Effects of adding dexmedetomidine,fentanyl,and verapamil to 0.5% ropivacaine on onset and duration of sensory and motor block in forearm surgeries:a randomized controlled trial
2021-04-02NazaninHashemiHesameddinModirEsmailMoshiriAmirHosseinMoradiAmirAlmasiHashiani
Nazanin Hashemi,Hesameddin Modir,Esmail MoshiriAmir Hossein Moradi,Amir Almasi-Hashiani
1 Student research committee Arak University of Medical Sciences Arak,Iran
2 Anesthesiology Department Arak University of Medical Sciences Arak,Iran
3 Department of Orthopedic Surgery,Arak University of Medical Sciences,Arak,Iran
4 Department of Epidemiology,School of Health,Arak University of Medical Sciences,Arak,Iran
Abstract This study was aimed to compare the onset and duration of axillary block with ropivacaine 0.5% plus either dexmedetomidine,fentanyl,or verapamil in forearm surgeries.This double-blind clinical trial enrolled three equal-sized block-randomized groups of patients (n = 105)scheduled for hand and forearm surgery at Arak,Iran in 2019,who received:(i) ropivacaine (40 mL/0.5%) + dexmedetomidine (1 μg/kg),(ii)ropivacaine (40 mL/0.5%) + fentanyl (1 μg/kg),and (iii) ropivacaine (40 mL/0.5%) + verapamil (2.5 mg),respectively.We recorded some vital signs such as mean arterial pressure,heart rate and oxygen saturation,onset of complete sensory and motor block,duration of the block,opioid use,as well as pain score at recovery and certain time points (2,4,6,12,and 24 hours post-operation).Adding dexmedetomidine to ropivacaine (40 mL/0.5%) prolonged the duration of sensory (P = 0.001) and motor block (P = 0.001) in compared to adding fentanyl and verapamil and it also shortens the time to onset of sensory (P = 0.001) and motor block (P = 0.001).There is a significant difference between three groups in terms of visual analog scale mean and the lowest pain score was obtained in the dexmedetomidine group (P = 0.001),significant time trend (P = 0.001),as well as the time and groups interaction (P = 0.001).Dexmedetomidine was concluded to be associated with alleviated pain; reduced opioid use; short onset of sensory block; and prolonged duration of sensory and motor block.It hence is recommended to lengthen the duration of axillary block and to help relieve postoperative pain and ultimately to move to cut down the postoperative opioid use in forearm surgery.The study was approved by the Ethical Committee of Arak University of Medical Sciences (approval No.IR.ARAKMU.REC.1397.266),and registered on Iranian Registry of Clinical Trials (registration No.IRCT20141209020258N111) on May 9,2019.
Key words:axillary block; dexmedetomidine; fentanyl; forearm surgeries; motor block; ropivacaine; sensory block; verapamil; visual analog scale
INTRODUCTION
The axillary plexus block is proved to be a beneficial choice for anesthesia in hand and forearm surgeries through which epinephrine can normally be co-administered with local anesthetics to induce local anesthesia,whereas offering abundant benefits engendered by its vasoconstrictive effects,covering a prolonged block duration and a decrease in peak plasma level of local anesthetic,thereby lessening side effects associated with these.1,2A local block reduces the risks with general anesthesia which may be allied with adverse events,even death,in some patients while creating surgical conditions and faster patient mobility postoperatively,and besides diminishes hospital-associated costs.3,4Axillary plexus block is frequently employed for anesthesia in hand and forearm surgeries.5
The regional block involves the injection of a local anesthetic in the vicinity of a major nerve trunk.6Further drugs,like opioids,bicarbonate,adrenaline,and dexamethasone are repeatedly co-administered in combination with anesthetics to enhance the severity,quality,and to prolong duration of anesthesia in such blocks.7-9Postoperative pain may raise the cost of treatment,as well as the length of hospitalization.Anesthesiologists have studied many ways to prolong block duration with diverse local anesthetics.The prolonged duration of analgesia ensures the patient's comfort and convenience.The possibility of peripheral opioid receptors has led to the use of various drugs in local blocks to prolong the duration of analgesia without increasing side effects.Various studies with a range of local anesthetics and opioids have revealed quite different results.10,11
Dexmedetomidine is considered to be an α2-adrenergic agonist,with analgesic,sedative and antihypertensive properties9and can be effective,if added to local anesthetics during peripheral nerve block.12,13Axillary block technique is more widely used in forearm and hand surgeries due to its ease,safety,and reliability.Bangera et al.14reported that dexmedetomidine is useful as an adjuvant for faster anesthesia and longer duration of anesthesia,and besides improves hemodynamic changes during forearm and hand surgeries.
Fentanyl had a potent analgesic effect with rapid onset and short duration of action which is used to relieve pain from repair of skin lesions in emergency wards and reported as a μ-opioid receptor agonist.15While mainly used as a premedication and analgesic in the operating room,fentanyl is a potent opioid analgesic that is 80 times as potent as morphine,which was introduced to medicine in the 1960s as an intravenous anesthetic.Today,opioids are widely used for anesthesia as well as pain relief.15,16
Verapamil,a calcium-channel blocker,was used in regional block since the early 1990s and helps potentiate the analgesic effect of local anesthetics,based on the available studies17and the effects of opioids may be exacerbated by concurrent administration of calcium-channel blockers.18Various studies have investigated separately the efficacy of bupivacaine and ropivacaine as local anesthetics for regional block alone or with adjuvants such as fentanyl or dexmedetomidine.19,20But so far,no comparative study,like the recent one,has been reported.Hence,this study was designed to compare the onset and duration of axillary block with 0.5% ropivacaine plus either dexmedetomidine,fentanyl,or verapamil in forearm surgeries.
SUBJECTS AND METHODS
Study design
The double-blind parallel group clinical trial study included 105 patients aged 20–70 years with American Society of Anesthesiologist I–II21who met the inclusion/exclusion criteria,underwent forearm and hand surgery,and referred to Valiasr Hospital,Iran in 2019,after demonstrating accurate knowledge of the procedure and completing the informed consent.Ethical committee of Arak University of Medical Sciences approved this study in December 2018 (approval No.IR.ARAKMU.REC.1397.266).For all participants,written informed consent was obtained.
Subjects Inclusion criteria
Patients with American Society of Anesthesiologist I–II,20–70 years,of both genders,scheduled for forearm and hand surgery under axillary block,forearm or hand fracture,absence of more than one fracture in the body,no blood coagulation and prothrombin time,partial thromboplastin time,and international normalized ratio disorders,body mass index< 35 kg/m2,no psychological problems,no pregnancy,and no neurological disorders.
Exclusion criteria
Lack of patient cooperation to perform the block,allergy to treatment drugs,infection at the block area,failure of the block,surgery time > 150 minutes,and need for sedation more than scheduled in the plan,chronic pain syndrome.
Surgical preparation
Patient candidate for surgery was hospitalized one night before surgery and kept nothing by mouth for 8 hours,then taken to the operating room the following morning.The axillary block was performed with the patient after intravenous lines were established,he/she received 10 mL/kg crystalloid serum,and an anesthetist recorded baseline vital signs,including mean arterial pressure (by non-invasive blood pressure),heart rate,and oxygen saturation.The drugs were prepared by an anesthetist in each group and were administered for block,whereas a medical student collecting data as well as subjects were unaware of group allocation to ensure blinding.All patients were injected with 2 mg midazolam (Caspian Tamin Co.,Rasht,Iran) before the block.The patient was first placed in the supine position to perform the block.
The arm to be blocked was abducted at a 90° angle to the trunk and the elbow of the same limb was maintained in 90° flexion in the supine position,putting his/her hand back on the pillow.We then disinfected the axillary area with a povidone-iodine solution.After the axillary artery is touched,through which the 5-cm 24-G block needle was inserted,the exact location of axillary block was determined using a nerve stimulator and a needle block.After assuring the location of the needle block,and finally,stimulation at 0.5 mA with multiple injection technique,the syringe containing the block solution (local anesthetic with the adjuvant in each group)was attached,and then injection of the drugs after negative aspiration was performed.
Interventions
The patients,divided into three equal groups using balanced block randomization,received:dexmedetomidine group:ropivacaine (40 mL/0.5%; L.Molteni & C.dei F.Iii Alitti Societa di Esercizio S.p.A./Italy) plus dexmedetomidine (1 μg/kg;Exir Co.,Lorestan,Iran); fentanyl group:ropivacaine (40 mL/0.5%) plus fentanyl (1 μg/kg; Caspian Tamin Co.,Rasht,Iran); and verapamil group:ropivacaine (40 mL/0.5%) plus verapamil (2.5 mg; Sopharma Co.,Sofia,Bulgaria),respectively.To induce complete analgesia and to tolerate tourniquet pain,the intercostobrachial nerve was blocked by placing a skin wheal of local anesthetic,using 5 mL of 2% lidocaine local anesthetic in each group,subcutaneously.The volume of adjuvant was calculated and adjusted to 5 mL with normal saline in all three groups,and finally,the total volume of drug administered to the patient was 45 mL.After the block,we placed the patient’s arm into the adduction position to ensure appropriate analgesia and anesthesia and then a tourniquet is applied to a patient.
Evaluation
It should be noted that the patient was monitored intraoperatively for adverse events,such as bradycardia,hypotension,and arrhythmia,whereas any appropriate remedial action was taken in each event.Initially,time for administration of local anesthetic was recorded in each patient.Sensory block was evaluated using pin prick with a 22 G needle every 30 seconds.Patient response in sensory dermatome of the nerves of the upper extremity was evaluated as a 3-point scale:0:normal sensation,1:loss of sensation to pinprick (analgesia),2:loss of sensation to touch (complete anesthesia).
Motor block was assessed along a 3-point scale13for motor function by thumb abduction (radial nerve),thumb adduction(ulnar nerve),thumb opposition (median nerve),and elbow flexion (musculocutaneous nerve):0:normal motor function,1:reduced motor strength but able to move fingers,and 2:complete motor block.13
We recorded the onset of complete sensory and motor block and then the duration of the block in the groups as well as the time to first postoperative analgesic administration in the patient and overall dose of analgesic administered,and opioid use in each group 24 hours postoperatively and the results were compared between the three groups.Block failures were all recorded in groups.
Pain scores were measured using the visual analog scale at recovery,2,as well as,4,8,12 and 24 hours postoperatively,as followed:0 represents the lowest and 10,the highest.50 mg meperidine was administered intramuscularly and the administration time was recorded when the subject had visual analog scale > 4.22
Moreover,their complications were controlled:hypoxia(oxygen saturation < 92%) by supplemental oxygen administration and hypotension (blood pressure < 20% baseline) by crystalloid serum administration or sympathomimetic drug administration,if needed.Bradycardia (heart rate < 40 beats/min)was besides controlled with atropine 0.5 mg intravenously while recording any other complication,if existed,and taking remedial action if needed.The data were collected by an intern who had no awareness of the group allocation.
Statistical procedure Randomization
Permuted balanced block randomization method with block size 6 was used to dividing participants into groups.Concealment (non-predictability of random sequence) was also assured because of the block randomization method.

Figure 1:The participant’s flowchart.
Blinding
All required data was gathered and recorded by a senior medical student who had not informed of the patients groups; also patients were unaware of research group they were in.The adjutants were prepared by an anesthesiologist,and a resident performed spinal anesthesia.
Sample size
With considering alpha 5%,study power 80%,the minimum acceptable clinical change (δ) at the time to achieve the sensory block 15 minutes,and a standard deviation of 22,23the minimum required sample size was estimated to be 33 in each group and we assigned 35 patients in each group.Stata version 13 (Stata Corp.,College Station,TX,USA) was used to calculate the sample size.
Statistical analysis
To summarize the categorical and continuous variables frequency (percent) and mean ± standard deviation (SD) were used,respectively.One-way analysis of variance and repeated measure analysis of variance was used to compare the clinical outcome between groups.Stata version 13 was used to analyze the data at significant level less than 0.05.
RESULTS
Baseline characteristics of enrolled patients
Patient’s recruitment process was displayed in Figure 1.A total of 183 patients who were scheduled for forearm and hand surgery and referred to Valiasr Hospital in Arak,Iran were assessed for eligibility,out of whom 78 patients did not meet the inclusion criteria or declined to participate and finally,and 105 cases were allocated to three groups (35 cases in each group).Table 1 showed the baseline comparison of demographic and clinical characteristics among the three groups.Mean age of participants was 36.5 ± 6.3 years old and 49% of them were female.The body mass index,mean arterial pressure and heart rate at baseline were 25.6 ± 2.5,90.7 ± 8.7 and 89.0 ±7.8,respectively.
Sensory and motor block of enrolled patients
As shown in Table 2,the results suggested that adding ropivacaine (40 mL/0.5%) to dexmedetomidine prolonged the duration of sensory (P= 0.001) and motor block (P= 0.001)in comparison to adding fentanyl and verapamil and it also shortened the time to onset of sensory (P= 0.001) and motor block (P= 0.001).
Pain relief of enrolled patients
As seen in Table 3,repeated measure analysis of variance revealed that there was a significant difference among three groups in terms of visual analog scale,and the lowest pain score was obtained in the dexmedetomidine group (P= 0.001).The differences were significant in time trend (P= 0.001),as well as the time and group interaction (P= 0.001; Figure 2).
Opioid use in enrolled patients
Opioid use among groups was compared and the results suggested less opioid use in dexmedetomidine group than the others.Among dexmedetomidine,fentanyl and verapamil groups,24 (69%),33 (94%) and 34 (100%) patients needed opioid use (P= 0.001).
Side effects in enrolled patients
Side effects of the drugs were also evaluated between the three groups and there was one case of vertigo in verapamil group and three cases of vomiting in fentanyl group.However,eight patients in fentanyl group and six patients in verapamil group needed two doses of opioid use.
DISCUSSION
The main results of current study suggested that adding dexmedetomidine to ropivacaine (40 mL/0.5%) lengthened the duration of sensory and motor block in compared to adding fentanyl and verapamil and it shortens the time to onset of sensory and motor block and also the lowest post-operative pain score was observed in the dexmedetomidine group.In addition,the need for opioid use was lower in the dexmedetomidine group.Overall,one can suggest that dexmedetomidine(i) relieves pain,(ii) reduces opioid use,(iii) shortens the onset of sensory and motor block,and (iv) prolongs the length and duration of sensory and motor block.
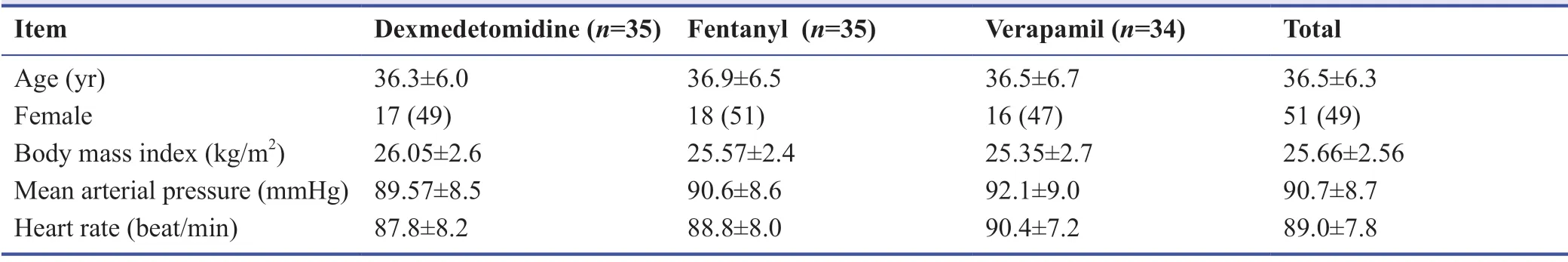
Table 1:Demographics and clinical characteristics at baseline among the forearm surgeries patients with 0.5%ropivacaine plus either dexmedetomidine,fentanyl,or verapamil axillary block
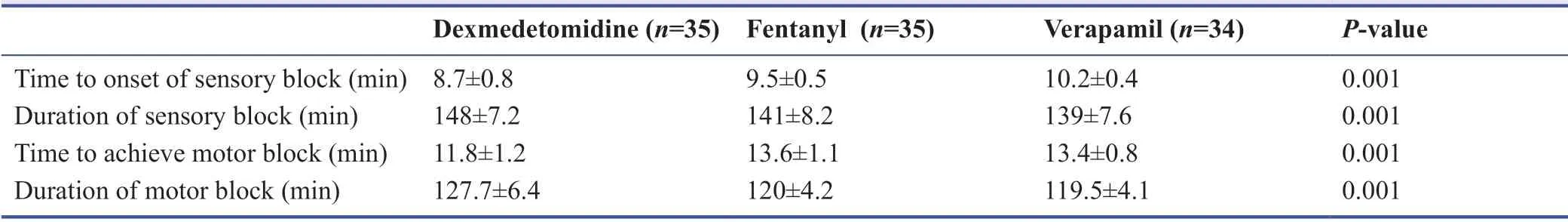
Note:Data are expressed as the mean ± SD,except female,which are expressed as number (percentage),and were analyzed by one-way analysis of variance.
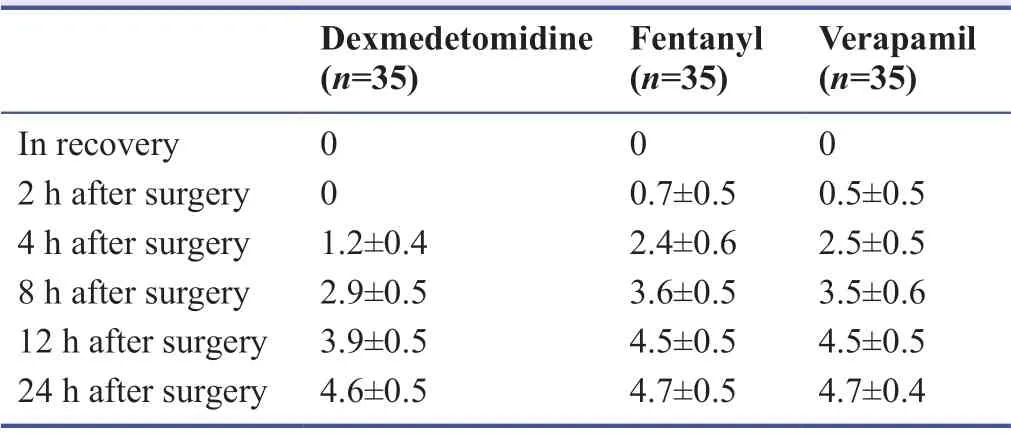
Note:Data are expressed as the mean ± SD,and were analyzed by repeated measure analysis of variance.
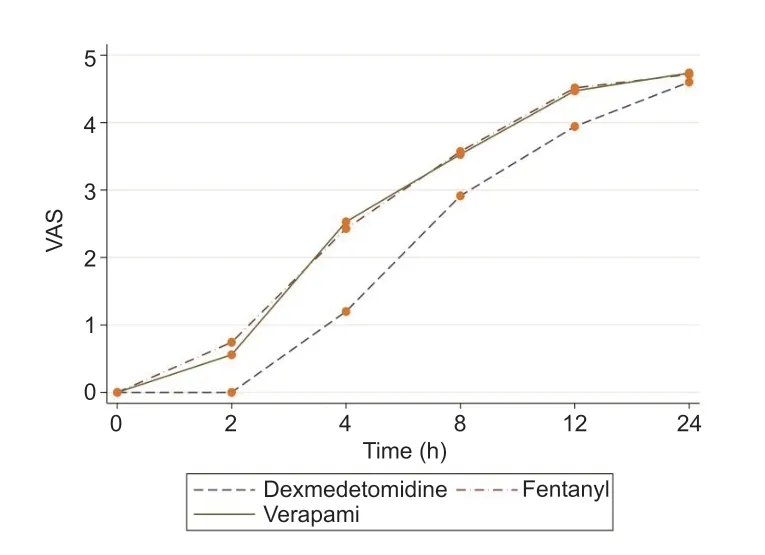
Figure 2:Comparison of pain scores among the forearm surgeries patients with 0.5% ropivacaine plus either dexmedetomidine,fentanyl,or verapamil axillary block.
Gerges’s study24comparing dexmedetomidine with verapamil as adjuvants to local anesthesia in intravenous anesthesia during upper limb orthopedic surgery reported that adding either of these produces faster onset of block,prolonged duration of sensory and motor block,and improvement of postoperative analgesia.Similarly,dexmedetomidine relieved pain,reduced opioid use,shortened the onset of sensory block,and prolonged the length and duration of sensory and motor block in our study where 1 μg/kg dexmedetomidine and 2.5 mg verapamil with ropivacaine were administered,while 0.5 μg/kg dexmedetomidine and 2.5 mg verapamil with lidocaine did in the Medhat’s trial.24Furthermore,the data were recorded up to 12 hours postoperatively in their study but it continued up to 24 hours in ours.
Bangera et al.14compared the effects of ropivacaine with/without dexmedetomidine in the axillary block,suggesting that adding dexmedetomidine to ropivacaine would cause faster,longer anesthesia and could be used in the forearm and/or hand surgeries,whose results were consistent with ours.Das et al.25conducted a study to compare the efficacy of dexmedetomidine and clonidine in hand surgery under axillary block,reporting that adding dexmedetomidine to local anesthetic in the block prolonged the duration of sensory and motor block and reduced overall analgesic use without any adverse effects and providing results consistent with ours.The 2014 study by Zhang et al.26to evaluate the duration of axillary block following infusion of dexmedetomidine with ropivacaine reflected that the adjuvant was effective in prolonging the duration of axillary block,if added,though complications such as bradycardia and hypotension,and hypertension may be observed and whose results regarding block quality are consistent with ours in which side effects of taking medication did not occur.
Chavan et al.27launched a study to assess the efficiency of adding fentanyl to local anesthetic in brachial block on the duration of analgesia,concluding that the duration is prolonged by adding fentanyl,but with no increase in side effects.Similarly,dexmedetomidine in our study relieved pain,reduced opioid use,shortened the onset of sensory block,and prolonged the length and duration of sensory and motor block.The findings of Alebouyeh et al.’s28study in 2009 conducted to assess the effect of adding different doses of verapamil to lidocaine 1.5% for sensory and motor axillary block demonstrated that verapamil 10 mg could be used in combination with lidocaine in patients receiving axillary anesthesia by prolonging the duration of sensory and motor block and postoperative analgesia,while had no similar hemodynamic changes induced by verapamil 5 mg.As mentioned earlier,dexmedetomidine affects pain scores,opioid use,the onset of sensory block,and the length and duration of sensory and motor block during our study where verapamil 2.5 mg with ropivacaine was used versus verapamil-lidocaine in theirs.
Karakaya et al.’s29study,aimed at adding fentanyl to bupivacaine to prolong the duration of anesthesia and axillary block,suggested that adding fentanyl 100 μg/mL to bupivacaine 0.25% doubled the duration of the axillary block compared to bupivacaine alone.In our study,dexmedetomidine was associated with decreased pain and opioid use,short onset of sensory block,and prolonged length and duration of sensory and motor block.
There were some limitations in this study.The current study was conducted on an Iranian sample.To better conclusion,it is recommended to perform a similar study in different population.However,some multicenter trials in recommended.
In summary,dexmedetomidine in compared to fentanyl and verapamil (i) relieves the pain,(ii) reduces the opioid use,(iii) shortens the onset of sensory and motor block,and (iv)prolongs the length and duration of sensory and motor block.Consequently,dexmedetomidine is recommended to prolong the duration of axillary block,to help relieve postoperative pain,and ultimately to lessen postoperative opioid use during forearm surgery.
Acknowledgements
We would like to express deep gratitude to the Clinical Research Council at Valiasr Hospital for their guidance,as well as the research deputy of Arak University of Medical Sciences for his assistance and support.
Author contributions
NH,HM,AAH,AHM and EM conceived the study.NH,EM,AHM and HM collected the data.All authors contributed equally to draft the manuscript.AAH and HM analyzed the data and all authors revised the manuscript and approved the final version.
Conflicts of interest
All the authors have no conflict of interest to declare.
Financial support
This study was funded by Arak University of Medical Sciences.
Institutional review board statement
The study was approved by Ethical Committee of Arak University of Medical Sciences in December 2018 (approval No.IR.ARAKMU.REC.1397.266),and registered on Iranian Registry of Clinical Trials (registration No.IRCT20141209020258N111) on May 9,2019.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms.In the form the patients have given their consent for patients images and other clinical information to be reported in the journal.The patients understand that their names and initials will not be published.
Reporting statement
The writing and editing of the article were performed in accordance with the CONsolidated Standards Of Reporting Trials (CONSORT)Statement.
Biostatistics statement
The statistical methods of this study were reviewed by the epidemiologist of Arak University of Medical Sciences,Iran.
Copyright transfer agreement
The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement
The data could be shared if requested but the patients completed the informed consent.
Plagiarism check
Checked twice by iThenticate.
Peer review
Externally peer reviewed.
Open access statement
This is an open access journal,and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
杂志排行
Medical Gas Research的其它文章
- Sevoflurane versus halothane for induction of anesthesia in pediatric and adult patients
- Tuberculosis incidence in area with sulfur dioxide pollution:an observation
- Electrolytic hydrogen-generating bottle supplies drinking water with free/combined chlorine and ozone repressed within safety standard under hydrogen-rich conditions
- The role of hyperbaric oxygen therapy in inflammatory bowel disease:a narrative review
- Recent advances in the protective role of hydrogen sulfide in myocardial ischemia/reperfusion injury:a narrative review
