The potential impact of moxidectin on onchocerciasis elimination in Africa:An economic evaluation based on the Phase II clinical trial data[J].Parasit Vectors,2015,8:1-12.
[3] AWADZI K,OPOKU N O,ATTAH S K,et al.A Randomized,single-ascending-dose,ivermectin-controlled,double-blind study of moxidectin in Onchocerca volvulus infection[J].PLoS Negl Trop Dis,2014,8(6):1-18.
[4] AWASTHI A,RAZZAK M,AL-KASSAS R,et al.Analytical profile of moxidectin[J].Profiles Drug Subst Excip,Relat Methodol,2013,38:315-366.
[5] FAZZIO L,MORENO L,GALVAN W,et al.Pharmacokinetic profile and anthelmintic efficacy of moxidectin administered by different doses and routes to feedlot calves[J].Vet Parasitol,2019,266:73-79.
[6] VADLEJCH J,MAKOVICKY P,CADKOVA Z,et al.Efficacy and persistent activity of moxidectin against natural Muellerius capillaris infection in goats and pathological consequences of muelleriosis[J].Vet Parasitol,2016,218:98-101.
[7] BERNIGAUD C,FANG F,FISCHER K,et al.Preclinical study of single-dose moxidectin,a new oral treatment for scabies:Efficacy,safety,and pharmacokinetics compared to two-dose Ivermectin in a porcine model[J].PLoS Negl Trop Dis,2016,10(10):1-18.
[8] BOWMAN D D,OHMES C M,HOSTETLER J A,et al.Efficacy of 10% imidacloprid + 2.5% moxidectin topical solution(Advantage Multifor Dogs)for the prevention of heartworm disease and infection all month long[J].Parasit Vectors,2017,10(S2):59-235.
[9] FAZZIO L E,STREITENBERGER N,GALVAN W R,et al.Efficacy and productive performance of moxidectin in feedlot calves infected with nematodes resistant to ivermectin[J].Vet Parasitol,2016,223:26-29.
[10] RIZK M A,OSMAN S A,AL-GAABARY M H,et al.Comparative clinical and parasitological efficacy of moxidectin pour-on,ivermectin,and piperazine citrate on Toxocara vitulorum infection in buffalo calves(Bubalus bubalis):A randomized clinical trial[J].Turk J Vet Anim Sci,2018,42:29-33.
[11] GIADINIS N D,FARMAKI R,PAPAIOANNOU N,et al.Moxidectin efficacy in a goat herd with chronic and generalized sarcoptic mange[J].Vet Med Int,2011,2011:1-4.
[12] DEMEULENAERE D,VERCRUYSSE J,DORNY P,et al.Comparative studies of ivermectin and moxidectin in the control of naturally acquired cyathostome infections in horses[J].Vet Rec,1997,141(15):383-386.
[13] VERONESI F,MORGANTI G,DI CESARE A,et al.A pilot trial evaluating the efficacy of a 10% imidacloprid/2.5% moxidectin spot-on formulation in the treatment of natural nasal capillariosis in dogs[J].Vet Parasitol,2014,200(1/2):133-138.
[14] BOWMAN D D,MCTIER T L,ADAMS E L,et al.Evaluation of the efficacy of ProHeart 6(moxidectin)against a resistant isolate of Dirofilaria immitis(JYD-34)in dogs[J].Parasites Vectors,2017,10(Suppl 2):53-235.
[15] MCTIER T L,SIX R H,PULLINS A,et al.Efficacy of oral moxidectin against susceptible and resistant isolates of Dirofilaria immitis in dogs[J].Parasit Vectors,2017,20(S2):39-235.
[16] PATERSON T E,HALLIWELL R E,FIELDS P J,et al.Canine generalized demodicosis treated with varying doses of a 2.5% moxidectin + 10% imidacloprid spot-on and oral ivermectin:Parasiticidal effects and long-term treatment outcomes[J].Vet.Parasitol,2014,205(3/4):687-696.
[17] DI CESARE A,VERONESI F,CAPELLI G,et al.Evaluation of the efficacy and safety of an imidacloprid 10 % / moxidectin 1 % spot-on formulation(Advocate),Advantagemulti)in cats naturally infected with Capillaria aerophila[J].Parasitol Res,2017,116(S1):55-64.
[18] FOURIE L J,KOK D J,HEINE J.Evaluation of the efficacy of an imidacloprid 10%/moxidectin 1% spot-on against Otodectes cynotisin cats[J].Parasitol Res,2003,90(S3):S112-113.
[19] BEASLEY A M,KAHN L P,WINDON R G.The periparturient relaxation of immunity in Merino ewes infected with Trichostrongylus colubriformis:Endocrine and body compositional responses[J].Vet Parasitol,2010,168(1/2):51-59.
[20] WILLIAMS A R,GREEFF J C,VERCOE P E,et al.Merino ewes bred for parasite resistance reduce larval contamination onto pasture during the peri-parturient period[J].Animal,2010,4(1):122-127.
[21] SARGISON N D,BARTRAM D J,WILSON D J.Use of a long acting injectable formulation of moxidectin to control the periparturient rise in faecal Teladorsagia circumcincta egg output of ewes[J].Vet Parasitol,2012,189(2/3/4):274-283.
[22] VARGAS-DUARTE J J,LOZANO-MRQUEZ H,GRAJALES-LOMBANA H A,et al.Effect of moxidectin treatment at peripartum on gastrointestinal parasite infections in ewes raised under tropical andes high altitude conditions[J].Vet Med Int,2015,2015:1-18.
[23] LANUSSE C,LIFSCHITZ A,VIRKEL G,et al.Comparative plasma disposition kinetics of ivermectin,moxidectin and doramectin in cattle[J].J Vet Pharmacol Ther,1997,20(2):91-99.
[24] MACKINTOSH C G,COWIE C,FRASER K,et al.Reduced efficacy of moxidectin and abamectin in young red deer(Cervus elaphus)after 20 years of moxidectin pour-on use on a New Zealand deer farm[J].Vet Parasitol,2014,199(1-2):81-92.
[25] ALVINERIE M,ESCUDERO E,SUTRA J F,et al.The pharmacokinetics of moxidectin after oral and subcutaneous administration to sheep.[J].Vet Res,1998,29(2):113-118.
[26] ESCUDERO E,CARCELES C M,DIAZ M S,et al.Pharmacokinetics of moxidectin and doramectin in goats[J].Res Vet Sci,1999,67(2):177-181.
[27] SALLOVITZF J,LIFSCHITZ A,IMPERIALE F,et al.Breed differences on the plasma availability of moxidectin administered pour-on to calves[J].Vet J,2002,164(1):47-53.
[28] COCQUYT C M,VAN AMSTEL S,COX S,et al.Pharmacokinetics of moxidectin in alpacas following administration of an oral or subcutaneous formulation[J].Res Vet Sci,2016,105:160-164.
[29] OUKESSOU M,BERRAG B,ALVINERIE M.A comparative kinetic study of ivermectin and moxidectin in lactating camels(Camelus dromedarius)[J].Vet Parasitol,1999,83(2):151-159.
[30] DEATH C E,TAGGART D A,WILLIAMS D B,et al.Pharmacokinetics of moxidectin in the southern hairy-nosed wombat(Lasiorhinus latifrons).[J].J Wildl Dis,2011,47(3):643-649.
[31] LIFSCHITZ A,VIRKEL G,IMPERIALE F,et al.Moxidectin in cattle:Correlation between plasma and target tissues disposition.[J].J Vet Pharmacol Ther,1999,22(4):266-273.
[32] LIFSCHITZ A,IMPERIALE F,VIRKEL G,et al.Depletion of moxidectin tissue residues in sheep[J].J Agric Food Chem,2000,48(12):6011-6015.
[33] CRUZ M D B A,FERNANDES MM,MONTEIRO A L G,et al.Tissue residue depletion of moxidectin in lambs(Ovis aries)following subcuianeous administration[J].Food Addit Contam:Part A,2018,35(7):1278-1285.
[34] ZENG Z,ANDREW N W,ARISON B H,et al.Identification of cytochrome P4503A4 as the major enzyme responsible for the metabolism of ivermectin by human liver microsomes[J].Xenobiotica,1998,28(3):313-321.
[35] AFZAL J,STOUT S J,DACUNHA A R,et al.Moxidectin:Absorption,tissue distribution,excretion,and biotransformation of14C-Labeled moxidectin in sheep[J].J Agric Food Chem,1994,42(8):1767-1773.
[36] ZULALIAN J,STOUT S J,DACUNHA A R,et al.Absorption,tissue distribution,metabolism,and excretion of moxidectin in cattle[J].J Agric Food Chem,1994,42(2):381-387.
[37] DUPUY J,ESCUDERO E,EECKHOUTTE C,et al.In vitro metabolism of 14C-moxidectin by hepatic microsomes from various species[J].Vet Res Commun,2001,25(5):345-354.
[38] KORTH-BRADLEY J M,PARKS V,CHALON S,et al.Excretion of moxidectin into breast milk and pharmacokinetics in healthy lactating women[J].Antimicrob Agents Chemother,2011,55(11):5200-5204.
[39] CAMPBELL B J,PAIRIS-GARCIA M D,CAMPLER M R,et al.An investigation of oral moxidectin carryover to nursing lambs via milk[J].Small Rumin Res,2017,154:9-12.
[40] OUKESSOU M,BERRAG B,ALVINERIE M.A comparative kinetic study of ivermectin and moxidectin in lactating camels(Camelus dromedarius)[J].Vet Parasitol,1999,83(2):151-159.
[41] CRAVEN J,BJRN H,HENNESSY D R,et al.The effects of body composition on the pharmacokinetics of subcutaneously injected ivermectin and moxidectin in pigs[J].J Vet Pharmacol Ther,2002,25(3):227-232.
[42] VANAPALLI S R,HUNG Y P,FLECKENSTEIN L,et al.Pharmacokinetics and dose proportionality of oral moxidectin in beagle dogs[J].Biopharm Drug Dispos,2002,23(7):263-272.
[43] LLOBERAS M,ALVAREZ L,ENTROCASSO C,et al.Comparative tissue pharmacokinetics and efficacy of moxidectin,abamectin and ivermectin in lambs infected with resistant nematodes:Impact of drug treatments on parasite P-glycoprotein expression[J].Int J Parasitol Drug Drug Resist,2013,3(12):20-27.
[44] PREZ R,NU'EZ M J,PALMA C,et al.Plasma disposition kinetics of moxidectin after subcutaneous administration to pregnant sheep[J].J Vet Pharmacol Ther,2014,37(6):550-555.
[45] Lespine A,Sutra JF,Dupuy J,et al.The influence of parasitism on the pharmacokinetics of moxidectin in lambs[J].Parasitol Res,2004,93(2):121-126.
[46] ALVINERIE M,SUTRA J F,CABEZAS I,et al.Enhanced plasma availability of moxidectin in fasted horses[J].J Equine Vet Sci,2000,20(9):575-578.
[47] BASSISSI M F,LESPINE A,ALVINERIE M.Enhancement of oral moxidectin bioavailability in rabbits by lipid co-administration[J].Parasitol Res,2004,94(3):188-192.
[48] COTREAU M M,WARREN S,RYAN J L,et al.The antiparasitic moxidectin:Safety,tolerability,and pharmacokinetics in humans[J].J.Clin Pharmacol,2003,43(10):1108-1115.
[49] KORTH-BRADLEY J M,PARKS V,CHALON S,et al.The effect of a high-fat breakfast on the pharmacokinetics of moxidectin in healthy male subjects:A randomized phase I trial[J].Am J Trop Med Hyg,2012,86(1):122-125.
[50] KORTH-BRADLEY J M,PARKS V,PATAT A,et al.Relative bioavailability of liquid and tablet formulations of the antiparasitic moxidectin[J].Clin Pharmacol Drug Dev,2012,1(1):32-37.
[51] BAPTISTA R C,FERNANDES M A M,GILAVERTE S,et al.Determination of moxidectin in serum by liquid chromatography-tandem mass spectrometry and its application in pharmacokinetic study in lambs[J].J Braz Chem Soc,2017,28(2):250-256.
[52] CRAVEN J,HENNESSY D R,FRIIS C.Does the rate of fat deposition influence the pharmacokinetic disposition of subcutaneously administered moxidectin and ivermectin in pigs?[J].J Vet Pharmacol Ther,2002,25(5):351-357.
[53] DUPUY J,SUTRA J F,ALVINERIE M.Pharmacokinetics assessment of moxidectin long-acting formulation in cattle[J].Vet Parasitol,2007,147(3/4):252-257.
[54] HORITA Y,DOI N.Comparative study of the effects of antituberculosis drugs and antiretroviral drugs on cytochrome P450 3A4 and P-glycoprotein[J].Antimicrob Agents Chemother,2014,58(6):3168-3176.
[55] PRICHARD R K,ROULET A.ABC transporters and beta-tubulin in macrocyclic lactone resistance:prospects for marker development[J].Parasitology,2007,134(Pt8):1123-1132.
[56] LIFSCHITZ A,VIRKEL G,SALLOVITZ J,et al.Loperamide-induced enhancement of moxidectin availability in cattle[J].J Vet Pharmacol Ther,2002,25(2):111-120.
[57] DUPUY J,LARRIEU G,SUTRA J F,et al.Enhancement of moxidectin bioavailability in lamb by a natural flavonoid:Quercetin[J].Vet Parasitol,2003,112(4):337-347.
[58] BALLENT M,MAT L,VIRKEL G,et al.Intestinal drug transport:ex vivo evaluation of the interactions between ABC transporters and anthelmintic molecules[J].J Vet Pharmacol Ther,2014,37(4):332-337.
[59] LESPINE A,MARTIN S,DUPUY J,et al.Interaction of macrocyclic lactones with P-glycoprotein:Structure-affinity relationship[J].Eur J Pharm Sci,2007,30(1):84-94.
[60] PRICHARD R,MNEZ C,LESPINE A.Moxidectin and the avermectins:Consanguinity but not identity[J].Int J Parasitol Drugs Drug Resist,2012,2:134-153.
[61] DE SOUZA L P,LELIS R T,GRANJA I R,et al.Efficacy of albendazole and moxidectin and resistance to ivermectin against Libyostrongylus douglassii and Libyostrongylus dentatus in ostriches[J].Vet Parasitol,2012,189(2/3/4):387-389.
[62] ALMEIDA G D,FELIZ D C,HECKLER R P A,et al.Ivermectin and moxidectin resistance characterization by larval migration inhibition test in field isolates of Cooperia spp.in beef cattle,Mato Grosso do Sul,Brazil[J].Vet Parasitol,2013,191(1/2):59-65.
[63] VAN DEN BROM R,MOLL L,BORGSTEEDE F H,et al.Multiple anthelmintic resistance of Haemonchus contortus,including a case of moxidectin resistance,in a Dutch sheep flock[J].Vet Rec,2013,173(22):1-2.
[64] WALLER P J,DASH K M,BARGER I,et al.Anthelmintic resistance in nematode parasites of sheep:Learning from the Australian experience[J].Vet Rec,1995,136(16):411-413.
[65] JIANG Y,ZHANG X M,MU H J,et al.Preparation and evaluation of injectable Rasagiline mesylate dual-controlled drug delivery system for the treatment of Parkinson's disease[J].Drug Deliv,2018,25(1):143-152.
[66] CAMPOS P P,FRACETO L F,FERREIRA M.Layer-by-layer films containing emodin or emodin encapsulated in liposomes for transdermal applications[J].Colloids Surf B:Biointerface,2018,162(1):69-75.
[67] RAJ R,RAJ P M,RAM A.Nanosized ethanol based malleable liposomes of cytarabine to accentuate transdermal delivery:formulation optimization,in vitro skin permeation and in vivo bioavailability[J].Artif Cells Nanomed Biotchnol,2018,46(S2):951-963.
[68] TOSATO M G,MAYA GIRN JV,MARTIN A A,et al.Comparative study of transdermal drug delivery systems of resveratrol:High efficiency of deformable liposomes[J].Mater Sci Eng C,2018,90(9):356-364.
