高效催化氧还原及氧析出反应的掺杂石墨炔的设计与理论计算
2021-02-26张珊珊黄仪珺张利鹏李亚平孙晓明夏振海
马 骏,钟 洋,张珊珊,黄仪珺,张利鹏,李亚平,孙晓明,夏振海
(1.北京化工大学化工资源有效利用国家重点实验室,北京100029;2.北德克萨斯州大学材料科学与工程系,丹顿TX 76203,美国)
1 Introduction
With mightily approving of sustainable development concept,the clean and efficient renewable energy storage and conversion devices,such as fuel cells,metal-air batteries and water electrolyzers,have aroused tremendous attention currently because of their advantages of no pollution,recyclability and high energy density[1—3].From the perspective of chemical reaction principles,the core of these technologies are two funda⁃mental oxygen-involving reactions,namely oxygen reduction reaction(ORR)and oxygen evolution reaction(OER)[4,5].However,their sluggish reaction rates restrict seriously the widespread applications[6].In response to the above problem,rare metal catalysts,including platinum(Pt),iridium(Ir)and ruthenium(Ru),are commonly used to accelerate the kinetics of the ORR and OER[7—9].However,such catalysts are not only expensive and rare in reserves,but also have the weaknesses of poor durability and environmental pol⁃lution[10].Consequently,it is of great significance to explore and design low-cost catalysts with high-activity for ORR and OER[11].
As the allotrope of carbon materials,graphdiyne(GDY)has emerged as a promising candidate in the field of ORR/OER electrocatalysis since 2010[12—16].The planar GDY skeleton is fabricated by the hexagonal ben⁃zene rings and diacetylenic linkages(—C≡C—C≡C—)[17].A large amount of experiments showed that the intrinsic ORR/OER activities of GDY were enhancedviaintroducingp-region elements(N,B and S)[18—20]into its matrix.For example,Zhanget al.[18]synthesized the N-doped GDY electrode,which showed ORR perfor⁃mance comparable to commercial Pt/C,and tolerance to cross-over effect.On this basis,tremendous efforts have been devoted to further precisely control the position and the content of doping heteroatoms.The highly controllable arrangements came into outstanding catalytic activities,whether forming the pyridinic nitrogen selectively[21]or anchoringsp-hybridized nitrogen atom through pericyclic replacement of the acetylene moieties[22].In addition,Wanget al.[23]gave a route to successfully control the relative sites between nitrogen and sulfur substitution,boosting the current density for OER,higher than the commercial RuO2.
Although doping different heteroatoms is an effective strategy to improve the ORR/OER properties under experimental reports,trial-and-error approach has still been adopted to search ideal catalysts up to date[24,25],which is extremely time-consuming and resource-wasteful.On the contrary,theoretical simulations make it possible to accelerate development of advanced catalysts based on high-throughput screening and predic⁃tion[26,27].Herein,we designed heteroatom-doped GDY catalysts,and systematically studied their electronic structures,active sites and catalytic activities.The correlation between the electrochemical activity and properties of the active sites was unveiled,which provides a guiding principle for rational design of twodimensional(2D)carbon-based catalysts for clean energy conversion and storage technologies.
2 Computational Methods
The first-principles calculations of all the heteroatom-doped GDY catalysts were made utilizing the Vien⁃naAb initioSimulation Package(VASP)software package[28].Taking the density functional theory(DFT)as the framework,the projection-augmented plane wave(PAW)[29]was employed to deal with the nuclei-electron interaction,and in the meanwhile the generalized gradient approximation(GGA-PBE)[30]was applied to de⁃scribe the electron-electron effects.The cutoff energy of the plane wave basis was set to 500 eV.Under the conjugate gradient algorithm,when the energy difference between the two electron steps was less than 10−5eV and the average force of each atom was less than 0.1 eV/nm,the relaxed process reached required end.In the first Brillouin zone,a 3×3×1k-point was generated automatically withΓpoint as the center according to the Monkhorst-Pack way.According to previous reports,with regard to carbon-based catalysts,the solvent effect contributed marginally and negligibly to catalytic performance compared to the effect of heteroatom doping[31].Hence,on account of the typical monolayer nanostructure of GDY,a 1.5 nm vacuum layer was imposed along thecaxis direction to eliminate interlayer forces.
3 Results and Discussion
3.1 p-Block Element Doped GDY Structures and Stability
Scheme 1 shows the geometric structure of periodic GDY arrays.After optimization,the lattice constantaandbequaled to 0.947 nm,which is consistent with the results in the literature[32—34].The 2D network of GDY are porous structures with triangular holes that not only impedes the erosion of larger acid ions,but also keep access for transporting small molecules(O2,H2O).This unique structure is beneficial to enhance the catalyst in the acid system.In addition,since the surface of the structure is filled withsp-sp2hybrid carbon,this un⁃even electron distribution may lead to highly active sites.For convenience of expression,the atoms in the model were numbered from 1 to 12.M-GDY means that the elements in thepregion are doped into the GDY ma⁃trix,where M=B,Si,Ge,N,P,As,Sb,S,Se,Te,Cl,Br,I.Due to the ultra-high symmetry,there are three typical positions as following:1(sp2-C),2 and 3(sp-C),respectively.Due to the difference in the num⁃ber of electrons in the outer layer,the halogen elements enter the carbon skeleton in the form of adsorption while the other elements mainly substitute the carbon.For every possible positions and doping elements,we calculated the possible adsorption sites from 1 to 12.aM/GDY-b represents that heteroatom M dopes at the site-a of GDY and then it reacts at site-b.

Scheme 1 Schematic diagram of a graphdiyne model with p⁃block element doping
In order to evaluate whether the carbon skeleton is stable or not after doping,the cohesive energies(Ecoh)were calculated.It is obtained by the formula[35]:Ecoh=(EDFT−XμC−YμM)/(X+Y),whereEDFTis the total energy obtained after optimizing.μCandμMrepresent the averaged chemical potential per atom in the most crystal phase of carbon atoms and heteroatoms(Table S1,see the Electronic Supplementary Matcrial of this paper),andXandYmean the number of carbon atoms and heteroatoms,respectively.As can be seen from Fig.1,in stably existing systems,P is the easiest element to enter the carbon skeleton,while Sb is the most difficult one.According to the relatively stable sites,all the elements can be divided into two categories,the first cate⁃gory includes B and Si,which are more inclined to replacesp2-hybridized carbon atoms,and the second one in⁃cludes Ge,N,P,As,Sb,S,Se,Te,Cl,Br and I,which prefer to dope adjacent tosp-hybridized carbon.Except 1Br/GDY and 1I/GDY,most of the doped GDYs haveEcohless than zero,indicating that they are thermodynamically stable and can be synthesized experimentally.The band structures of pristine and doped GDY were further investigated as it is significant to transport electron during catalytic reactions(Fig.S1—Fig.S14,see the Electronic Supplementary Material of this paper).For pure GDY,the band gap is about 0.50 eV,which is consistent with the result previously reported[36].On the one hand,some doped structures,such as B-GDY,2Ge-GDY,N-GDY,etc.,exhibit metallic features for a certain amount of energy states at the Fermi level,while others form a band gap and spin polarization near the Femi level.However,it still re⁃mains unaffected on their conductivity because the electron is facile to jump the narrow band gap.
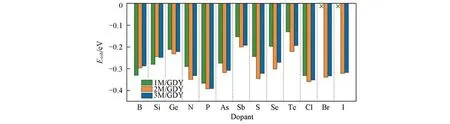
Fig.1 Cohesion energies of different heteroatoms⁃doped graphdiyne
3.2 Catalytic Performance of Doped GDYs for ORR/OER
For the above-mentioned stable structures,ORR/OER pathways were explored.According to Norskov’s theory[37,38],the ORR reaction under acidic conditions is that O2transfers four electrons to generate H2O.The mechanism mainly includes four steps:
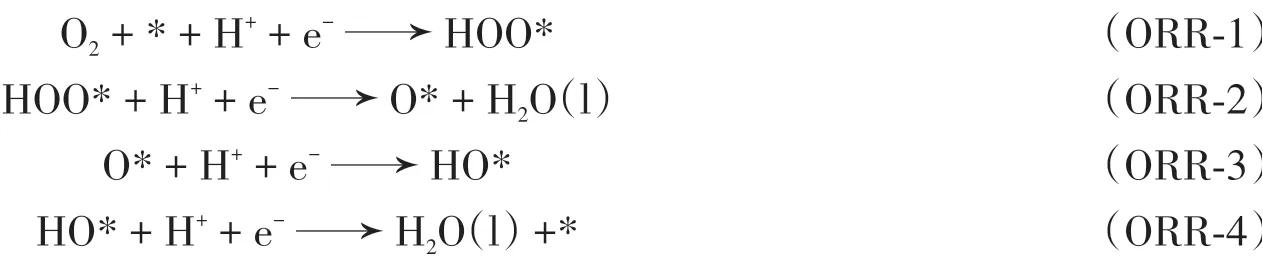
where*represents the active site,HOO*,O*,and HO*represents the adsorbed intermediate,g and l refers to the gas and liquid states,respectively.The OER can be regarded as the reverse process of the ORR.Hence,the mechanism is opposite to ORR:

The free energy change(ΔG)of the elementary reactions in every steps is calculated by the following formula[39]:ΔG=ΔE+ΔZPE−TΔS−eU−kbTln[H+],whereΔE(eV),ΔZPE(eV)andΔS(eV/K)refer to the calculated energy,zero point energy and entropy changes after reactions,respectively,T(K),e(e),U(V),kb(eV/K)and[H+]denote the temperature,charge number,electric potential,Boltzmann constant and hydro⁃gen ion concentration(pH=0),respectively.ΔG1,ΔG2,ΔG3andΔG4,referred to free energy changes of the elementary reactions from(ORR/OER-1)to(ORR/OER-4),respectively.Similar to classic Norskov’s method,the overpotential parameterηwas also introduced as a measure for the intrinsic activity of M-GDY.For the ORR,η=ΔGmin/e+1.23 V;for the OER,η=ΔGmax/e−1.23 V,whereΔGmaxandΔGminare the maximum and minimum values of free energy changes in the four-step elementary reactions,respectively[40].Correspondingly,this step is the reaction limit step.
In order to compare the performance before and after doping,the ORR/OER pathways of pristine GDY was first calculated and shown in Fig.2(A)and(B).The catalytic activity of carbon in the ethynyl units is higher than that of aromatic moiety rings(Table S2,see the Electronic Supplementary Material of this paper).The overpotentialsηof the pristine GDY are 1.33 V(ORR)and 1.10 V(OER).Under the thermodynamic equilibrium potential(U=1.23 V),steps(ORR-1)and(OER-4)are“uphill”processes,indicating that they cannot be spontaneous.In particular,the conversion of adsorbed O2to HOO*is the rate-limiting step of the ORR.Similarly,there is rate-limiting step in OER:O*combining with H2O to form HOO*and releases H+.In both ORR and OER,pure GDY has a large thermodynamic energy barrier in the catalytic processes,which is required to be further modified to boost its catalytic activities.
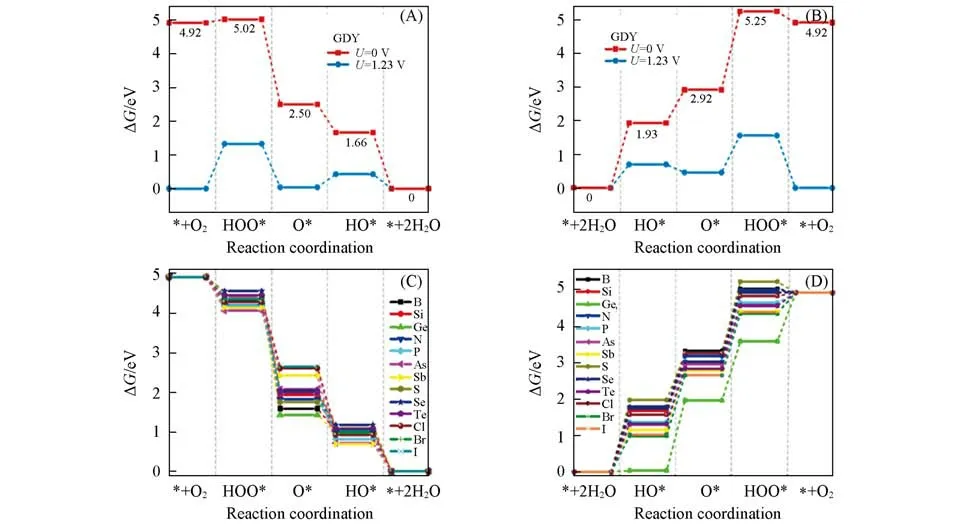
Fig.2 Free energy diagrams for ORR(A)and OER(B)of GDY at different electrode potential U,and ORR(C)and OER(D)of a series of doped GDY(U=0 V)
After introducing heteroatoms into the GDY system,a large number of possible active sites were calculated,and the best catalytic performance of the M-GDY structure was selected for each doping element,as shown in Fig.2(C)and(D).For ORR,those M-GDYs with N and Sb substitution or Cl,Br,and I adsorption onsp-C site exhibit the improved catalytic performance,but the substitution of other atoms forsp2-C results in better performance.The improvement of the catalytic activities is attributed to the heteroatom-doping that breaks the highly symmetrical electronic structure,and thus change the adsorption strength of the intermediates on the catalyst surface.This doping effect significantly enhances the ORR activity of M-GDY.Among all the doping structures,pnictogen group doping shows the best catalytic effect:1P/GDY-9 has the lowest overpotential(0.50 V),followed by 1As/GDY-4(0.51 V)<3N/GDY-4(0.52 V)<2Sb/GDY-2(0.53 V).In OER,heteroatoms can be divided into three types according to the optimal doping sites:(1)at site-1:Ge,S,Se,Te,and Cl;(2)at site-2(sp-C):Si,P,As,Br,and I;(3)at site-3(sp-C):B,N and Sb.Com⁃pared with GDY,the rate-limiting steps of the GDY doped with all the elements except for B and S elements,change from the first step(OER-1)due to the different intermediates adsorption.The GDY with Ge,P,As and Cl change to the second step(OER-2)while the limiting step become the third step(OER-3)for those with Si,N,Sb,Se,Te,Br and I.Among those doping structures,the OER overpotential of 3Sb/GDY-1 is the lowest(0.41 V),while for the other doping structures,including 2P/GDY-12(0.43 V),2As/GDY-12(0.43 V),1Cl/GDY-6(0.45 V),2Br/GDY-1(0.47 V),2I/GDY-1(0.50 V)and 3B/GDY-7(0.50 V),the OER overpotemtial is within 0.50 V.It was noted that the above overpotentials were obtained from an idealized model as most reports[40,41].Although some experimental studies indicated that macroscopic struc⁃tures had no noticeable changes before and after electrochemical measurements[23],they may still variate at the atomic level in which the carbon materials were easily oxidized.The stabilities and activities of GDY catalysts were evaluated by an hydroxyl adsorbed on the surface,one of the simplest models,in an alkaline electrolyte,as the pervious work[42].In this reaction,the performance decayed from 0.41 V to 1.12 V(Fig.S15,see the Electronic Supplementary Material of this paper),indicating that we should pay more attention to the dynamic degradation mechanisms to prevent performance downgrading in the future.For experiments,it is significant to develop more advanced characterization tools to determine how they evolve during reactions.For theoretical predictions,the model should be developed to study the detail how the GYD is oxidized in the reactions.
3.3 Scaling Relationship and Intrinsic Descriptors
Firstly,the adsorption energy of intermediates was used as a descriptor to evaluate the catalytic activity of different doped GDY structures.The adsorption energy of the reaction intermediates HOO*,O*and HO*can be calculated by the following equations[43,44]:
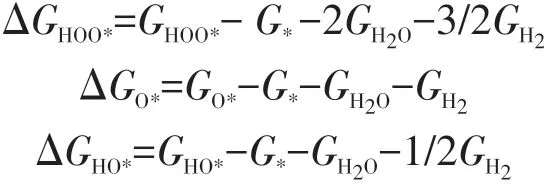
Fig.3 shows the scaling relationship betweenΔGHOO*andΔGHO*.There exists a linear correlation between them and the specific relationship is described by ΔGHO*=ΔGHOO*−3.35.Thus,in GDY system,the ad⁃sorption free energy of all the intermediates are corre⁃lated with each other and the minimum value of the po⁃tential the system could reach can be estimated from the relationship.According to the chemical principle,ΔG1+ΔG2+ΔG3+ΔG4=4.92 eV.This equation and the above scaling relation betweenΔGHO*andΔGHOO*,joint⁃ly set a constraint onΔG2+ΔG3andΔG1+ΔG4as fol⁃lows:ΔG2+ΔG3=ΔGHOO*−ΔGHO*=3.35 eV,ΔG1+ΔG4=4.92−(ΔG2+ΔG3)=1.57 eV.Hence,the ORR/OER overpotential has the minimum value,ηmin=0.44 V.Viadoping heteroatoms,the overpotentials of GDY-based catalysts almost reached the minimum value in the OER process.
As shown in Fig.4(A)and(B),multitudinous calculations and statistical results indicate that the ORR and OER overpotentials show a“volcanic”trend withΔGHO*,andΔGO*−ΔGHO*,respectively.WhenΔGHO*isca.0.8 eV,the ORR overpotential is close to the“volcano”peak,about 0.4 V.Similarly,the catalysts achieve its best OER performance(ηca.0.5 V)if the value ofΔGO*−ΔGHO*is near a region of 1.6 eV.Al⁃though the above descriptors are regarded as tools to predict the ORR/OER overpotentials of different catalytic sites in different models,it is still inconvenient to use the adsorption energies of the reaction intermediates.
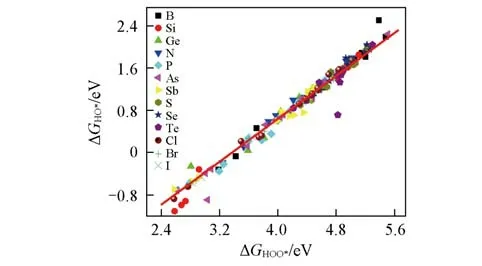
Fig.3 Relationship between HOO*and HO*adsorp⁃tion free energies of doped GDY structures at different sites
A simple intrinsic descriptorφwas found after a lot of testing and exploration.First of all,since the intro⁃duction of dopants will affect the electronic structure of carbon atoms in GDY,it is critical to effectively ex⁃press the electron transfer utilizing electronegativity,first ionization energy and electron affinity.Secondly,the difference between the radius of the heteroatoms and the carbon atom deforms the GDY structure,which eventually changes the effect on the electronic structure.Therefore,the catalytic activity is also related to the relative radius of the heteroatom and the carbon atom.Based on the above two aspects,we defined the mathe⁃matical expression of the intrinsic descriptor asφ=(χM/χC)×(IMYC/ICYM)0.5×(RM/RC),whereχ,I,YandRrepre⁃sent the electrical negativity,first ionization energy,electron affinity energy,and atomic radius,subscripts M and C indicate heteroatoms and carbon atom,respectively.The regulations of catalytic activities originates from the variation of electronic structure,which is related to the above intrinsic factors of dopants.To better investigate how the dimensionless descriptor denotes the effect of these characteristics,we calculated the elec⁃tron charge distributions,represented by 1P-GDY and 3Sb-GDY.As can be seen in Fig.S16 and Fig.S17(see the Electronic Supplementary Material of this paper),doping gave rise to the charge transfer,which altered the adsorption energies of reactive intermediates.The electron flow,from P and Sb to carbon framework,made the charge accumulated at active sites,which accordingly brought the more positive charge values for P(+1.71)and Sb(+1.03).Furthermore,the spin density redistributions were also altered by the heteroatoms substitution(Fig.S17).It is well known that the electron-rich and high-spin states are beneficial to catalytic re⁃action.Hence,it is essential to introduce parameters associated with electron transfer,such as electrical nega⁃tivity,first ionization energy,electron affinity energy and so on,so that the charge and spin density can be ex⁃pressed indirectly.In addition,unlike the graphene,there is the extreme distortion at atomic level in doped GDY chains(Fig.S18,see the Electronic Supplementary Material of this paper),which is related to the dop⁃ant radius.These basic parameters in the descriptor correlate material structure with electrochemical reactivi⁃ty.Fig.4(C)and(D)show the overpotential as a function of the intrinsic descriptor.A volcano-shaped rela⁃tionship is established between the catalytic activities(overpotential)and the descriptor.When 1.0<φ<1.3,the overpotential of ORR/OER is at the“volcanic peak”(within 0.5 V).Compared with the energy descrip⁃tors,this intrinsic descriptor can measure and predict the reactivity of ORR/OER only through the fundamen⁃tal physical and chemical parameters of the dopant and carbon atoms,which has more physical meaning and practical value for application.
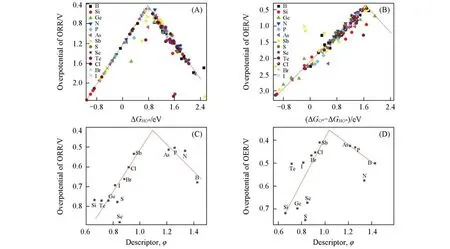
Fig.4 ORR(A)and OER(B)overpotentials versus adsorption free energiesΔGHO*andΔGO*-ΔGHO*and ORR(C)and OER(D)minimum overpotentials versus the intrinsic descriptor
We compared the catalytic characteristics of GDY and graphene,since they are two typical carbon allo⁃tropes.Firstly,heteroatom doping is a general strategy for both to facilitate the catalytic performance.According to the previous reports[40,41],N and P elements are ideal dopants to achieve highly bifunctional activ⁃ities for graphene,while P,Sb and As atoms for GDY.It is only for ORR that N-GDY exhibits satisfactory per⁃formance than noble-metal counterparts.Secondly,the electrochemical activities for GDY and graphene sys⁃tems were described in two similar descriptors,both of which were related with electrical negativity and elec⁃tron affinity energy[40,41].However,attributed to the co-exist of two hybrid types and the flexibility of its numer⁃ous diacetylenic chains,the GDY catalyst correlate more intrinsic material factors in the descriptor than gra⁃phene.Last but not the least,unlike graphene,GDY with non-uniform hybrid structures provide more modu⁃late possibilities to accelerate the catalytic processes.The network framework of GDY is not only more benefi⁃cial to the diffusion of small molecules involved in the ORR/OER,but also favorable for the design of next generation of foldable and stretchable energy devices[45,46].As an emerging candidate for ORR/OER catalysts,GDY is gaining more and more attention,and our predictions provide a theoretical base for GDY as an excel⁃lent catalyst.
4 Conclusions
We have carried out the first-principles calculations,and systematically studied the ORR and OER cata⁃lytic properties of GDY doped with differentp-block elements.The formation energy calculations show that these doping structures can exist stably.The catalysts with good ORR/OER performance are predicted by cal⁃culating the reaction pathways and overpotentials,with the priority orders of 1P/GDY-9>1As/GDY-4>3N/GDY-4 for ORR,and 3Sb/GDY-1>2P/GDY-12>2As/GDY-12 for OER.In order to efficiently predict its catalytic ac⁃tivity,we found an intrinsic descriptorφ=(χM/χC)×(IMYC/ICYM)0.5×(RM/RC),which provides a guiding principle in the design of low-cost GDY even other carbon-based catalysts for the development of clean energy conver⁃sion devices in the future.
Acknowledement
The authors thank the Sino-Foreign Cooperative Training Project of BUCT.We are also grateful to Prof.LIN Wen-feng(Loughborough University)for his insightful suggestions.
The supporting information of this paper see http://www.cjcu.jlu.edu.cn/CN/10.7503/cjcu20200332.
This work is supported by the National Natural Science Foundation of China(Nos.21675007,21676015,21520102002,91622116,51973174),the National Key Research and Development Project,China(Nos.2018YFB1502401,2018YFA0702002,2017YFA0206500)and the Royal Society and the Newton Fund Through the Newton Advanced Fellowship Award(No.NAFR1191294).
