Sevoflurane versus halothane for induction of anesthesia in pediatric and adult patients
2021-04-02GouriKangralkarParbatiBaburaoJamale
Gouri Kangralkar,Parbati Baburao Jamale
Department of Anesthesiology,Krishna Institute of Medical Sciences (Deemed to be) University,Karad,Maharashtra,India
Abstract Induction of anesthesia using an inhalation agent remains a fundamental technique due to its rapid induction and emergence.Sevoflurane is preferred over halothane for its faster induction of anesthesia and lesser complications.Studies on sevoflurane in pediatrics have established it as safe and effective.However,its effectiveness in adults is very limited.Hence,this study was conducted to compare the induction and intubating conditions,hemodynamic profiles,and emergence from anesthesia with sevoflurane and halothane in adults and pediatric patients.This randomized clinical study was carried out for a period of 2 years (November 2006–September 2008) in the Anesthesiology Department of a Krishna Institute of Medical Sciences (Deemed to be) University.Eighty patients of American Society of Anesthesiologists Class I and II were randomly assigned to halothane group and sevoflurane group with 40 patients in each group.Patients were induced and intubated with increasing concentrations of halothane from 0.5% to 5% and sevoflurane 1% to 7% in 50% nitrous oxide and 50% oxygen mixture.Recordings of vitals including induction and intubation time,recovery characteristics,and recovery and discharge time was also recorded.There was a statistically significant difference between sevoflurane and halothane in the induction and intubation time indicating that sevoflurane had faster induction and shorter intubation time compared to that of halothane.Patients in halothane group had more incidence of coughing,intolerance,salivation,breathe holding,rigidity,and movement as compared to sevoflurane group.The mean time to consciousness,response to verbal commands,orientation,and recovery room discharge time was significantly shorter in sevoflurane group as compared to halothane group.Sevoflurane can be a suitable alternative to halothane for induction of anesthesia in patients with a shorter induction and intubation time with better hemodynamic stability.This study was approved by the Institutional Ethics Committee (KIMSDU/IEC-307/028/14/11/2006).
Key words:anesthesia; arterial pressure; consciousness; halothane; heart rate; hemodynamics; intubation; oxygen; salivation; sevoflurane
INTRODUCTION
The main objective of anesthesia is to facilitate surgery with minimal risk to the patient and to ensure smooth induction and optimal recovery after the surgical procedure.Anesthesia induction and recovery are mainly influenced by the choice of the volatile agent.Agents with lower blood gas solubility showed faster times of induction and recovery.1-3A perfect inhaled anesthetic agent should have less blood gas solubility,negligible pungency,minimal respiratory irritation,withstand metabolic and physical degradation,and be easily available.4Various researches have been conducted by improving the quality of drugs,instruments,and different procedures to impart a smooth induction of anesthesia and for better operative conditions.The most widely used volatile inhalational anesthetics include nitrous oxide,desflurane,isoflurane,halothane,enflurane,etc.Among these,halothane is often used as an anesthetic agent in pediatrics.It is preferred for its relatively nonirritant nature and smooth and rapid induction in children.5However,it has disadvantages like myocardial depression,cardiac arrhythmias,hepatitis,and malignant hyperthermia.6,7Sevoflurane,a substitute to halothane,was introduced because it has minimal cardiac and hepatic side effects and the induction time was lesser.8It has a lower blood solubility which permits for faster recovery and undergoes minimal metabolism.9
The anesthesiologists generally choose anesthetic techniques associated with a smooth and faster induction and smooth,uneventful postoperative recovery.Although the use of halothane is limited in tertiary care centers,studies are conducted with halothane as an induction agent in smaller centers.5,10Most of the studies related to induction,recovery characteristics,and hemodynamic variability of halothane and sevoflurane are conducted in pediatrics.6,11-13Pediatric patients require unique anatomic,physiologic,and pharmacologic considerations compared to adults for the management of anesthesia.Age-related changes could result in various alterations in the body which could have clinical implications for different stages of anesthesia.The current study was conducted in pediatric patients and adults in order to observe the impact of anesthetic agents in various age groups.To our knowledge,this could be the first study carried out in patients of all age groups to compare the induction characteristics,intubating conditions,hemodynamic profiles,and emergence from anesthetic effect with halothane and sevoflurane in adults and children.
SUBJECTS AND METHODS
This randomized,clinical,comparative study was carried out for a period of 2 years (November 2006–September 2008)in the Anesthesiology Department of a tertiary care teaching hospital (viz.Krishna Institute of Medical Sciences [Deemedto-be] University) in Karad,Maharashtra,India post approval from the Institutional Ethics Committee (KIMSDU/IEC-307/028/14/11/2006).A prior written informed consent was taken from the subjects and their parents (in case of children).Calculation of sample size was based on the induction time for sevoflurane and halothane with 5% level of significance and 85% power to obtain a final sample size of 40 subjects per group.14
Selection criteria
A total of 40 patients of either gender,undergoing general anesthesia and belonging to American Society of Anesthesiologists Class I and II were included.15Patients with allergy or sensitivity to either halothane or sevoflurane and those with known or suspected to develop malignant hyperthermia were excluded.Patients with prior history of any bleeding disorder,known respiratory,cardiovascular,neurological or gastrointestinal ailments were also exempted from the study.
Sample randomization and intervention
A total of 80 patients were randomly (by computer-generated program using a block size of 6) assigned to halothane and sevoflurane groups with 40 patients in each.Preanesthetic evaluation (which includes evaluation of co-morbidities,breathing difficulty,blood hemoglobin,details of previous anesthesia and surgeries,fluid status,and physical status) was done a day before the proposed surgery in case of elective surgeries along with routine investigations.Baseline (pre-induction)pulse rate,electrocardiogram,blood oxygen saturation levels,and non-invasive blood pressure were measured post shifting of patients to the operation theater.The flow of participants through the study is presented in Figure 1.

Figure 1:Consort flow chart.
Inhalation induction of anesthesia was accomplished in all patients and preoxygenation with 100% oxygen was done for 3 minutes.Patients were induced with 50% nitrous oxide and 50% oxygen mixture with gradually increasing concentrations of the volatile anesthetic halothane or sevoflurane through respective vaporizers.Halothane group patients were intubated with incremental increase in the concentration of halothane from 0.5% to 5% in 50% nitrous oxide and 50% oxygen mixture.Sevoflurane group patients were induced and intubated with gradually increasing concentration of sevoflurane from 1% to 7% in nitrous oxide and oxygen (50:50) mixture.After loss of eyelash reflex,endotracheal intubation was carried out.The patients were maintained on balanced anesthesia technique with the use of muscle relaxant.They were continued to be maintained on nitrous oxide and oxygen (50:50) mixture with 0.5% to 1% halothane or 1% to 2% sevoflurane till the end of the surgical procedure.During the final surgical procedure,100% oxygen was given,and reversal of neuromuscular blockade done with neostigmine and glycopyrrolate.
Electrocardiogram was monitored throughout the procedure for the presence of any type of cardiac arrhythmias.Also,the first post-operation induction time,intubation time,analgesic demand and recovery characteristics,and discharge time were noted.Adverse events,such as excitatory movements,coughing,intolerance,salivation,laryngospasm,vomiting,breath holding,rigidity,and shivering were noted with graded severity (0:none; 1:mild; 2:moderate; 3:severe).16Assessment of quality of intubating conditions was performed using Viby-Mogensen Scale based on patency of vocal cords,ease of laryngoscopy,jaw relaxation,coughing/bucking,and limb movement.17Overall intubating conditions were defined as excellent,good,or poor based on the nature of the intubation characteristics.
Outcome measures Primary outcomes
Mean induction time and induction characteristics:This was assessed based on the time interval between the placement of face mask and loss of eyelash reflex.
Mean intubation time and intubation characteristics:This was assessed based on the time interval between the placement of face mask and centrally placed mid-dilated pupils.
Secondary outcomes
Mean heart rate (HR),blood oxygen saturation levels (3800 Pulse Oximeter; GE Healthcare Private Limited,Mumbai,India),and mean arterial pressure (MAP; B20 Patient Monitor,GE Healthcare Private Limited):This was assessed based on the time during induction,at intubation,and immediately after intubation at 1,2,3,4,and 5 minutes,and during maintenance and continued till emergence.
Emergence:This was measured based on the time of discontinuation of nitrous oxide and potent agent to eye opening on command.Time to attaining discharge and recovery profile was recorded.
Statistical analysis
Data was analyzed using statistical software R version 3.6.3(R Foundation for Statistical Computing,Vienna,Austria).Categorical variables are represented by frequency tables,and continuous variables are presented as the mean ± standard deviation (SD).Statistical analysis of data was performed using Student’s unpairedt-test,Chi-square test,and correlation test.Comparison of induction,intubation,and emergency characteristics between the groups was analyzed using Chi-square test with Monte Carlo stimulation and Mann-WhitneyUtest.APvalue of < 0.05 was regarded statistically significant.
RESULTS
Demographics of pediatric and adult patients under halothane and sevoflurane induction
The frequency distribution of basic demographic variables is presented in Table 1.Majority of the patients were males(52%) in halothane group and females (58%) in sevoflurane group,who were < 15 years old in both groups (halothane group:68%; sevoflurane group:45%).Most of the patients in halothane group (58%) and sevoflurane group (68%) belonged to American Society of Anesthesiologists I category.
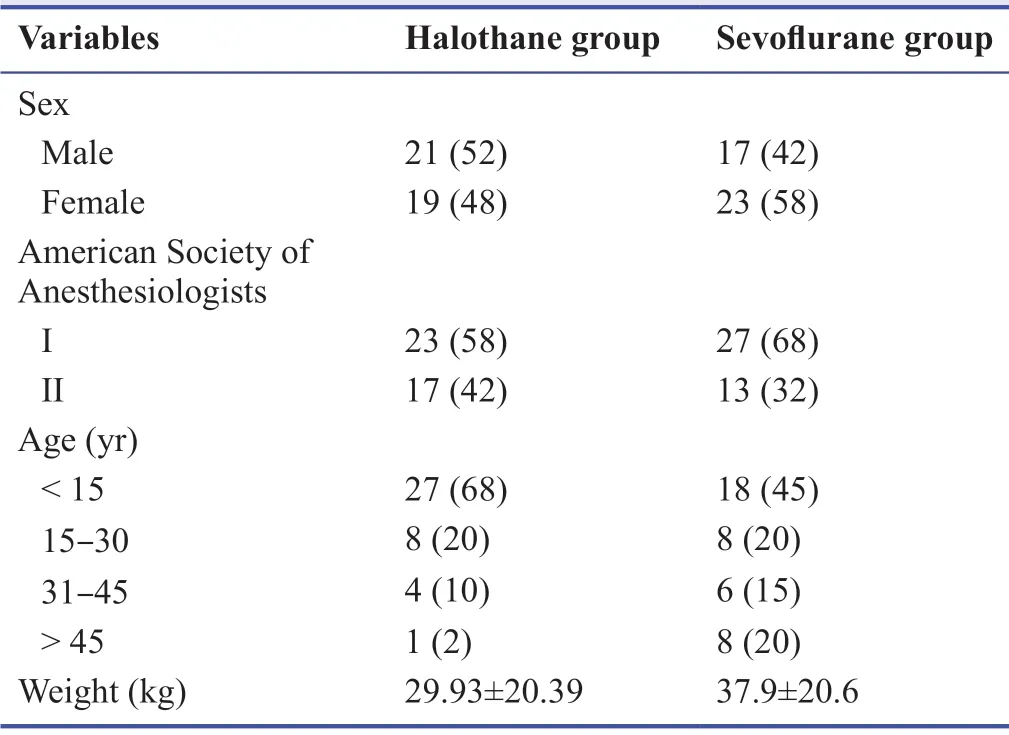
Table 1:Demographic characteristics of pediatric and adult patients under halothane and sevoflurane induction
Induction and intubation in pediatric and adult patients under halothane and sevoflurane induction
There were significant differences in induction time and intubation time between sevoflurane and halothane groups(P< 0.001) indicating that sevoflurane had faster induction and shorter intubation time compared to that of halothane(Table 2).
Patients in the halothane group had increased incidence of coughing,intolerance,salivation,breath holding,rigidity,and movement as compared to sevoflurane.However,the difference was not statistically significant (Table 3).
With regard to intubation characteristics between the groups,no statistically significant difference was observed between halothane and sevoflurane groups (Table 4).
Hemodynamic responses of pediatric and adult patients under halothane and sevoflurane induction
The distributions of mean HR and MAP at different time points are depicted in Figure 2.Induction of anesthesia in the halothane group resulted in a progressive decrease in the HR compared to basal value,whereas there was no significant variation in the HR in the sevoflurane group (P= 0.076).After intubation,both the groups observed a gradual increase in the HR.With regard to MAP,induction of anesthesia resulted in a progressive decrease of MAP in both the groups whereas,after intubation,an increase in MAP was observed in both the groups.
Emergency and recovery characteristics of pediatric and adult patients under halothane and sevoflurane induction
Table 5 presents the comparison of emergency and recovery characteristics between the groups.The mean time to consciousness,response to verbal commands,orientation,and for discharge from recovery room was shorter in the sevoflurane group as compared to halothane group (P< 0.001).There was no significant difference in the total time of inhalation,incidence of agitation,first post-operation analgesic demand,retching or vomiting,and shivering between the two groups.

Figure 2:Trend of mean heart rate and arterial pressure of pediatric and adult patients under halothane and sevoflurane induction.
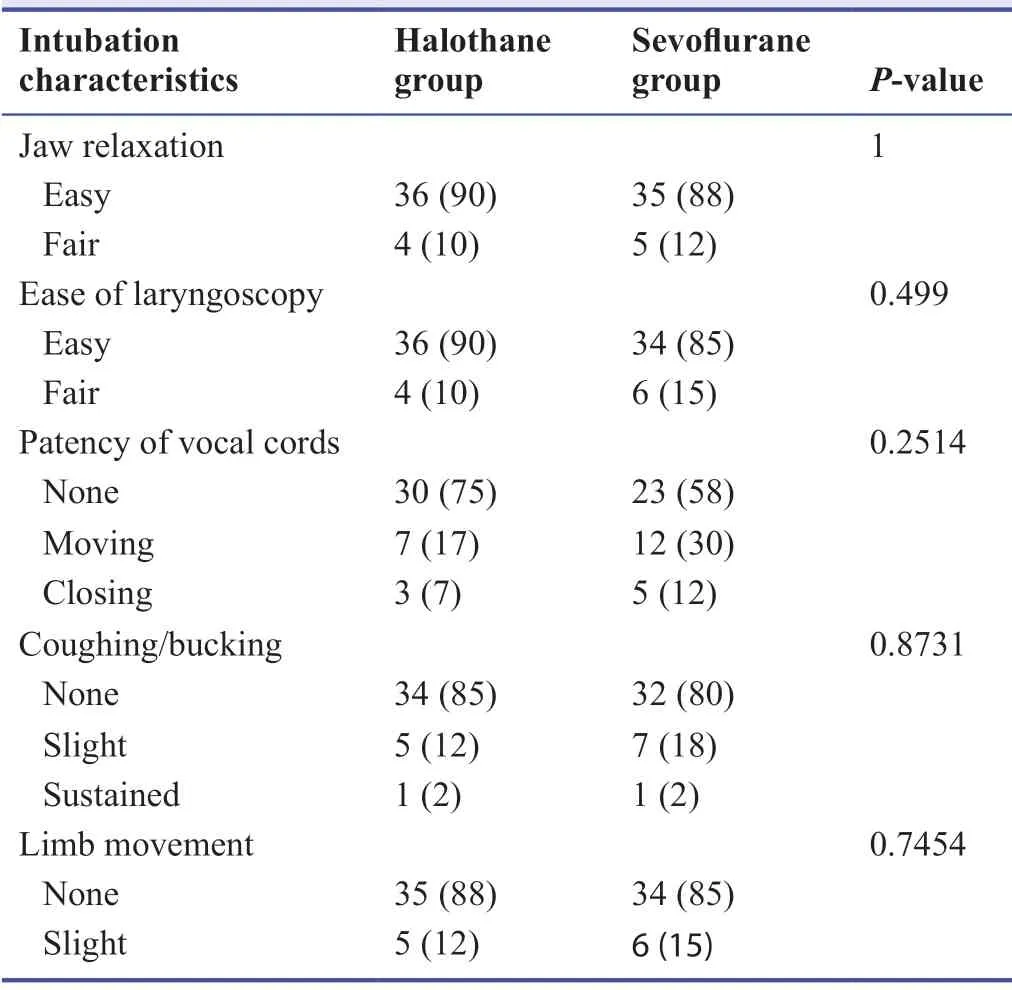
Table 4:Comparison of intubation characteristics of pediatric and adult patients under halothane and sevoflurane induction
DISCUSSION
Inhalational induction of anesthesia is one of the common methods of induction employed in medical practice.This comparative study was conducted in healthy adult and pediatric patients to evaluate the induction of anesthesia,maintenance,and emergence from anesthetic effect with sevoflurane and halothane.Overall induction and intubation characteristics were observed to be better in the sevoflurane group as compared to halothane similar to the study conducted by Ravi et al.18
In the present study,the mean induction time of halothane was significantly more as compared to sevoflurane group.Shareena et al.19reported the mean induction time to be significantly more than double in the halothane group as compared to sevoflurane.Bhaskar et al.5also reported a statistically significant difference in the induction time between halothane and sevoflurane groups.Faster induction time of sevoflurane could be attributed to low potency of sevoflurane due to low blood and lipid solubility as compared to halothane which has high potency due to its high blood and lipid solubility.
In terms of intubation time,halothane demonstrated significantly more intubation time as compared to sevoflurane.These results are in accordance with the study conducted by Bhaskar et al.5with doses similar to the current study’s in children.Another study conducted by Redhu et al.13in pediatrics also reported similar results with similar doses as in the current study with significantly shorter intubation time in the sevoflurane group as compared to halothane group.Paris et al.20suggested that the limitation of sevoflurane’s induction time could be a function of the vaporizer.The maximum concentration of anesthetics that could be delivered were 5%halothane and 8% sevoflurane which is in line with our study.In addition,sevoflurane would have had greater anesthetic depth which could have resulted in slower uptake of vapor compared to halothane.21
With regard to the HR,the present study reported increased HR in both the study groups after intubation.However,the difference was found to be statistically insignificant.In contrast,a study conducted by Shareena et al.19reported a significant difference in the mean elevated HR between halothane and sevoflurane groups during induction.Wadud et al.22reported a significant reduction in the HR in halothane group,whereas no changes were noted in sevoflurane group during the induction period.An increase in MAP was observed in both the groups after intubation although it was not found to be statistically significant.Wadud et al.22in a study reported significant decrease in MAP in halothane group,while no significant changes were observed in sevoflurane group.The hemodynamic results in the current study demonstrated that the stability of HR provided by sevoflurane was desirable as it neither increased the myocardial oxygen consumption nor decreased the time available for myocardial perfusion.
At the time of emergence from anesthesia,recovery was significantly faster in the sevoflurane group compared to halothane.Similar results were reported by Redhu et al.13with significantly rapid recovery in the sevoflurane group compared to halothane group.Also,time to respond to verbal commands,consciousness,and orientation time were shorter for sevoflurane as compared to halothane which was in accordance to the study conducted by Walker et al.23The faster recovery and precise control of sevoflurane over delivery of anesthesia could be attributed to its lower blood and lipid solubility.18
The use of sevoflurane guaranteed a faster emergence from anesthetic state.It also enabled faster recovery and met discharge criteria significantly earlier compared to halothane.Also,sevoflurane showed better hemodynamic stability.However,the limitation of our present study was the small sample size; hence,findings of our study are limited in terms of its generalizability.Hence,a larger number of subjects need to be incorporated in future studies to re-emphasize our results of sevoflurane as an effective agent for inhalational induction of anesthesia with low incidence of airway-related complications.
Sevoflurane provides a rapid and smooth induction with effective intubating conditions.Therefore,it can be considered as a suitable alternative to halothane for inhalational induction of anesthesia in children and adults.However,more studies in future are recommended for evaluation of efficacy of sevoflurane in comparison to other anesthetic agents.
Author contributions
Study design,implementation,and manuscript writing:GK,PBJ;data analysis:GK.Both authors approved the final version of the manuscript.
Conflicts of interest
There are no conflicts of interest to declare.
Financial support
This research received no specific grant from any funding agency in the public,commercial,or any profit sectors.
Institutional review board statement
This research was conducted after attaining ethical clearance (KIMSDU/IEC-307/028/14/11/2006) from the Institutional Ethics Board of Krishna Institute of Medical Sciences (Deemed to be) University in Karad,Maharashtra,India.
Declaration of patient consent
The authors certify that they have obtained patients and their parents’(in case of children) consent forms.In the form,patients and their parents have given their consent for patients’ images and other clinical information to be reported in the journal.The patients and their parents(in case of children) understand that patients’ names and initials will not be published and due efforts will be made to conceal their identity.
Reporting statement
The writing and editing of the article were performed in accordance with the CONsolidated Standards Of Reporting Trials (CONSORT)Statement.
Biostatistics statement
The statistical methods of this study were conducted and reviewed by the biostatistician of Krishna Institute of Medical Sciences (Deemed to be) University,India.
Copyright transfer agreement
The Copyright License Agreement has been signed by both authors before publication.
Data sharing statement
Datasets analyzed would be provided on reasonable request.
Plagiarism check
Checked twice by iThenticate.
Peer review
Externally peer reviewed.
Open access statement
This is an open access journal,and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
杂志排行
Medical Gas Research的其它文章
- Tuberculosis incidence in area with sulfur dioxide pollution:an observation
- Electrolytic hydrogen-generating bottle supplies drinking water with free/combined chlorine and ozone repressed within safety standard under hydrogen-rich conditions
- Effects of adding dexmedetomidine,fentanyl,and verapamil to 0.5% ropivacaine on onset and duration of sensory and motor block in forearm surgeries:a randomized controlled trial
- The role of hyperbaric oxygen therapy in inflammatory bowel disease:a narrative review
- Recent advances in the protective role of hydrogen sulfide in myocardial ischemia/reperfusion injury:a narrative review
