Effects of Early- and Late-Sowing on Starch Accumulation and Associated Enzyme Activities During Grain Filling Stage in Rice
2021-03-20WangWentingCuiWenpeiXuKeGaoHuiWeiHaiyanZhangHongcheng
Wang Wenting, Cui Wenpei, Xu Ke, Gao Hui, Wei Haiyan, Zhang Hongcheng
Research Paper
Effects of Early- and Late-Sowing on Starch Accumulation and Associated Enzyme Activities During Grain Filling Stage in Rice
Wang Wenting1, 2, Cui Wenpei1, 2, Xu Ke1, 2, Gao Hui1, 2, Wei Haiyan1, 2, Zhang Hongcheng1, 2
()
The environmental temperature occurring during the grain filling stage is an important factor affecting starch synthesis and accumulation in rice. We investigated starch accumulation, amylase activity and starch granule size distribution in two low-amyloserice varieties, Nanjing 9108 and Fujing 1606, grown in the field at different filling temperatures by manipulating sowing date. The two rice varieties exhibited similar performances between two sowing dates. Total starch, amylose and amylopectin contents were lower at the early-filling stage of T1 treatment (Early-sowing) compared with those at the same stage in T2 treatment (Late-sowing). In contrast, at the late-filling stage, when field temperatures were generally decreasing, total starch and amylopectin contents in T1 were higher compared to those in T2. The ideal temperature for strong activity of ADP-glucose pyrophosphorylase and soluble starch synthase was about 22 ºC. A higher temperature from the heading to maturity stages in T1 increased the activities of starch branching enzyme and suppressed the activities of granule bound starch synthetase and starch debranching enzyme. We found that rice produced larger-sized starch granules under the T1 treatment. These results suggested that due to the early-sowing date, the high temperature (30 ºC) occurring at the early-filling stage hindered starch synthesis and accumulation, however, the lower temperatures (22 ºC) at the late-filling stage allowed starch synthesis and accumulation to return to normal levels.
rice; grain filling; starch accumulation; starch synthesis enzyme; starch granule size; temperature
Rice is one of the main food crops consumed worldwide, especially in Asian countries. Therefore, increasing yield and improving rice grain quality are the most important goals for rice breeders. Rice starch, which comprises more than 90% of the weight of polished rice grains, is an important factor affecting yield and quality (Syahariza et al, 2013). Starch primarily consists of two branched glucose polymers, amylopectin and amylose. Amylose is primarily a linear molecule composed of glucose units linked by α-1,4-glycosidic bonds, and amylopectin is a highly- branched glucose linked by α-1,6-glycosidic bonds, and both are considered determinants of rice eating and cooking quality (Chung et al, 2011; Lin et al, 2011;Gilbert et al, 2013). In the process of starch accumulation,more than 30 major enzymes participate in the metabolism of carbohydrates during endosperm development in rice. Among them, five enzymes play important roles in this process, which are ADP-glucose pyrophosphorylase (AGPase), granule bound starch synthetase (GBSS), soluble starch synthase (SSS), starch branching enzyme (SBE) and starch debranching enzyme (DBE) (Yang et al, 2001; Hannah and James, 2008; Chen and Bao, 2017). The main function of AGPase is to transform hexose, formed after sucrose decomposition, into adenosine diphosphate glucose (ADPG), a starch synthesis substrate. This is the first and limiting step of the starch synthesis reaction (Tuncel et al, 2014). Increasing AGPase activity can regulate starch synthesis and increase seed weight (Smidansky et al, 2003; Tuncel and Okita, 2013). GBSS is mainly responsible for the synthesis of amylose, SSS mainly synthesizes amylopectin, SBE catalyzes the formation of α-1,6- glycoside bond, while DBE hydrolyzes α-1,6-glycoside bond (Satoh et al, 2003; Fujita et al, 2009; Jeon et al, 2010). The activities of these five enzymes are closely related to total starch, amylose and amylopectin accumulation in rice endosperm.
Enzymes involved in starch accumulation are not only affected by genotype but also the growing environment of rice (Halford et al, 2015; Mayer et al, 2016). Many previous studies found that abiotic factors such as fertilizer application, soil moisture and environmental temperature can affect enzyme activity and subsequently, change total starch, amylose and amylopectin contents (Sun et al, 2018; Cheng et al, 2019; Prathap et al, 2019). Among these factors, enzyme activities are reportedly very sensitive to environmental temperature from the heading to maturity stages (Cao et al, 2015). Moreover, increasing temperatures due to global climate change will likely affect plant enzyme activity and starch content (Sacks et al, 2010). Cheng et al (2019) concluded that high, medium and low daily average temperatures of 31 ºC, 26 ºC and 21 ºC can significantly affect starch synthetase activity and consequently, affect starch accumulation. Xia et al (2016) found that a low temperature during the grain filling stage can decrease the activities of SSS and SBE. Ahmed et al (2015) found that the activity of SBE in rice endosperm increases at the early grain filling stage but decreases thereafter by heat stress.
Although many studies have investigated changes in starch accumulation in response to different environmental temperatures during grain filling, results are not consistent. Also, most of those studies were carried out in controlled greenhouse. Greenhouse studies are unlikely to accurately imitate long-term rice cultivation in the field (Patindol et al, 2015). Thus, we conducted a field study to investigate the effects of environmental temperature on rice starch accumulation and enzymes associated with starch accumulation. Moreover, because of the gap of knowledge in the literature and the importance of the stage in rice production, we examined temperature effects on field- grown rice at the grain filling stage.We selected two rice varieties with low-amylose content, Nanjing 9108 (NJ9108) and Fujing 1606 (FJ1606), because they have been bred and widely cultivated (especially in Jiangsu Province, China in recent years) for their good eating and cooking quality (Wang et al, 2009). The overall objective of this study was to examine the effect of high temperatures during the early-filling stage on starch accumulation and the activities of five enzymes associated with starch accumulation in two low-amylose rice varieties grown in field conditions.
RESULTS
Starch accumulation
Total starch contents of NJ9108 and FJ1606 increased rapidly within 25 DAH (days after heading) and increased slowly from 40 DAH to maturity (Fig. 1). Total starch contents of the two varieties in T1 treatment (sowing date was 10 May, 2018) were initially lower than those in T2 treatment (sowing date was 21 June, 2018) within 20 DAH, and then the contents were nearly the same between the two treatments at 25 DAH. Beyond 25 DAH, total starch contents in T1 were higher compared with those in T2 in the both varieties.At 55 DAH, total starch contents of NJ9108 and FJ1606 were 5.6% and 4.2% higher in T1 than in T2, respectively. Amylose contents of NJ9108 and FJ1606 increased rapidly within 35 DAH, and increased slowly from 40 DAH to maturity. Amylose contents of NJ9108 and FJ1606 in T1 were 22.7% and 11.8% lower than those of the corresponding varieties in T2 at 55 DAH. Amylopectin contents of the two varieties increased rapidly within 25 DAH, and increased slowly from 35 DAH to maturity. Amylopectin contents of the two varieties in T1 were initially lower than those in T2 within 20 DAH, and then were nearly the same at 25 DAH. After 25 DAH, amylopectin contents in T1 were higher compared with those in T2. At the end of sampling times, amylopectin contents of NJ9108 and FJ1606 in T1 were 9.5% and 6.4% higher than those in T2. This pattern of amylopectin accumulation as affected by different temperature treatments was very similar to that of total starch accumulation (Fig. 1).
Key enzyme activities in starch synthesis
Activities of AGPase and SSS first increased and then decreased after heading (Fig. 2).Of both varieties, AGPase and SSS activities in T1 were lower than respective activities in T2 within 25 DAH, but higher after 30 DAH. The highest AGPase and SSS activities of NJ9108 and FJ1606 occurred in T1 were 13.8% and 9.8% higher than those in T2, respectively. The highest SSS activities of NJ9108 and FJ1606 also occurred in T1 and were 4.3% and 3.6% higher than those in T2, respectively.
Fig. 1. Total starch, amylose and amylopectin accumulation during grain filling stage of two rice varieties sown at different dates.
NJ9108, Nanjing 9108; FJ1606, Fujing 1606;T1, Rice seeds were sown at the early-sowing date (10 May, 2018); T2, Rice seeds were sown at the late-sowing date (21 June, 2018).
Data are Mean ± SD (= 3). *,< 0.05.
Activities of GBSS and DBE initially increased and then decreased after heading (Fig. 2). In the both varieties, GBSS and DBE activities peaked at 30 DAH. Additionally, GBSS and DBE activities of the both varieties in T1 were lower than those in T2 from heading to maturity. The highest levels of GBSS activity of NJ9108 and FJ1606 in T1 were 23.6% and 6.7% lower than those in T2, and the highest levels of DBE activity of the two varieties in T1 were 26.8% and 14.3% lower than those in T2, respectively. The pattern of SBE activity increased and then decreased (Fig. 2). The SBE activities in the both varieties in T1 were higher than those in T2 from heading to maturity. The highest SBE activities of NJ9108 and FJ1606 in T1 were 40.3% and 29.2% higher than those in T2, respectively.
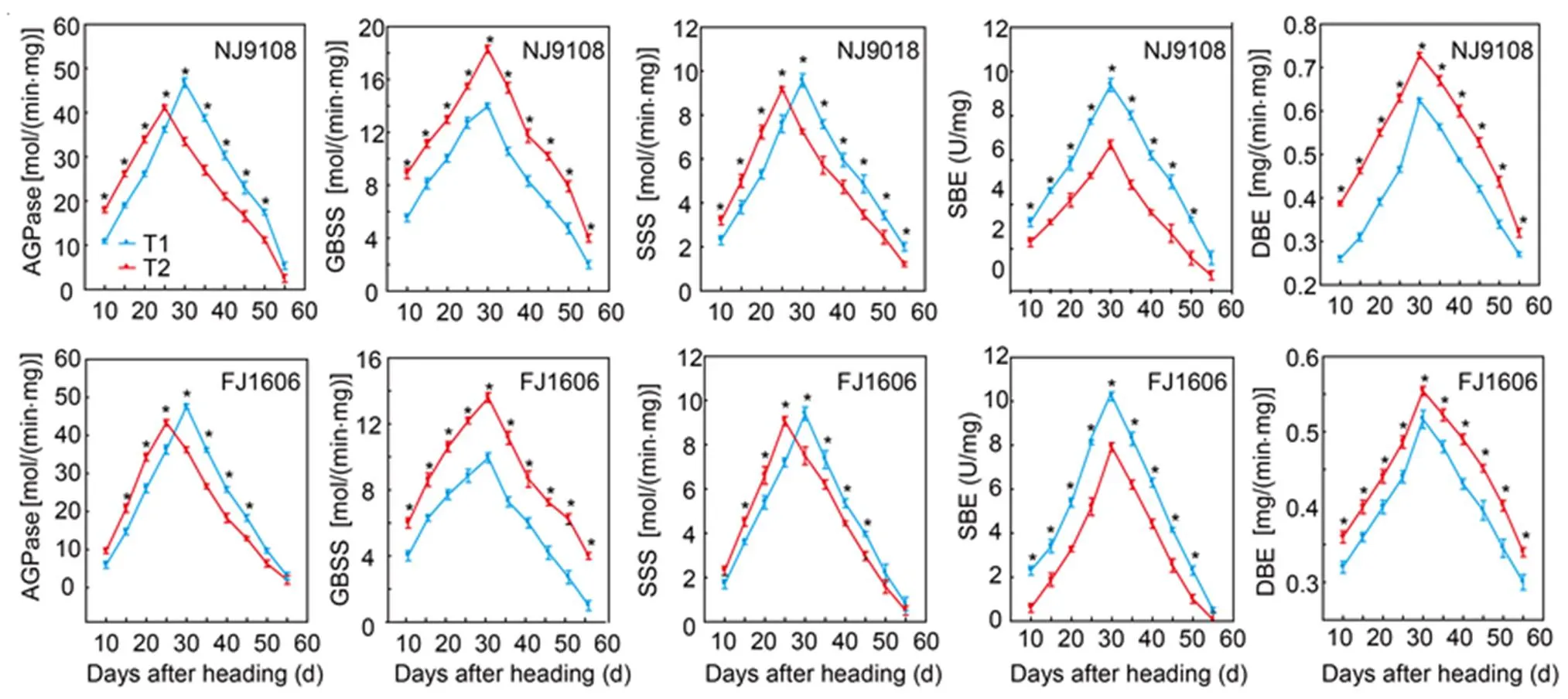
Fig. 2. Enzyme activities of ADP glucose pyrophosphorylase (AGPase), granule bound starch synthetase (GBSS), soluble starch synthase (SSS), starch branching enzyme (SBE) and starch debranching enzyme (DBE) in two rice varieties sown at different dates.
NJ9108, Nanjing 9108; FJ1606, Fujing 1606; T1, Rice seeds were sown at the early-sowing date (10 May, 2018); T2, Rice seeds were sown at the late-sowing date (21 June, 2018).
Data are Mean ± SD (= 3). *,< 0.05.
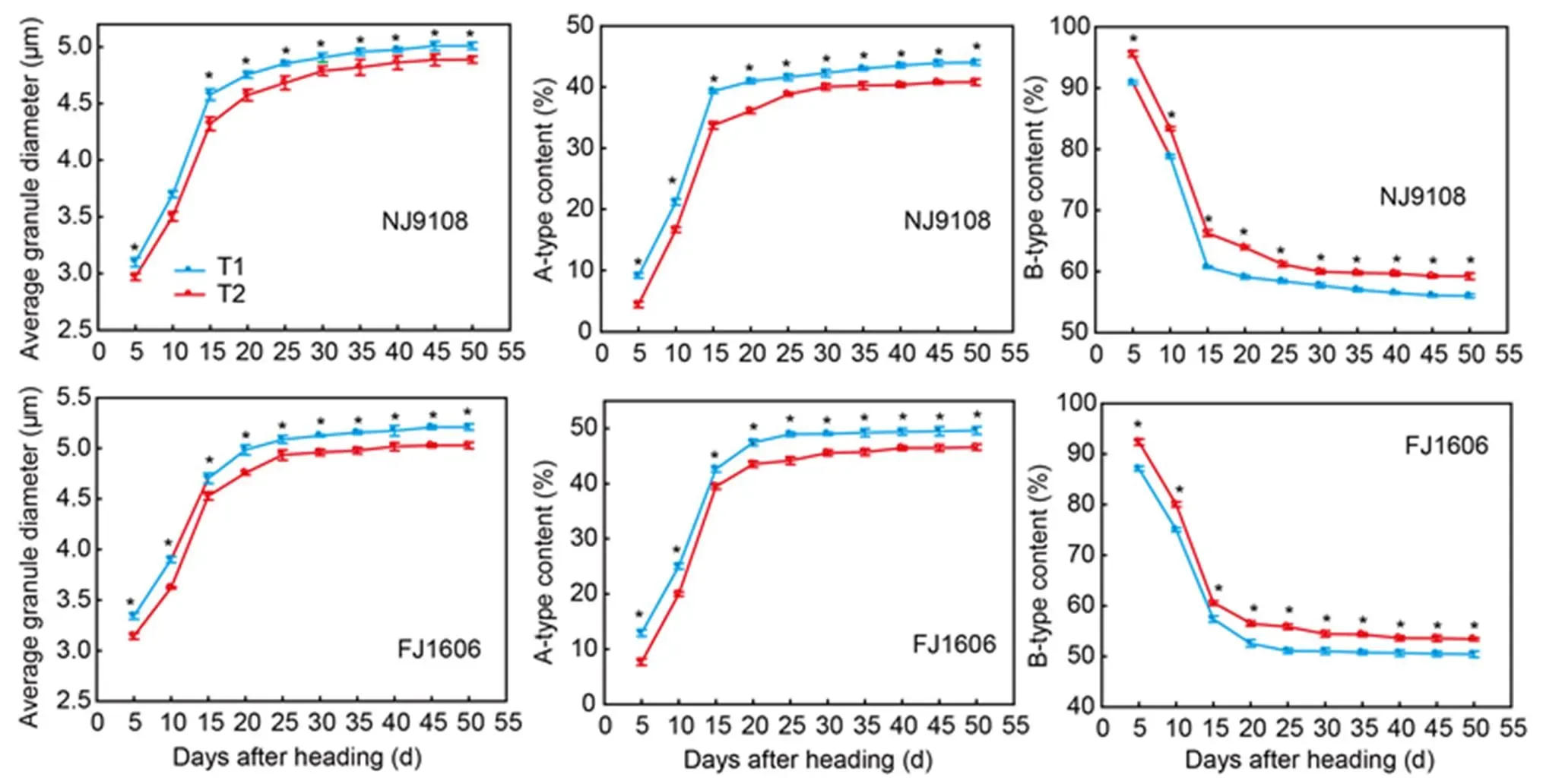
Fig. 3. Starch granule size distribution and average granule diameter from two rice varieties sown at different dates.
NJ9108, Nanjing 9108; FJ1606, Fujing 1606; T1, Rice seeds were sown at the early-sowing date (10 May, 2018); T2, Rice seeds were sown at the late-sowing date (21 June, 2018).
Data are Mean ± SD (= 3). *,< 0.05.
Starch granule size distribution
Average granule diameter of the two varieties rapidly increased within 15 DAH. Diameters of the two varieties in T1 were 6.4% and 4.4% lower than the respective diameters in T2 (Fig. 3). In the both varieties, the contents of B-type starch granules quickly declined in contrast to the increase contents of A-type starch granules within 15 DAH. The contents of B-type starch granules of the two varieties in T1 were respectively 5.7% and 6.0% higher than those in T2.
DISCUSSION
Effects of early- and late-sowing on starch accumulation and enzyme activity
Our results suggested that total starch, amylose and amylopectin accumulation were differentially affected between the two different filling-temperature groups. Total starch accumulation in T1 was lower than that in T2 at the early-filling stage (within 25 DAH), and then higher than that in T2 at the late-filling stage (Fig. 1). Ahmed et al (2015) found that a higher temperature (32 ºC) during grain development can decrease total starch content in rice. A similar result was found in maize (L.) by Yang et al (2017). They reported waxy maize exposed to heat stress (29 ºC) during the grain filling stage decreases grain dry weight and total starch content. A lower total starch content under higher temperature is mainly caused by low efficiency of starch biosynthesis (Liao et al, 2014; Cao et al, 2015). The pattern of AGPase activity was in consistent with that of total starch accumulation in the T1 and T2 groups. At the early-filling stage of NJ9108 and FJ1606, the average daily temperatures in T1 were 27.37 ºC and 26.88 ºC, and the averages of daily highest temperatures reached 31.69 ºC and 31.18 ºC, respectively. The high temperature (˃ 30 ºC) likely suppressed the activity of AGPase at the early-filling stage. This finding is similar to previous studies of Ahmed et al (2015) and Cao et al (2015), which indicated that a high temperature (32 ºC) can suppress AGPase activity in rice endosperm after about 10 DAH. By contrast, Zahedi et al (2003) determined that wheat grown at 30 ºC showed no changes in enzyme activities compared with those grown at a lower temperature (20 ºC). The contrasting results may be caused by two possibilities. Firstly, the two different crop species, rice and wheat, suggests that the effect of higher temperatures on the activity of AGPase may be species dependent. Secondly, based on the results of Zahedi et al (2003), we suspect that AGPase activity is not only affected by high temperatures but also inhibited by low temperatures. This point is further supported by the study of Xia et al (2016) who concluded that exposure of three rice varieties to a low temperature (17 ºC) lowers AGPase activity during grain filling. Our study also provided supportive evidence from the late-filling stage data in the both rice varieties. The activity of AGPase was lower in the lower temperature of T2 (approximately 16 ºC) than in T1 (approximately 22 ºC).
Amylose accumulation in the two varieties in T1 was lower than that in T2 from heading to maturity stages (Fig. 1). Consistent with our finding, previous studies have also shown that amylose content is reduced by higher temperature during grain filling (Ahmed et al, 2014; Cheng et al, 2019). Xia et al (2016) reported that amylose content significantly increases even under low temperature stress (17 ºC) during the grain filling stage. Lower amylose content is mainly caused by lower activity of GBSS from the heading to maturity stages (Zhang et al, 2016). Although one study demonstrated that exposed wheat to high temperature in a short time produces small effects on the activity of GBSS in wheat grains (Yan et al, 2008), more studies have shown that high temperatures occurring at grain filling decrease the activities of GBSS in wheat and rice (Kaur et al, 2017; Duan et al, 2019). Furthermore, SBE, a key enzyme involved in amylopectin synthesis, can also cleave α-1,4-glycoside bonds in amylose and then catalyze to branches of amylopectin (Abe et al, 2014; Brummell et al, 2015). Higher SBE activity in grains was found under higher temperatures during grain filling compared with that under lower temperatures. In this study, the higher SBE activity in T1 than in T2 from the heading to maturity stages was associated with the higher daily mean temperature. Therefore, more amylose was likely converted to amylopectin due to the high SBE activity in T1.
Previous studies have reported that high grain- filling temperature reduces starch content. Luo et al (2007) determined that when daily mean temperatures higher than 24 ºC, amylopectin content decreases with increasing grain-filling temperature. Zhao et al (2008) also found lower amylopectin content in wheat grains exposed to a high grain-filling temperature (28 ºC) than to a low temperature (20 ºC). Consistent with those results, we observed lower amylopectin accumulation at the early-filling stage when daily mean temperatureswere 27.32 ºC and 26.88 ºC (T1) than when daily mean temperatures were 22.49 ºC and 22.11 ºC (T2) for the two rice varieties. However, amylopectin accumulation in T1 was higher than that in T2 at the late-filling stage (Fig. 1), and the trend was similar to total starch accumulation. Inukai and Hirayama (2010) suggested that amylopectin content per grain innear- isogenic lines for thelocus is greater at a high grain-filling temperature (32 ºC). The different results imply that the effect of high temperature on amylopectin accumulation may be variety dependent.
Amylopectin synthesis is mainly catalyzed by SSS, DBE and SBE, which are responsible for modifying the branches of amylopectin. The trend of SSS activity was similar to amylopectin accumulation from the heading to maturity stages. The SSS activities of the two rice varieties in T1 were lower than those in T2 at the early-filling stage, however, at the late-filling stage, the SSS activities in T1 were higher than those in T2. Other researchers have also shown that SSS activity decreases under a higher grain-filling temperature (Zhao et al, 2008; Cheng et al, 2019) or a low temperature (17 ºC) during grain filling (Xia et al, 2016). Altogether, these results suggest that SSS activity likely peaks at moderate temperatures at about 22 ºC.Xu et al (2015) studied starch synthesis enzymes in maize grains and found that the time for AGPase, SSS and SBE activities to reach their peaks differed. Their results are consistent with our results where the activities of AGPase and SSS peaked earlier than those of GBSS and DBE in T2. However, in T1, the activities of AGPase, SSS, GBSS and DBE climaxed at about the same times. Similar results were observed in rice variety 9311 by Cao et al (2015), who found similar time lengths are needed for AGPase, SSS and SBE activities to reach their maximums, and the time is affected by filling-temperature. Therefore, the differences of peak times in T1 and T2 were probably caused by filling-temperature and the low- amylose content varieties used in this study.
Effects of early- and late-sowing on starch granule size development and distribution
Starch granule size distribution is an important factor that influences rice eating and cooking quality (Park et al, 2009). We found that the higher temperature during the grain filling stage increased the percentage of large-sized starch granules and theaverage granule diameter. We also observed that the average granule diameter increased rapidly within 15 DAH. This supported a previous study by Zhang et al (2010), who found the mean diameter of wheat starch granules dramatically increased within 16 DAH. The SBE activity is reportedly responsible for changes in starch granule size, and lack of it hinders granules from reaching normal sizes (He et al, 2018). Therefore, the lower grain-filling temperature might have reduced SBE activity, which in turn, decreased average granule diameter.
Many previous researchers have demonstrated that higher temperatures (more than 30 ºC) during grain filling hamper starch synthesis in grain-producing crops through greenhouse or artificial climate chamber studies. In this study, we used two different sowing dates to create a high and low temperature difference while maintaining the natural changes in temperature during the grain filling period and to determine the more suitable temperature for starch synthesis and accumulation. In Jiangsu Province of China, an early- sowing date risks high-temperature stress during grain filling. Yet, our results suggested that the risk may be unwarranted. Starch synthesis was suppressed by the high temperature in T1 at the early-filling stage, however, at the late-filling stage, when daily mean temperature was lower at approximately 20 ºC, starch synthesis and accumulation in T1 were greater than those in T2. We concluded that the high temperature stress caused by early-sowing (about 10 May) resulted in little to no damage to starch accumulation and synthesis in rice grains with low-amylose content.
METHODS
Rice materials
Two varieties ofL. ssp.(Nanjing 9108 and Fujing 1606) were planted at the farm of Yangzhou University, China (119º42′ E, 32º39′ N) during rice growing season in 2018. The soil type was sandy loam and the preceding crop was wheat. Total availability of nitrogen, phosphorous and potassium were 1.4 g/kg, 35.1 mg/kg and 88.3 mg/kg, respectively. Plots were arranged in a randomized block pattern with three replicates. Hill spacing was 30 cm × 12 cm with four seedlings per hill. The plot area was 15 m2(5 m × 3 m).
Sowing date
Using the natural temporal changes in environmental temperature, we investigated the potential effects of temperature at the grain filling stage by growing the two rice varieties at different periods. The rice varieties were sown in 2018 at the earliest (10 May, T1 treatment) and the latest (21 June, T2 treatment) sowing dates practiced by local rice producers, respectively. Then rice seedlings were transplanted on 30 May and 11 July, respectively. Thus, grain filling of T1 rice plants occurred in August to October with higher temperatures and that of T2 rice plants encountered in September to November with lower temperatures.In this study, we divided the filling period into two stages: early-filling and late-filling stages, where each stage totaled approximately 25 d according to total starch and amylopectin accumulation curve. Heading and maturity periods for each sowing treatment and averages of daily, daily highest, and daily lowest temperatures during the grain filling stage are listed in Table 1 and Fig. S1.

Table 1. Averages of daily, daily highest and daily lowest temperatures of two rice varieties grown at different time periods.
Sampling
After flowering, about 200 panicles were chosen on the same day and tagged for each plot. After full heading, the tagged panicles were sampled from each plot every 5 d at 10:00 am, and the grains in the middle of the tagged panicles were collected (20 g). These collected grains were divided into two groups. Half of them were used to determine total starch, amylose and amylopectin contents, and the other half were put in liquid nitrogen for 3 min and then stored at -80 ºC for enzymatic analysis.
Total starch, amylose and amylopectin analyses
Total starch from rice flour was performed using commercial kits manufactured by Sigma-Aldrich (St. Louis, MO, USA) according to Zhu et al (2017). Amylose and amylopectin contents were determined according to He (1985) withminor changes. Amylose content was quantified at 620 and 479 nm, while amylopectin content was quantified at 556 and 737 nm, respectively.
Enzyme extraction and assay
The assays of enzymes were optimized for substrate concentrationand pH. Ten grains were hand- homogenized by grinding at 4 ºC with 5 mL extraction buffer (100 mmol/L Tricine-NaOH, pH 7.5, 8 mmol/L MgCl2, 2 mmol/L EDTA, 12.5% glycerol, 1% PVP-40 and 50 mmol/L β-mercaptoethanol).After centrifugation at 10 000 ×at 4 ºC for 10 min, the supernatant was used to determine enzyme activities. The activities of AGPase, GBSS and SSS were analyzed as described by Yang et al (2003) with minor changes. The analysis of SBE and DBE activities followed Nakamura et al(1989) and Nakamura et al (1996) with minor changes, respectively.
Starch granule distribution analysis
Rice starch was isolated from flour as described by Wei et al (2010) with minor modifications. Starch granule size distribution was investigated using a Mastersizer 2000 laser diffraction particle size analyzer (Malvern Panalytical, Malvern, England) as described by Zhu et al (2017). The starch samples were immersed in absolute ethyl alcohol and stirred at 2 000 r/min. Starch granule size was classified into A-type (≥ 5 μm) and B-type (< 5 μm) according to Liu et al (2017).
Statistical analyses
Means of triplicate experimental values were used. All analyses were conducted and analyzed by using Microsoft Excel 2016 and SPSS. 23. Figures were drafted by SigmaPlot 10.0.
ACKNOWLEDGEMENTS
This study was supported by the National Key Research Program of China (Grant No. 2016YFD0300503), the National Rice Industry Technology System of China (Grant No. CARS0127), the National Natural Science Foundation of China (Grant No. 31971841), the Key Research Program of Jiangsu Province, China (Grant No. BE2018355), the Earmarked Fund for Jiangsu Agricultural Industry Technology System, China (Grant No. JATS [2020]450), and the Priority Academic Program Development of Jiangsu Higher Education Institutions, China.
SUPPLEMENTAL DATA
The following material is available in the online version of this article at http://www.sciencedirect.com/science/journal/rice-science; http://www.ricescience.org.
Fig. S1. Daily mean temperature (DMT), daily mean high temperature (ADHT), and daily mean low temperature (ADLT) of two rice varieties in two treatments.
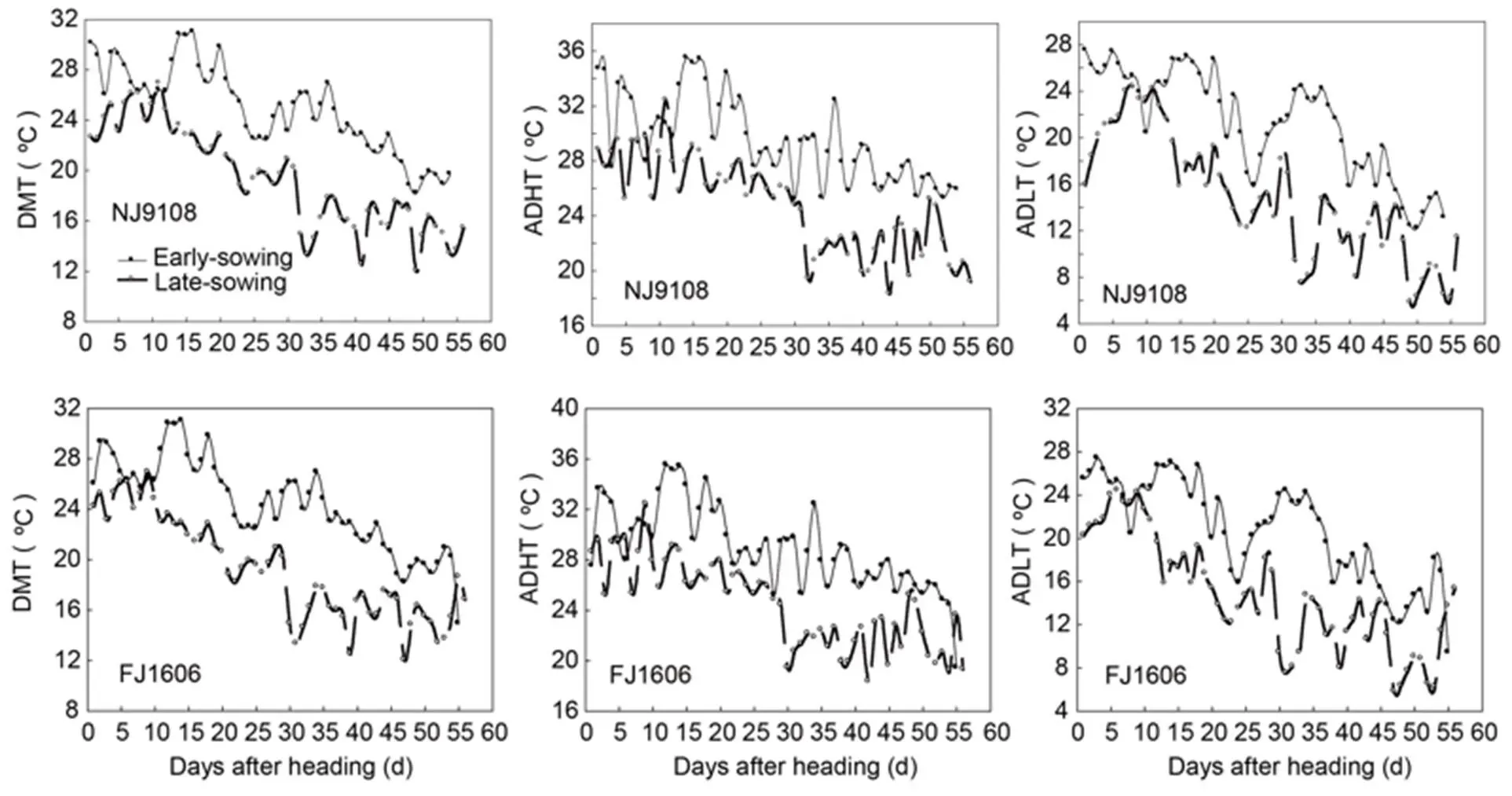
Fig. S1. Daily mean temperature (DMT), daily mean high temperature (ADHT), and daily mean low temperature (ADLT) of two rice varieties in two treatments.
NJ9108, Nanjing 9108; FJ, Fujing 1606.
Abe N, Asai H, Yago H, Oitome N F, Itoh R, Crofts N, Nakamura Y, Fujita N. 2014. Relationships between starch synthase I and branching enzyme isozymes determined using double mutant rice lines., 14: 1–12.
Ahmed N, Tetlow I J, Nawaz S, Iqbal A, Mubin M, Rehman M S N, Butt A, Lightfoot D A, Maekawa M. 2015. Effect of high temperature on grain filling period, yield, amylose content and activity of starch biosynthesis enzymes in endosperm of Basmati rice., 95: 2237–2243.
Brummell D A, Watson L M, Zhou J, McKenzie M J, Hallett I C, Simmons L,Carpenter M, Timmerman-Vaughan G M. 2015. Overexpression of starch branching enzyme II increases short- chain branching of amylopectin and alters the physicochemical properties of starch from potato tuber., 15: 1–14.
Cao Z Z, Pan G, Wang F B, Wei K X, Li Z W, Shi C H, Geng W, Cheng F M. 2015. Effect of high temperature on the expressions of genes encoding starch synthesis enzymes in developing rice endosperms., 14(4): 642–659.
Chen Y L, Bao J S. 2017. Progress in structures, functions and interactions of starch synthesis related enzymes in rice endosperm., 31(1): 1–12. (in Chinese with English abstract)
Cheng C, Zeng Y J, Chen H H, Tan X M, Shan Q Y, Zeng Y H, Shi Q H. 2019. Effects of different temperature from full heading to milking on grain filling stage on grain hormones concentrations, activities of enzymes involved in starch synthesis and accumulation in rice Nanjing 9108., 33(1): 57–67. (in Chinese with English abstract)
Chung H J, Liu Q, Lee L, Wei D Z. 2011. Relationship between the structure, physicochemical properties and in vitro digestibility of rice starches with different amylose contents.,25: 968–975.
Duan H, Tong H, Liu Y Q, Xu Q F, Ma J, Wang C M. 2019. Research advances in the effect of heat and drought on rice and its mechanism., 33(3): 206–218. (in Chinese with English abstract)
Fujita N, Toyosawa Y, Utsumi Y, Higuchi T, Hanashiro I, Ikegami A, Akuzawa S, Yoshida M, Mori A, Inomata K, Itoh R, Miyao A, Hirochika H, Satoh H, Nakamura Y. 2009. Characterization of pullulanase (PUL)-deficient mutants of rice (L.) and the function of PUL on starch biosynthesis in the developing rice endosperm., 60: 1009–1023.
Gilbert R G, Witt T, Hasjim J. 2013. What is being learned about starch properties from multiple-level characterization., 90: 312–325.
Halford N G, Curtis T Y, Chen Z, Huang J. 2015. Effects of abiotic stress and crop management on cereal grain composition: Implications for food quality and safety., 66: 1145–1156.
Hannah L C, James M. 2008. The complexities of starch biosynthesis in cereal endosperms.,19: 160–165.
He W, Lin L S, Wang J, Zhang L, Liu Q Q, Wei C X. 2018. Inhibition of starch branching enzymes in waxy rice increases the proportion of long branch-chains of amylopectin resulting in the comb-like profiles of starch granules., 277: 177–187.
He Z F. 1985. Grain Quality and Its Analysis Technology. Beijing, China: Agricultural Press: 274–294. (in Chinese)
Inukai T, Hirayama Y. 2010. Comparison of starch levels reduced by high temperature during ripening inrice lines near-isogenic for thelocus., 196: 296–301.
Jeon J S, Ryoo N, Hahn T R, Walia H, Nakamura Y. 2010. Starch biosynthesis in cereal endosperm., 48: 383–392.
Kaur V, Madaan S, Behl R K. 2017. ADP-glucose pyrophosphorylase activity in relation to yield potential of wheat: Response to independent and combined high temperature and drought stress., 45: 181–191.
Liao J L, Zhou H W, Zhang H Y, Zhong P A, Huang Y J. 2014. Comparative proteomic analysis of differentially expressed proteins in the early milky stage of rice grains during high temperature stress., 65: 655–671.
Lin J H, Singh H, Chang Y T, Chang Y H. 2011. Factor analysis of the functional properties of rice flours from mutant genotypes., 126: 1108–1114.
Liu J C, Zhao Q, Zhou L J, Cao Z Z, Shi C H, Cheng F M. 2017. Influence of environmental temperature during grain filling period on granule size distribution of rice starch and its relation to gelatinization properties., 76: 42–55.
Luo Q, Zhu Y, Peng G Z, Zhang M S, Wang X F. 2007. A study on relationship between starch content of rice and climate conditions in Yunnan Province., 28(3): 303–307. (in Chinese with English abstract)
Mayer L I, Savin R, Maddonni G A. 2016. Heat stress during grain filling modifies kernel protein composition in field-grown maize., 56: 1890–1903.
Nakamura Y, Yuki K, Park S Y, Ohya T. 1989. Carbohydrate metabolism in the developing endosperm of rice grains., 30: 833–839.
Nakamura Y, Umemoto T, Ogata N, Kuboki Y, Yano M, Sasaki T. 1996. Starch debranching enzyme (R-enzyme or pullulanase) from developing rice endosperm: Purification, cDNA and chromosomal localization of the gene., 199: 209–218.
Park S H, Wilson J D, Seabourn B W. 2009. Starch granule size distribution of hard red winter and hard red spring wheat: Its effects on mixing and bread making quality., 49: 98–105.
Patindol J A, Siebenmorgen T J, Wang Y J. 2015. Impact of environmental factors on rice starch structure: A review., 67: 42–54.
Prathap V, Ali K, Singh A, Vishwakarma C, Krishnan V, Chinnusamy V, Tyagi A. 2019. Starch accumulation in rice grains subjected to drought during grain filling stage., 142: 440–451.
Sacks W J, Deryng D, Foley J A, Ramankutty N. 2010. Crop planting dates: An analysis of global patterns., 19: 607–620.
Satoh H, Nishi A, Yamashita K, Takemoto Y, Tanaka Y, Hosaka Y, Sakurai A, Fujita N, Nakamura Y. 2003. Starch-branching enzyme I-deficient mutation specifically affects the structure and properties of starch in rice endosperm., 133: 1111–1121.
Smidansky E D, Martin J M, Hannah L C, Fischer A M, Giroux M J. 2003. Seed yield and plant biomass increases in rice are conferred by deregulation of endosperm ADP-glucose pyrophosphorylase., 216: 656–664.
Sun T, Tong L G, Zhao S Y, Wang H W, Han Y F, Zhang Z C, Jin Z X. 2018. Effects of nitrogen fertilizer application on starch quality, activities and gene expression levels of related enzymes in rice endosperm., 32(5): 475–484. (in Chinese with English abstract)
Syahariza Z A, Sar S, Hasjim J, Tizzotti M J, Gilbert R G. 2013. The importance of amylose and amylopectin fine structures for starch digestibility in cooked rice grains., 136: 742–749.
Tuncel A, Okita T W. 2013. Improving starch yield in cereals by over-expression of ADPglucose pyrophosphorylase: Expectations and unanticipated outcomes., 211: 52–60.
Tuncel A, Kawaguchi J, Ihara Y, Matsusaka H, Nishi A, Nakamura T, Kuhara S, Hirakawa H, Nakamura Y, Cakir B, Nagamine A, Okita T W, Hwang S K, Satoh H. 2014. The rice endosperm ADP-glucose pyrophosphorylase large subunit is essential for optimal catalysis and allosteric regulation of the heterotetrameric enzyme., 55: 1169–1183.
Wang C L, Zhang Y L, Zhu Z, Chen T, Zhao L, Lin J, Zhou L H. 2009. Development of a newrice variety Nan-jing 46 with good eating quality by marker assisted selection., 17: 1070–1076.
Wei C X, Qin F L, Zhou W F, Yu H G, Xu B, Chen C, Zhou L J, Wang Y P, Gu M H, Liu Q Q. 2010. Granule structure and distribution of allomorphs in C-type high-amylose rice starch granule modified by antisense RNA inhibition of starch branching enzyme., 58: 11946–11954.
Xia N, Zhao H W, Lv Y C, Zhao Z D, Zou D T, Liu H L, Wang J G, Jia Y. 2016. Effect of cold-water stress at grain-filling stage on starch accumulation and related enzyme activities in grains ofrice in cold-region., 30(1): 62–74. (in Chinese with English abstract)
Xu Y J, Gu D J, Qin H, Zhang H, Wang Z Q, Yang J C. 2015. Changes in carbohydrate accumulation and activities of enzymes involved in starch synthesis in maize kernels at different positions on an ear during grain filling., 41(2): 297–307. (in Chinese with English abstract)
Yan S H, Yin Y P, Li W Y, Liang T B, Li Y, Wu Y H, Wang P, Geng Q H, Dai Z M, Wang Z L. 2008. Effect of high temperature during grain filling on starch accumulation, starch granule distribution, and activities of related enzymes in wheat grains., 34(6): 1092–1096. (in Chinese with English abstract)
Yang H, Huang T Q, Ding M Q, Lu D L, Lu W P. 2017. High temperature during grain filling impacts on leaf senescence in waxy maize., 109: 906–916.
Yang J C, Zhang J H, Wang Z Q, Zhu Q S, Liu L J. 2001. Water deficit-induced senescence and its relationship to the remobilization of pre-stored carbon in wheat during grain filling., 93: 196–206.
Yang J C, Zhang J H, Wang Z Q, Zhu Q S, Liu L J. 2003. Activities of enzymes involved in sucrose-to-starch metabolism in rice grains subjected to water stress during filling., 81: 69–81.
Zahedi M, Sharma R, Jenner C F. 2003. Effects of high temperature on grain growth and on the metabolites and enzymes in the starch-synthesis pathway in the grains of two wheat cultivars differing in their responses to temperature., 30: 291–300.
Zhang C H, Jiang D, Liu F L, Cai J, Dai T B, Cao W X. 2010. Starch granules size distribution in superior and inferior grains of wheat is related to enzyme activities and their gene expressions during grain filling., 51: 226–233.
Zhang C Q, Zhao D S , Li Q F, Gu M H, Liu Q Q. 2016. Progresses in research on cloning and functional analysis of key genes involving in rice grain quality., 49(22): 4267–4283. (in Chinese with English abstract)
Zhao H, Dai T B, Jiang D, Cao W X. 2008. Effects of high temperature on key enzymes involved in starch and protein formation in grains of two wheat cultivars., 194: 47–54.
Zhu D W, Wei H Y, Guo B W, Dai Q G, Wei C X, Gao H, Hu Y J, Cui P Y, Li M, Huo Z Y, Xu K, Zhang H C. 2017. The effects of chilling stress after anthesis on the physicochemical properties of rice (L.) starch., 237: 936–941.
23 March 2020;
8 June 2020
Zhang Hongcheng (hczhang@yzu.edu.cn); Wei Haiyan (wei_haiyan@163.com)
Copyright © 2021, China National Rice Research Institute. Hosting by Elsevier B V
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/)
Peer review under responsibility of China National Rice Research Institute
http://dx.doi.org/10.1016/j.rsci.2021.01.008
(Managing Editor: Wu Yawen)
杂志排行
Rice Science的其它文章
- Genetic Interaction of Hd1 with Ghd7, DTH8 and Hd2 Largely Determines Eco-Geographical Adaption of Rice Varieties in Southern China
- Drought Tolerance in Rice: Focus on Recent Mechanisms and Approaches
- Genome Editing Strategies Towards Enhancement of Rice Disease Resistance
- RAVL1 Activates IDD3 to Negatively Regulate Rice Resistance to Sheath Blight Disease
- Osa-miR439 Negatively Regulates Rice Immunity Against Magnaporthe oryzae
- Exogenous Peroxidase Mitigates Cadmium Toxicity, Enhances Rhizobial Population and Lowers Root Knot Formation in Rice Seedlings
