基于过渡金属硫化物/还原氧化石墨烯复合物的高性能超级电容器
2021-02-26黄东雪张媛媛万其进杨年俊
黄东雪,章 颖,曾 婷,张媛媛,万其进,杨年俊
(1.武汉工程大学化学与环境工程学院,武汉430074;2.德国锡根大学材料工程研究所,锡根57076,德国)
1 Introduction
Owing to the serious population growth and environmental pollution,the demand for clean energy produc⁃tion and utilization is increasingly urgent.Such energy crisis drives the demands of new and innovative materials to develop alternative energy sources and manufacture innovative energy storage devices[1,2].Supercapaci⁃tor(SC)is one of promising and emerging electrochemical energy devices for future clean energy technology[3],due to its several unique features,including its fast charging rate,high power density,long cycle life,and simple configuration.Actually,it bridges the energy gap between conventional capacitors and batteries or fuel cells[4,5].
To assemble a SC,the capacitor electrode is of great importance.Among various capacitor electrodes or electrode materials(e.g.,carbon materials,conducting polymers,metal oxides/hydroxides),transition metal sulfides(TMSs)have attracted tremendous attention due to their high specific capacities,fast rate perfor⁃mance,and long cycle life.More exactly,the 3dorbitals of transition metal atoms have not been filled,and thus TMSs generally have multiple valence states.In other words,their valence states can generate more elec⁃trons,generating pseudocapacitances during the charging/discharging processes[6,7].On the other hand,TMSs have their shortcomings as the electrode materials for electrochemical energy storage applications.Taking copper monosulfide(CuS)as an example,it exhibits serious volume changes during the cycling processes when it is used as a battery electrode material[8,9].Molybdenum disulfide(MoS2)has a poor electron conductivity,although its thin layers own a large space for the electrolyte-ion transportation where a shortened length is available for the transfer of both ions and electrons[10—12].The cycling performance of nickel sulfide(NiS)is poor[13,14],although it has the features of a high electronic conductivity,low cost,ease of fabrication and low toxicity.To achieve full usage of TMSs for electrochemical energy storage applications,the composites/hy⁃brids of binary-or triple-TMSs thus need to be formed.Such composites are expected to integrate the advantag⁃es of each TMS components and meanwhile to overcome their shortcomings[6].To synthesize homogeneous TMS hybrids,the supporting materials need to be also considered.Among various supporting materials,car⁃bon nanomaterials will be one of the best choices,due to their high conductivities,high surface areas,and low densities.In this way,TMS electrode materials with high surface-to-volume-ratios can be fabricated and their high performance is expected to be obtained during electrochemical energy storage processes[15].
In this regard,we design TMS hybrids and further explore their applications for electrochemical energy storage(here,SCs)with simultaneously improved specific energy and power performance.To synthesize ho⁃mogeneous and highly conductive TMS hybrids,reduced graphene oxide(rGO)was utilized as the supporting material.Microwave assisted exfoliation was applied to produce hierarchical rGO(MErGO)in that this simple yet versatile method can simultaneously achieve the exfoliation and reduction of graphitic oxide(GO).A selftemplate strategy was further developed to prepare the MErGO coated CuS/MoS2hollow cubic hybrid(CuSMoS2/MErGO),where MErGO provides active sites to form chemical bonds between the graphene nano sheets and two TMSs.Moreover,the MErGO supported NiS nanoparticles(NiS/MErGO)were synthesizedviaa hydrothermal method.To assemble a TMS based asymmetric SC(ASC)device,the CuS-MoS2/MErGO and NiS/MErGO hybrids act as the negative and positive electrodes,respectively.Due to the combined features of both CuS and MoS2,the CuS-MoS2/MErGO hybrid is expected to have a high surface area,a high electronic conductivity,the features of the pseudocapacitive materials.Together this pseudocapacitive hybrid with the pseudocapacitive NiS/MErGO hybrid,the assembled ASCs are possible to own both high power density and energy density.By means of cyclic voltammetry,the galvanostatic charging-discharging(GCD)method,elec⁃trochemical impedance spectroscopy(EIS),the performance of such an ASC device was investigated,includ⁃ing its capacitance,capacitance retention,power-and energy-densities.The practical applications of this ASC was testified to light LEDs.
2 Experimental
2.1 Materials Synthesis
Graphite(≥99.85%)with chemically pure grade was purchased from China National Medicines Corpora⁃tion Ltd.The other mentioned chemicals were of analytical grade and thus used without further purification unless specifically mentioned.Concentrated sulfuric acid(≥98%),potassium permanganate(≥99.5%),hy⁃drogen peroxide 30%,ethanol(≥99.7%),thiourea(≥99%),sodium sulfide(≥99%),sodium thiosulfate pentahydrate(≥99%),hydrochloric acid(36%—38%),nickel nitrate hexahydrate(≥99%),ethylenediamine(≥99%),sodium hydroxide(99%),N-methyl-pyrrolidone(≥99%)were obtained from China National Medi⁃cines Corporation Ltd.Ascorbic acid(≥99.7%)was brought from Shanghai Aladdin Reagent Co.,Ltd.Cop⁃per sulphate(≥99%)was original from Tianjin Fuchen Chemical Reagent Factory.Ammonium molybdate(≥99%)was purchased from Tianjin Guangfu Fine Chemical Research Institute.Citric acid sodium(≥99%)was purchased from Beijing Chemical Plant.
Graphene oxide(GO)was synthesizedviaa modified Hummer’s method[16].Microwave exfoliated gra⁃phene oxide(MEGO)was prepared according to the following steps[17,18].In the first step,50 mg GO was dis⁃solved in 40 mL of ethanol inside a sonication bath for 30 min.The resulted solution was then dried at 80ºC in oven for 2 h.Subsequently,the product was transferred into a microwave oven at 700 W for 90 s under the am⁃bient conditions.The MEGO powders were then obtained.The synthetic methods of MoS2nanosheets[19]and hollow CuS nanocubes[20,21]were the same as reported.To obtain the CuS-MoS2/MErGO hybrid,0.06 mg assynthesized CuS nanobubes,0.0072 g(NH4)6Mo7O24·4H2O,and 0.06 g CH4N2S were dispersed in 60 mL of water.With aid of a ultrasound bath,a homogeneous solution was formed within 30 min.This solution was then transferred to a 100 mL Teflon-lined stainless steel autoclave and thermally treated at 180℃for 24 h.The product was washed with water and ethanol for several times.It was then mixed with 50 mg MEGO.Note that,such treatment conditions(e.g.,microwave power,treatment time)were varied once the amount of used GO was altered.A second thermal treatment was conducted on this mixture in an another autoclavation at 120℃for 8 h,leading to the production of the CuS-MoS2/MErGO hybrid.
To synthesize the NiS/MErGO hybrid,1.45 g Ni(NO3)2·6H2O was dissolved in 70 mL water.The resul⁃tant solution was stirred for 20 min before 1 mL hydrazine hydrate,2.0 g sodium sulfide,and 20 mL water were added.After a reaction time of 30 min,as-obtained black precipitate was collected and washed copiously with water.Subsequently,50 mg MEGO and 60 mL water were added.Such a mixture was then treated in a ultrasound bath for 30 min.The obtained suspension was then transferred to a 100 mL Teflon-lined stainless steel autoclave and thermally treated at 120℃for 6 h.After the final product was washed and dried at 60℃,the NiS/MErGO hybrid was obtained.
2.2 Characterization
Field enhanced scanning electron microscopic(FESEM)images were recorded on a Germini SEM 300 mi⁃croscope(Zeiss,Germany).Transmission electron microscopic(TEM)images were obtained on JREOL-JEM-2100 microscope(JEOL,Japan).Energy-dispersive X-ray spectroscopy(EDS)was conducted on an Oxford IE250 energy dispersive spectrometer(Zeiss,Germany).The X-ray photoelectron spectroscopy(XPS)analy⁃sis was carried out on an AXIS-ULTRA DLD-600W X-ray photoelectron spectrometer(SHIMADZU-Kratos company,Japan).
2.3 Electrochemical Measurements
All electrochemical measurements were carried out on a CHI 760E electrochemical workstation(Shanghai Chenhua Apparatus Corporation,China)at room temperature.For a three-electrode system,a graphite paper electrode and a saturated calomel electrode(SCE)electrode were used as the counter electrode and the reference electrode,respectively.The working electrode was a graphite paper(1 cm×1 cm)coated with homoge⁃neous pastes.These pastes were the mixtures of as-prepared hybrids,acetylene black,and polytetrafluoroeth⁃ylene(PTFE)that were with a mass ratio of 80∶10∶10 inN-methyl-2-pyrrolidone(NMP)solvent.Acetylene black and PTFE were used as the conductive agent and binder,respectively.The pasted coated graphite paper was dried in a vacuum oven at 80°C for 12 h.The mass loading of the active materials on graphite paper was varied as required from 1.0 mg to 2.0 mg.The specific capacitance(C)of the working electrodes were deter⁃mined from as-recorded cyclic voltammograms(CVs)and the GCD curves according to the reported methods[22].To assemble an asymmetric SC(ASC),the NiS/MErGO hybrid was used as the positive electrode and the CuS-MoS2/MErGO hybrid was used as the negative electrode.To optimize the charges between two electrodes,the optimal mass ratio of two electrodes was calculated according to the reported approach[23].The energy density(E,W·h·kg−1)and power density(P,W/kg)of this ASC were calculated using the reported method[24,25].
3 Results and Discussion
3.1 Characterization of TMS Hybrids
In this study,two types of composites/hybrids were synthesized,namely the CuS-MoS2/MErGO and NiS/MErGO hybrids.Prior to their characterization,as-synthesized GO and MErGO were checked by means of scanning electron microscopy(SEM).The GO film consists of many nanosheets,which are crumpled and rip⁃pled[Fig.S1(A),see the Supporting Information of this paper].These nanosheets are loosely associated with each other.The as-obtained rGO(MErGO)film has a wrinkled and folded texture where irregular edges are clearly noticed[Fig.S1(B)].More interestingly,it exhibits a porous structure,consisting of different thin car⁃bon layers.It is similar as that reported[26].This porous structure does not even vary when the MErGO film is hybridized with binary TMSs(here,CuS and MoS2)since one can clearly see in that the MErGO film is covered with diあerent sized grains[Fig.1(A)and(B)].On such a porous or nested structure,MoS2nanosheets are expected to be grown outside of the CuS cubes,leading to the formation of nanoboxes.The average size of these nanoboxes is about 400 nm.In contrast,the CuS/MErGO hybrid has numerous CuS parti⁃cles,which are embedded into the porous structure of the MErGO film.These particles are hollow(e.g.,meso⁃pores)[Fig.S1(C)and(D)].Those cubic and rough particles own an average size of approximate 600 nm,the same as that reported[21].The MoS2/MErGO hybrid owns a 2D architecture,where a large number of MoS2nanosheets are densely distributed on the MErGO film[Fig.S1(E)and(F)].To further confirm the ho⁃mogeneity of the CuS-MoS2/MErGO hybrid,its EDS mapping of Cu,Mo,C,and S[Fig.1(C)]was conduct⁃ed,where homogeneous distribution of these elements are observed.The microstructure of the CuS-MoS2/MEr⁃GO hybrid was also examined by means of TEM.The size of CuS-MoS2on the MErGO film appears to be small⁃er than that of CuS nanoparticles[Fig.S2(A),see the Supporting Information of this paper].This results from the formation of a thin MoS2layer wrapped on the CuS surface during the hydrothermal process,which com⁃presses the size of CuS nanoparticles.The hierarchical shells are composed of randomly assembled MoS2nanosheets.The well-defined hollow configuration of the CuS nanocubes on the MErGO film is verified[Fig.S2(B)].The typical morphology of NiS nanosheets is seen on the MErGO film[Fig.S2(C)],consistent with that observed from the SEM image.
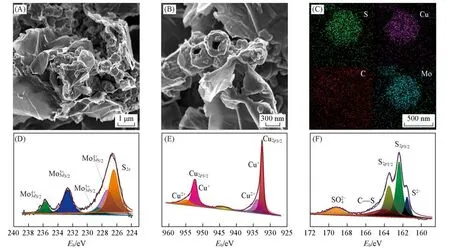
Fig.1 SEM images(A,B),EDS elemental mappings(C),high resolution XPS spectra of Mo3d(D),Cu2p(E)and S2p(F)of the CuS⁃MoS2/MErGO hybrid
To further confirm the composition of CuS-MoS2/MErGO hybrid,it was analysed by means of XPS.In its survey spectrum[Fig.S3(A),see the Supporting Information of this paper],the presence of C,S,Cu,and Mo elements is confirmed.In the Mo3dXPS spectrum[Fig.1(D)],the major peaks at 227.2 and 232.7 eV are assigned to Mo3d3/2and Mo3d5/2,respectively.Both are assumed to be resulted from the Mo4+ions[27].The Cu2pXPS spectrum[Fig.1(E)]shows two prominent peaks cantered at 932.3 and 952.5 eV.They are associated with Cu+2p3/2and Cu+2p1/2,respectively.An additional small peak located at 943.8 eV stems from the Cu2+oxida⁃tion state[28].Moreover,the S2ppeak is observed at 226.4 eV in the S2pXPS spectrum[Fig.1(F)].The peaks located at 162.4 eV(2p3/2)and 163.4 eV(2p1/2)and two more peaks appeared at 169.2 and 164.1 eV are pre⁃sumably due to SO42−and C—S[29,30],respectively.The high-resolution C1sXPS spectrum[Fig.S3(B)]consists of three main components,including the C—C peak located at 284.7 eV,the C—O peak at 285.6 eV,and the C=O peak at 286.6 eV[17,31].The above XPS data evidently confirms the co-existence of the elements of Mo,Cu,S and C as well as their chemical states.In other words,the CuS-MoS2/MErGO hybrid has been suc⁃cessfully synthesized.Due to the presence of these metal centres,it is also expected to be electrochemically active.
Using similar techniques,as-synthesized NiS/MErGO hybrid was characterized.The NiS nanosheets are aggregated into the clusters[Fig.2(A)]and attached on the entire MErGO surface[Fig.S4(A),see the Sup⁃porting Information of this paper]in the form of crinkled textures[Fig.S4(B)].From the XPS full survey spec⁃trum of the NiS/MErGO hybrid[Fig.S4(C)],the presence of Ni,S,and Cu elements is confirmed.In the high-resolution Ni2pXPS spectrum[Fig.2(B)],the Ni2p3/2and Ni2p1/2peaks appear around at 855.7 and 873.3 eV,respectively.Both correspond to the Ni3+state.In contrast,the binding energies at 856.9 and 874.5 eV are related with Ni2p3/2and Ni2p1/2of the Ni2+state,respectively.The satellites at 861.7 and 879.9 eV,two shake-up peaks of Ni are originated from its surface oxidation states[32,33].The high-resolution S2pXPS spectrum[Fig.2(C)]has three peaks of 163.8 eV(S2p1/2),164.8 eV(S2p3/2),and 187.8 eV,an indication of the presence of the S2−state.The deconvoluted C1speak[Fig.S4(D)]of the NiS/MErGO hybrid displays similar fit⁃ting peaks as that of the CuS-MoS2/MErGO hybrid.Namely,the peaks at 284.7,285.6,286.5 and 289.3 eV are related to C—C,C—O,C=C and O—C=O,respectively[34,35].Consequently,the NiS/MErGO hy⁃brid has been synthesized as expected.The presence of Ni2+state in this hybrid will result in electrochemical activities of the NiS/MErGO hybrid.
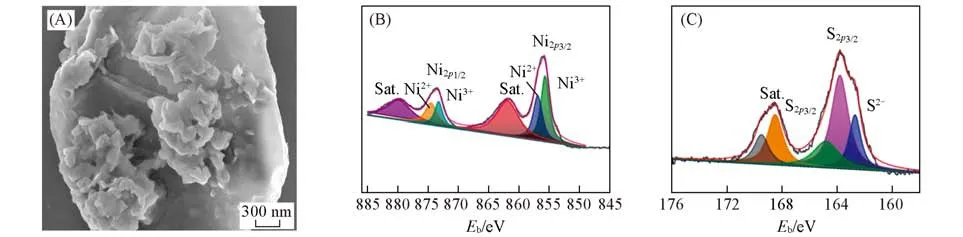
Fig.2 SEM image(A),high⁃resolution XPS spectra of Ni2p(B)and S2p(C)of the NiS/MErGO hybrid
3.2 Capacitive Performance of TMS Hybrids
The capacitive performance of the CuS-MoS2/MErGO and NiS/MErGO hybrids was firstly investigated by means of cyclic voltammetry and EIS.As control experiments,similar experiments were conducted on the CuS/MErGO and MoS2/MErGO hybrids.In their Nyquist plots recorded in either 1 mol/L Na2SO4or 0.15 mol/L NaOH[Fig.S5(A),see the Supporting Information of this paper],the CuS-MoS2/MErGO hybrid exhibits the lowest electron transfer resistance(Rct).Meanwhile,the CVs of these hybrids in 0.15 mol/L NaOH solution[Fig.S5(B)]are different within the scanned potential window from−1.0 V to 0.2 V.Within such a negative potential range,the CV of the MoS2/MErGO hybrid is almost rectangular,a typical characteristic of an electri⁃cal double-layer capacitor(EDLC).Such a feature is fully different from its CV recorded in the positive poten⁃tial ranges,where the MoS2/MErGO hybrid acts as a pseudocapacitor since the faradaic redox waves are visible[36,37].In the CVs of the CuS/MErGO and CuS-MoS2/MErGO hybrids,two pairs of redox peaks are seen,located at−0.08/−0.75 V and−0.40 V/−0.38 V,respectively.They result from the reversible faradaic redox processes of CuS+OH−⇌CuSOH+e−and CuSOH+OH−⇌CuSO+e−,respectively[38].In both cases,the CVs are nearly symmetric,an indication of good reversibility of these redox reactions.The peak currents of the CuS-MoS2/MErGO hybrid is slightly higher,when compared with those of the CuS/MErGO hybrid.A syner⁃gistic effect might thus exist between the components inside the CuS-MoS2/MErGO hybrid.
The CVs of the CuS-MoS2/MErGO hybrid were then recorded at different scan rates[Fig.3(A)].As the scan rate increase from 5 mV/s to 200 mV/s,the oxidation and reduction peak potentials shift to more positive and negative values,respectively.Namely,the difference of these redox peak potentials is enlarged as an in⁃crease of the scan rate.Such a result is due to the limited ion-diffusion rates inside this surface-controlled fara⁃daic processes.Calculated from these CVs,the specific capacitances of the CuS-MoS2/MErGO hybrid are 851.1,664.8,467.2,285.6,212.1,183.0 and 124.8 F/g at the scan rates of 5,10,20,50,100 and 200 mV/s,respectively.Meanwhile,the GCD curves of the CuS-MoS2/MErGO hybrid,measured at the cur⁃rent densities ranging from 2 A/g to 10 A/g[Fig.3(B)]exhibits two distinct plateaus,corresponding to two re⁃dox reactions of the CuS component in this hybrid.The calculated specific capacitances are 861.5,570.0,441.2,236.6 and 195.1 F/g at the current densities of 2,4,5,8 and 10 A/g.The maximum specific capaci⁃tance is 861.5 F/g and obtained at a current density of 2 A/g,obviously resulting from a sufficient charging/discharging time.As control experiments,the capacitive performance of the CuS/MErGO(Fig.S6,see the Supporting Information of this paper)and MoS2/MErGO(Fig.S7,see the Supporting Information of this paper)hybrids was studied by use of different scan rates and current densities.Stemming from the faradaic reactions of CuS in an alkaline solution,the CuS/MErGO hybrid features the pseudo-capacitive behaviour.Therefore,in its CVs clear peaks are clearly seen at different scan rates[Fig.S6(A)],together with clearly plateaus in its GCD curves[Fig.S6(B)].From these CVs,the calculated specific capacitances are 721.9,552.8,399.4,247.5,175.2 and 126.0 F/g at the scan rates of 5,10,20,50,100 and 200 mV/s.From the GCD curves,the calculated specific capacitances are 751.1,458.3,356.2,204.6 and 180.8 F/g at the current densities of 2,4,5,8 and 10 A/g.Differently,the MoS2/MErGO hybrid displays as a typical EDLC since its CVs re⁃corded at different scan rates are nearly rectangular[Fig.S7(A)].Meanwhile,its GCD curves recorded at the different current densities are almost linear and symmetric[Fig.S7(B)].From these CVs,the calculated spe⁃cific capacitances are 340.5,305.3,264.9,218.1,195.7,185.0 and 150.0 F/g at the scan rates of 5,10,20,50,100 and 200 mV/s.From the GCD curves,the calculated specific capacitances are 318.8,265.1,250.8,221.3 and 206.0 F/g at the current densities of 2,4,5,8 and 10 A/g.The comparison of the specific capacitances of three hybrids obtained by use of cyclic voltammetry at different scan rates[Fig.S8(A),see the Supproting Information of this paper]and the GCD method at different current densities[Fig.S8(B)]reveals clearly that the CuS-MoS2/MErGO hybrid always has a higher capacitance than another two hybrids when identified conditions are applied.
As an important index to evaluate the practical applications of these hybrids,their cycling stability was tested by means of cyclic voltammetry(e.g.,at a scan rate of 100 mV/s for 4500 cycles).As shown in Fig.3(C),the capacitance stability of three hybrids is fully different.The capacitance retention of the CuS/MErGO hybrid is poor(only 33.3% of initial capacitance is remained after 4500 cycles),probably due to the volume expansion and/or the loss of CuS during the charging/discharging process.The capacitance retention of the CuS-MoS2/MErGO hybrid is up to 75.3% of the initial capacitance under the identified conditions.The CuS-MoS2/MErGO hybrid shows the best cycling stability,where 84.2% of initial capacitance is remained after 4500 cycles.Such a feature is benefited from the unique nano-boxes structure of the CuS-MoS2/MErGO hybrid.
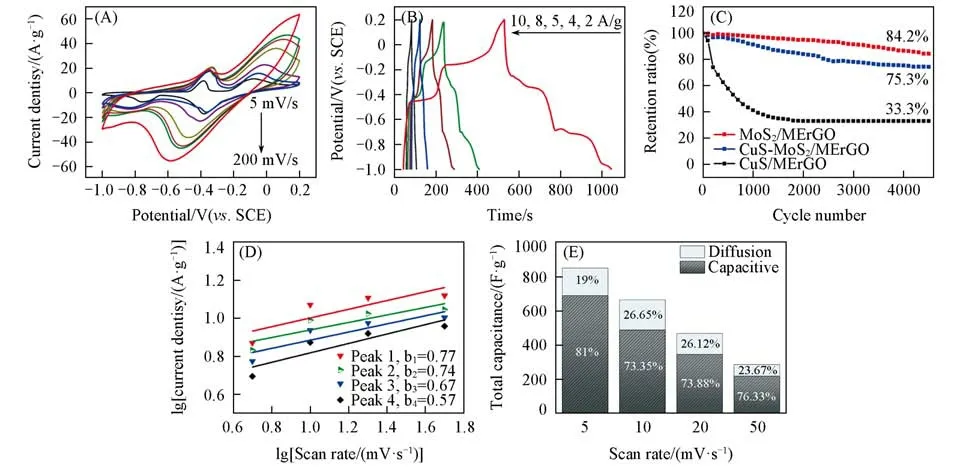
Fig.3 CVs at different scan rates(A),GCD curves at different current densities(B),the capacitance reten⁃tion as a function of charging/discharging cycles(C),variation of the logarithms of scan rates with those of corresponding peak currents in(A)(D)and contribution ratios of faradaic and capacitive pro⁃cesses during the charge storage processes(E)of the CuS⁃MoS2/MErGO hybrid
In order to investigate the charge-storage mechanism of the CuS-MoS2/MErGO hybrid,the kinetics analy⁃sis based on the as-recorded CVs[Fig.3(A)]was performed.Namely,the plot of the logarithms of scan rates versus those of corresponding peak currents was re-drawn[Fig.3(D)].The capacitive effect was characterized by analyzing peak current and the scan rate according to the formula ofi=avb,whereiis the current,νis the scan rate.Bothaandbare adjustable values[39,40].Thebvalues were then determined from the slope of Fig.3(D).The calculatedbvalues for all redox waves fall in the range of 0.6~0.8,demonstrating that the ca⁃pacitance of the CuS-MoS2/MErGO hybrid is simultaneously influenced by the mixed capacitive effect with the contributions from faradic redox reactions[21,32,39].When the scan rate is varied from 5 mV/s to 50 mV/s,the pseudocapacitive contribution rates are altered from 81.0% to 76.3%[Fig.3(E)].The results again confirm the characteristics of capacitance-controlled kinetics of the CuS-MoS2/MErGO hybrid.A higher capacitive con⁃tribution is mainly due to a more pronounced pseudocapacitive feature of hollow cubic CuS nanoparticles.Note that the MoS2coating layer is expected to increase the stability of the CuS nanoparticles during the charging/dis⁃charging processes[39,40].With the support from the porous graphene,this hybrid also has a large specific sur⁃face area and is possible to be fully contacted with the electrolyte.
The capacitive performance of the NiS/MErGO hybrid was also evaluated by use of cyclic voltammetry and the GCD method.The CVs of the NiS/MErGO hybrid in 1 mol/L Na2SO4and 0.15 mol/L NaOH solution exhibit broad redox waves[Fig.4(A)]and its GCD curves have plateaus[Fig.4(B)].They result from redox reac⁃tion between nickel ions associated with the OH−anions in the alkaline solutions[33,41].In other words,the NiS/MErGO hybrid also exhibits the pseudocapacitive characteristics.From these CVs,the calculated specific ca⁃pacitances are 580.7,409.5,266.5,144.0,102.8,87.1 and 53.5 F/g at the scan rates of 5,10,20,50,100 and 200 mV/s.From the GCD curves,the calculated specific capacitances are 848.1,768.2,635.2,574.4,420.8 and 344.2 F/g at the current densities of 1,2,4,5,8 and 10 A/g,respectively.The highest capacitance(e.g.,580.7 F/g at a scan rate of 5 mV/s or 848.1 F/g at a current density of 1 A/g)is due to a suf⁃ficient charging/discharging time.Moreover,the capacitance of the NiS/MErGO hybrid is relatively stable.For example,after the 2000 cycles at a scan rate of 100 mV/s,the capacitance of the NiS/MErGO hybrid is still remained approximately 80.8%of its initial value[Fig.4(C)].Furthermore,the NiS/MErGO hybrid has a very lowRct,as confirmed from the negligible semicircle at a high frequency region in its Nyquist plot.
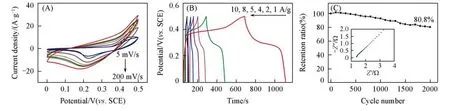
Fig.4 CVs at different scan rates(A),GCD curves at different current densities(B)and the capacitance retention(C)of the NiS/MErGO hybrid
3.3 Performance of an ASC Using TMS Hybrids
An ASC was then assembled using the positive electrode of the NiS/MErGO hybrid and the negative elec⁃trode of the CuS-MoS2/MErGO hybrid,where the electrolytes of 1 mol/L Na2SO4and 0.15 mol/L NaOH solu⁃tions were used,respectively.As-expected,the peak-shaped CVs[Fig.5(A)]are seen for the positive and negative electrodes at a scan rate of 5 mV/s within the potential ranges of 0—0.5 V and−1.0—0.2 V,respectively.The CVs of this ASC device at different voltage ranges(e.g.,varied from 0 to 1.2 V or 1.7 V)were further recorded at a scan rate of 100 mV/s[Fig.5(B)].Stable and expected capacitive behaviour is seen till 1.6 V.A higher cell voltage generally leads to a higher capacitance.However,the ASC device fails in the galvanostatic charging/discharging process when the potential is extended further to 1.7 V.Therefore,the maximum cell voltage of 1.6 V was applied to this NiS/MErGO//CuS-MoS2/MErGO ASC device for its perfor⁃mance investigation.From its CVs recorded at different scan rates[Fig.5(C)],the calculated capacitances of this ASC device are 145.0,135.7,126.1,95.1,79.5,72.3 and 53.0 F/g at the scan rates of 5,10,20,50,80,100 and 200 mV/s,respectively.Here,the mass of the hybrids in the positive and negative elec⁃trodes was used for these calculations.From the GCD curves of this ASC device recorded at different current densities[Fig.5(D)],the calculated specific capacitances are 152.5,100.1,74.5,66.2,47.0,and 39.3 F/g at current densities of 1,2,4,5,8,and 10 A/g,respectively.When the scan rate increases from 5 mV/s to 200 mV/s,the capacitance decreases from 145.0 F/g to 53.0 F/g[Fig.S9(A),see the Supporting Informa⁃tion of this paper].Similarly,the capacitance reduces from 152.5 F/g to 39.3 F/g when the current density in⁃creases from 1 A/g to 10 A/g[Fig.S9(B)].The capacitance stability of this ASC was measured within 2000 cy⁃cles at a scan rate of 200 mV/s.Even after 2000 cycles,the capacitance of this ASC remains its initial value of 84.5%[Fig.5(E)].The Ragone plot of this NiS/MErGO//CuS-MoS2/MErGO ACS device was drawn with itsEandPvalues,obtained from the GCD curves at different current densities.Here,the total mass of two electrodes was used.The ASC device delivers a maximum gravimetricEof 54.2 W·h·kg−1at aPof 1.28 kW/kg.TheEvalue of the ASC device decreases slowly with an increase of itsPvalue.At aPof 12.8 kW·kg−1,theEvalue of this ASC is still kept as 14.0 W·h·kg−1.Consequently,this ASC exhibits excellent rate capabil⁃ity.TheEandPvalues of as-fabricated ASC device is thus better than those of previously reported,including the G/CoS2/Ni3S4//GF SC device[42],the CuS-AC//AC SC device[43],the NiCo-LD@NiOOH//AC SC device[44],the CuS//AC SC device[45],the CoS2/NSC-CNT//AC SC device[46],and the MnO2/GS//graphene SC device[47].The practical application of this ASC device was testified by lighting commercial light-emitting diodes(LEDs)with a voltage of 3 V.For such tests,a demonstrator,consisting of two ASC devices in series was fabricated.This demonstrator lights this commercial LED for more than 10 min[Fig.5(G)].The different colors of the LED indicates altered charging states of this demonstrator.Therefore,this ASC is promising for some practical applications,such as renewable application in energy storage[48—50].
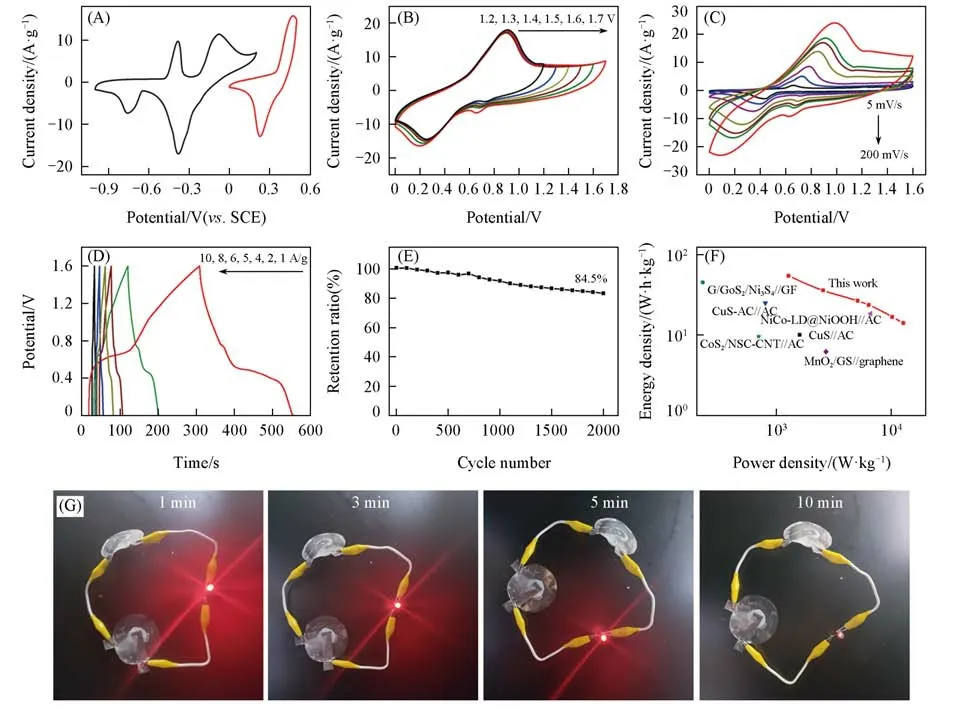
Fig.5 CVs of the positive and negative electrodes at a scan rate of 5 mV/s(A),CVs at a scan rate of 100 mV/s within altered cell voltages(B),CVs at different scan rates(C),GCD curves at different current densities(D),the capacitance retention over 2000 cycles(E),comparison of the Ragone plot of this de⁃vice with other reported devices(F)and demonstration of LED lighting at different charging times(G)of the CuS⁃MoS2/MErGO//NiS/MErGO ASC device
4 Conclusions
Transition metal sulfides supported on porous graphene have been synthesized and utilized as both posi⁃tive and negative electrodes to assemble asymmetric supercapacitors.These supercapacitors feature big and stable capacitances as well as high power-and energy-densities.Such high performance of these supercapaci⁃tors originates from the pseudocapacitive characteristics of these transition metal sulfides,a big surface area of porous graphene,and the synergistic effect of different components in these hybrids.Further studies on the influence of various transition metal sulfides and/or carbon nanomaterials on the performance of as-fabricated supercapacitors can be conducted.In summary,the transition metal sulfide hybrids proposed in this work are promising electrode materials for various electrochemical energy storage applications such as for asymmetric supercapacitors.
The supporting information of this paper see http://www.jlu.edu.cn/CN/10.7503/cjcu20200641.
This work is supported by the National Natural Science Foundation of China(Nos.61701352).
