二维材料用于电催化析氢的研究进展
2021-02-26史江维孟楠楠郭亚梅于一夫
史江维,孟楠楠,郭亚梅,于一夫,张 兵
(1.天津大学化工学院工业催化系,理学院,2.天津大学分子+研究院,天津300072)
1 Introduction
Energy crisis and environmental pollution have become the serious challenges for the sustainable develop⁃ment of human society[1—4].Seeking for clean and renewable energy is of great interest to solve these problems[5—9].Molecular hydrogen(H2)emerges as a promising energy source due to its high energy density,small molecular mass,and no pollution,has received more and more attention in recent decades[10,11].Current⁃ly,there are main three kinds of hydrogen production approaches,including methane reforming,water-gas shift,and water electrolysis[12,13].Among them,water electrolysis,especially driven by clean wind electricity and photo electricity,shows promising potential[14—16].Although precious metals(such as Pt,Ir,and Ru)show the excellent activity towards hydrogen evolution reaction(HER),the scarcity and high price hindered their wide applications[17—20].Thus,exploiting novel and cheap electrocatalysts with high HER performance is of great importance[21,22].During recent years,two-dimensional(2D)nanomaterials have been widely used in the field of electrocatalysis due to their low cost,abundant low-coordination surface atoms and large specific surface area[23—27].Consequently,the development of advanced 2D nanomaterials with excellent electrocataly⁃sis performance for HER is of great importance[28,29].
In this review,we provided a summary of recent progress in 2D materials for electrocatalytic HER.A series of synthetic methods was firstly summarized,including top-down and bottom-up strategies.Then,we discussed the recent strategies to enhance HER activity of 2D materials,such as edge engineering,defect engineering and doping engineering.Finally,some challenges and opportunities in this field were provided.
2 Synthesis Methods
Enormous efforts have been made by researchers to enrich the synthetic approaches for 2D materials[30].Up to now,they could be classified into top-down and bottom-up strategies[31].Top-down strategy referred to a process in which bulk crystals were physically broken into 2D pieces.Mechanical cleavage and liquid exfolia⁃tion were typical top-down synthesis strategies.In comparison,bottom-up synthesis adopted chemical reac⁃tions to produce 2D materials,including chemical vapor deposition(CVD),solvothermal synthesis,and tem⁃plate-assisted synthesis[32].
2.1 Top-down Synthesis
In layer-structured materials,chemical bonds existed in each layer,while adjacent layers combined to⁃getherviavan der Waals force.Compared to bonding energy,the van der Waals force was much weaker.The external force could break the van der Waals force and drive the exfoliation of bulk layer-structured material into 2D nanosheets.Based on the type of external force,the top-down synthesis strategies could be divided in⁃to mechanical cleavage and liquid exfoliation.
2.1.1 Mechanical Cleavage
2D materials prepared by mechanical cleavage were often used for research on their physical,electronic,and optical properties owing to their clean surface,excellent crystal quality,and minimum defects[33].In 2004,Novoselov and coworkers[34]firstly used the Scotch-tape method to synthesize single-layer graphene nanosheets.Since then,this synthesis method has been widely used to obtain ultrathin 2D nanomaterials,in⁃cluding CuInP2S6[35],BP[36],h-BN[37],transition metal dichalcogenides(TMDs),metal oxides,etc.The typi⁃cal mechanical cleavage process for producing graphene was intuitively shown in Fig.1[38].The fresh surface of the bulk crystals was pasted on the Scotch tape,and then another Scotch tape peeled it into a thin sheet.A suitable thin slice could be obtained after repeating this process[33].But,the low production and non-uniformi⁃ty of the produced 2D nanosheets severely retarded the wide application of this method.In addition,the me⁃chanical exfoliation is an important method to prepare individual nanosheet-based electrocatalytic device[39].In 2016,Wanget al.[40]produced a field-tuned HER device with a single MoS2nanosheet by mechanical peeling.The influence of field effect on catalysis was explored through this electrocatalytic device.When an electric field was employed,the catalytic performance of MoS2nanosheets was significantly improved.Subsequently,they developed an individual MoS2nanosheet-based device to conduct a systematic study to understand the relationship between HER performance and electrode conductivity[41].The experiment found that as the electrode conductivity increased,the initial overpotential continued to decrease.
2.1.2 Liquid Exfoliation
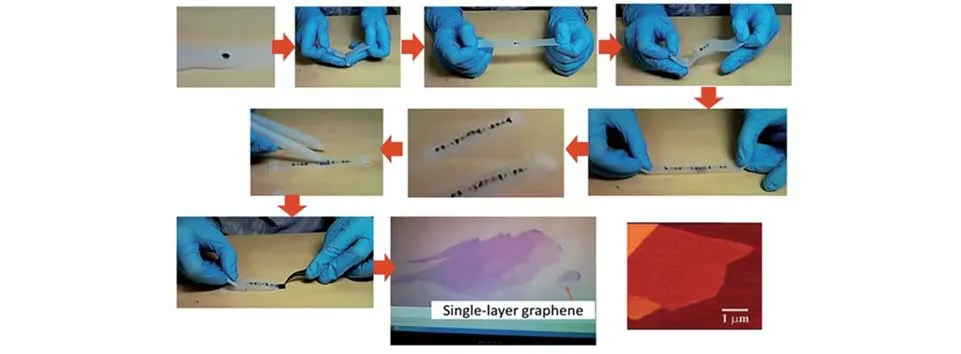
Fig.1 An intuitive review of mechanical cleavage for the production of graphene[38]
Ultrasonic treatment was widely used to break the van der Waals force between adjacent nanosheets in a proper solvent,as shown in Fig.2(A)[42].In 2008,graphene was synthesized with a liquid-phase exfoliation method by using the ultrasonic treatment inN-methyl-pyrrolidone[43].Subsequently,various solvents were studied.Kimet al.[44]used ultrasound to synthesize 2D MoS2,WS2,TaS2,and TiS2nanosheets withN-vinyl⁃pyrrolidone as a solvent.Recently,Wang and coworkers[45]developed a facile liquid-phase exfoliation strategy to produce few-layer BiI3nanosheets in green solvents such as methyl salicylate,triacetin,ethyl benzoate,and diethyl phthalate as well as their binary mixtures.This method could produce well-dispersed 2D materials with about 30% yield and a concentration of 1.56 mg/mL.Ions intercalation could further extend the liquid phase exfoliation method to synthesize various kinds of 2D nanosheets with high quality.For example,Zhanget al.[46]utilized the charging process of lithium battery to intercalate lithium ions into the interspacing of layered bulk crystals[Fig.2(B)].The distance between adjacent layers was widened,and the van der Waals force becomes weak.During ultrasonic treatment,lithium ions reacted with water solvent to produce hydrogen gas.As a result,the pressure in the interspacing increased,and bulk crystals could be easily exfoliated into 2D ultrathin nanosheets in a high yield.Using this ions-intercalation assisted liquid exfoliation method,they successfully prepared high-yield and high-quality single-layer TiS2and TaS2nanosheets[47].Then,Pt and Au nanoparticles(NP)were deposited on the obtained nanosheets with high surface coverage.The Pt-TiS2composite showed excellent electrocatalytic activity in HER.
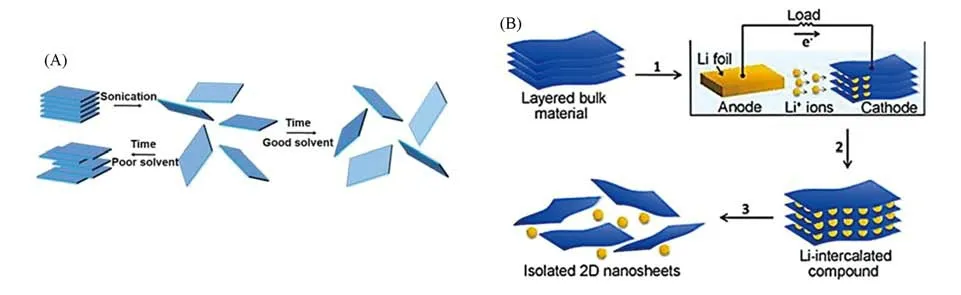
Fig.2 Scheme for the liquid exfoliation of bulk materials into 2D nanosheets by ultrasonic treatment(A)[42]and electrochemical lithium ions intercalation process for the fabrication of 2D nanosheets from the layered bulk materials(B)[46]
2.2 Bottom-up Synthesis
The top-down synthesis method was usually suitable for layer-structured materials,while bottom-up synthesis could be used to prepare layered and non-layered materials.In a typical bottom-up synthesis proce⁃dure,proper precursors proceeded chemical reaction under given conditions to grow in 2D orientation.As a result,2D nanosheets were obtained.For non-layered structured materials,the key for the formation of 2D materials lied in the limitation of axial growth.Based on the type of chemical reaction,the bottom-up synthesis strategies could be divided into solvothermal synthesis,chemical vapor deposition(CVD),and template-assisted synthesis.2.2.1 Solvothermal Synthesis
During the solvothermal process,the precursors were mixed in a reactor containing water and/or organic solvents.The reaction temperature was generally higher than the boiling point of the solvent,resulting in a high-pressure environment in the reactor.The formation of 2D materials contained two processes of nucleation and growth[48].The key lied in the adjustment in the growth process to form 2D materials.Xieet al.[49—52]syn⁃thesized a variety of 2D materials by directly using solvothermal method,including Co3O4,ZnS,CeO2and Co9Se8nanosheets.Moreover,the solvothermal process could also produce 2D precursors for chemical conver⁃sion synthesis of novel 2D materials[53].For example,we synthesized Sub-1.1 nm ultrathin porous CoP nanosheets[Fig.3(A)]by the combination of solvothermal and chemical conversion[54].The CoP nanosheets were endowed with a unique porous structure,ultrathin morphology,and a high proportion of reactive{200}planes,which greatly improved the catalytic activity for HER.The required overpotential was only 131 mV for a current density of 100 mA/cm2.The high mass activity achieved 151 A/g at an overpotential of 100 mV and maintained well over 20 h[Fig.3(B)and(C)].Recently,metal-based 2D materials have also shown excellent performance for electrocatalytic HER.Sunet al.[55]synthesized CoFe alloy nanosheets on the Ni foam substrate by the combination of hydrothermal method andin situchemical reduction.After loading a small amount of Pt,the as-obtained novel 2D materials exhibited high catalytic activity for HER.Yinet al.[56]proposed a novel hydrothermal synthesis strategy to achieve simultaneous adjustment of crystalline phase and disorder in 1TMoSe2nanosheets,thereby significantly enhancing its HER catalytic activity.Luet al.[57]reported a simple and cost-effective hydrothermal strategy using polypyrrole(PPy)as a template to synthesize MoS2nanosheets,which had sufficient active edge sites and advanced HER performance.Besides,Zhenget al.[58]reported a solvothermal method to synthesize RuPd metal hydride through the chemical release of H from the formalde⁃hyde solution during the synthesis process[(Fig.3(D)].Hydrogen atom stabilized the RuPd bimetallic materi⁃al and improved its activity for HER.2.2.2 Chemical Vapor Deposition

Fig.3 TEM image of CoP nanosheeets(A),the corresponding Tafel plots of CoP ultrathin porous nanosheets(UPNSs),CoP nanoparticles(NPs)and 20% Pt/C in 0.5 mol/L H2SO4 at a scan rate of 2 mV/s(B),mass activity as a function of the overpotential for CoP UPNSs and NPs(C)[54]and scheme for the growth process of RhPd⁃H(D)[58]
CVD could be used to prepare 2D nanomaterials and thin films on solid substrates.In a typical CVD pro⁃cess,the precursor was placed on high-temperature zone and vacuum chamber,and the substrate was placed on the low-temperature zone.Under the condition of high temperature and high vacuum,the reactants vapor⁃ized and the product of the desired 2D material or film deposited on the substrate[59].In 2009,Betonet al.[60]synthesized single-layer grapheneviathe CVD method.Subsequently,researchers synthesized a series of 2D nanosheets with different sizes and morphologies by controlling the CVD parameters,including temperature,pressure,time,substrate,and precursors.Zhanget al.[61]synthesized uniform single-layer ReSe2nanosheets with variable morphology(sunflower shape and frustotriangular shape)on SiO2/Si substrate under different en⁃vironmental pressures by CVD.The as-prepared polycrystalline ReSe2sheets were completely transferred to the Au foil electrode for HER test.The results showed that the sunflower-shaped slices exhibited superior elec⁃trocatalytic HER activity compared to the frusto-triangular ReSe2nanosheets due to their edge-abundant sites.Gaoet al.[62]synthesized MoS2vertical nanosheets with the CVD method[Fig.4(A)].Matsueet al.[63]synthe⁃sized MoS2and WS2hetero-nanosheets using the CVD method.They used high-resolution scanning electro⁃chemical cell microscopy(SECCM)to image and quantify the HER catalytic active sites,and provided local cyclic voltammograms with nanoscale spatial resolution[Fig.4(B)].These results revealed the difference in HER activity at the edges,steps,and heterojunctions of MoS2and WS2as shown in Fig.4(C)and(D).2.2.3 Template⁃assisted Synthesis

Fig.4 Schematic diagram for dual temperature zone CVD synthesis of vertical MoS2 nanosheets on glassy carbon(A)[62],the current image of MoS2 nanosheets on highly oriented pyrolytic graphite(HOPG)substrate with 15μm×15μm of scan sizes and 130 V/s of scan rate at-1.3 V vs.RHE sweep voltage(B),overpotential(C)and Tafel slope(D)on the MoS2 edge(red),terrace(green),and HOPG edge(grey)regions[63]
The flat substrate could serve as a template to guide the 2D growth of materials[64].It was reported the growth of hexagonal close-packed(hcp)gold nanosheets on graphene oxide(GO)nanosheet template in 2011[65].As shown in Fig.5(A),the Au nanosheet showed an edge length of 200—500 nm and a thickness ofca.2.4 nm(ca.16 Au atomic layer).Subsequently,Fanet al.[66]reported a method of synthesizing 4H/fcc trimetallic Au@PdAg core-shell nanoribbons(NRBs)by adopting 4H/fcc Au@Ag NRBs as template.The asobtained 4H/fcc Au@PdAg NRBs exhibited excellent electrocatalytic activity for hydrogen evolution,even close to commercial Pt black[Fig.5(B)and(C)].The bulk salts were promising template candidates owing to their high thermal stability and solubility in water.Huanet al.[67]used sodium chloride crystal powder as a tem⁃plate to prepare TMDs nanosheets,including TaS2,NbS2,and V5S8.The synthesis process of TaS2was shown in Fig.5(D).Cubic NaCl crystals were prepared through recrystallization[67].Nanosheets grew on NaCl cubic crystals,and then they were obtained by dissolution of salt in water.The as-produced TaS2nanosheets with the uniform thickness could serve as HER catalysts[Fig.5(E)].

Fig.5 Typical TEM image of Au nanosheets on graphene oxide(GO)with a scale bar of 500 nm(A)[65],onset po⁃tentials and overpotentials(at the current density of 10.0 mA/cm2)of 4H/fcc Au@PdAg NRBs,Pd black,and Pt black(B)and the corresponding Tafel plots(C)[66],schematic illustration for the growth of 2D TaS2 nanosheets on micron⁃sized NaCl crystals(D)and schematic illustration of TaS2 for the HER process(E)[67]
3 Strategy for Improving HER Activity
Generally,an ideal HER catalyst possessed abundant active sites,and the active sites exhibited high in⁃trinsic activity.2D materials,especially ultrathin 2D nanosheets,were furnished with abundant low-coordina⁃tion surface atoms and large specific surface areas[68].The optimization of the atomic structure of 2D materials could further improve their HER activity.Moreover,2D materials could serve as an excellent platform for HER mechanism investigation because they could be easily and clearly characterized.Coupling with oxidative reaction was also a way to promote HER activity.For example,the anodic oxidation of hydrazine and tetrahy⁃droisoquinoline was developed to raplace the oxygen evolution reaction[69,70].In the following part,we will mainly discuss the effect of edge engineering,defect engineering,and heteroatom doping on HER activity by adopting 2D materials as model electrocatalysts.
3.1 Edge Engineering
As we all know,HER active site located at the edge of TMDs[12].Edge engineering aimed to expose as many as edge sites.Cui group[71]successfully grew MoS2and MoSe2thin films with vertically aligned layers,thereby maximally exposing the edges on the film surface.At the same time,they further confirmed the catalytic activity sites of the exposed edge.Subsequently,they realized the vertical layer orientation growth of MoS2and MoSe2films on curved and rough surfaces[Fig.6(A)][72].Compared with flat substrates,MoSe2and WSe2nano-films on carbon fiber paper exhibited more efficient electrocatalysis activity for HER,and both showed extremely high stability in acidic solutions.Zhanget al.[73]reported the controllable synthesis of single-layer MoS2flakes with dendritic fractal shapes on specific insulator SrTiO3[Fig.6(B)and(C)].The dendritic mono⁃layer MoS2with a large number of edges could show a relatively low Tafel slope of 73 mV/decade in 0.5 mol/L H2SO4.Xuet al.[74]reported a new strategy for the production of a strongly coupled MoS2nanosheet-carbon macroporous hybrid catalyst(S-MoS2@C)as shown in Fig.6(D).The engineered unsaturated sulfur edge en⁃hanced HER activity.

Fig.6 Scheme of MoSe2 nanofilm with molecular layers perpendicular to a curved surface(A)[72],schematic view illustrating the edges of fractal monolayer MoS2 flakes as active catalytic sites for HER(B),polarization curves of bare Au foil and MoS2/Au foils(a,b and c represent the coverage of 12%,40%,and 60% for MoS2,respectively)(C)[73]and schematic illustration of the preparation process and microstructure of un⁃saturated sulfur edge MoS2nanosheet⁃carbon macroporous(D)[74]
3.2 Defect Engineering
Defect could adjust the electronic structure of materials and thus affect their HER activity.Theoretical calculations showed that with the increase of S vacancy concentration,theΔGH*on the defective MoS2kept de⁃creasing.The optimal value reached when the S vacancy concentration was between 12%and 16%[Fig.7(A)and(B)][75].When the S vacancy was introduced,a new band appeared in the gap near the Fermi level.More vacancies induced the band closer to the Fermi level,resulting in the generation of dangling bond states[75].Jinet al.[76]synthesized a new type of porous metallic-phase 1T-MoS2nanosheets by liquid ammonia assisted route.The porous sheets had larger edges and S vacancies than conventional 1T-phase MoS2,so there was ex⁃cellent HER catalytic activity in porous 1T-phase MoS2nanosheets,as shown in Fig.7(C).Recently,plasma emerged as a powerful tool to create defects in the materials.We proposed a plasma-induced dry exfoliation method to prepare Co3S4ultrathin porous nanosheets with a large number of sulfur vacancies(Co3S4PNSvac)[Fig.7(D)][77].The nanosheet had an initial overpotential of only 18 mV and a superior mass activity of 1056.6 A/g under an overpotential of 200 mV.Liet al.[78]manufactured S vacancies on the 2H-MoS2basal surface through plasma treatment.The optimized MoS2nanosheets showed excellent HER activity,with an overpotential of 128 mV at 10 mA/cm2and a Tafel slope of 50 mV/dec.

Fig.7 Scheme for the top(upper panel)and side(lower panel)views of MoS2 with strained S⁃vacancies on the basal plane(A)and free energy vs.the reaction coordinate of HER for the S⁃vacancy range of 0—25%(B)[75],scheme for mesoporous 1T⁃MoS2 nanosheets for catalyzing HER(C)[76]and scheme for the preparation of Co3S4 PNSvac(D)[77]
3.3 Heteroatoms Doping
The doping of heteroatoms could change thed-band electronic structure and reduce the free energy of hydrogen adsorption.The basal plane of pure graphene was generally considered to be inert for the HER due to its relatively largeΔGH*(1.85 eV).Experiments proved that doping with non-metal atoms could activate graphene for HER[79].Thus,graphene doped with nitrogen and phosphorus was successfully preparedviaa one-step solid-state reaction of urea,glucose,and phosphoric acid[80].The as-prepared material showed excel⁃lent electrocatalytic HER activity with an onset overpotential of 0.12 V and a low Tafel slope of 79 mV/dec.Xie and coworkers[81]succeeded in construct disordered structure and oxygen doping into MoS2.The disor⁃dered structure provided sufficient unsaturated sulfur atoms as active sites for HER,and the introduction of ox⁃ygen effectively changed the electronic structure,as shown in Fig.8(A)and(B).The optimized catalyst showed excellent activity for HER with an initial overpotential as low as 120 mV,extremely high cathode cur⁃rent density,and excellent stability[Fig.8(C)].Moreover,the doping of metal heteroatoms into 2D materials was efficient to improve catalytic performance for HER.Chenet al.[82]successfully synthesized graphene nanosheets doped with nickel single atoms[Fig.8(D)].Thesp-dorbital charge transfers between the Ni dop⁃ant and the surrounding carbon atoms produced a local structure with empty C-Ni hybrid orbitals[Fig.8(E)].It exhibited excellent HER catalytic performance in a 0.5 mol/L H2SO4solution,including a low overpotential of about 50 mV,a Tafel slope of 45 mV/dec,and excellent cycle stability.Yanget al.[83]reported a two-step method to obtain 2H-MoS2doped with different metals[Fig.8(F)].This method prevented the doped metal atoms from aggregation,phase separation,and edge enrichment.Doping of cobalt and palladium on MoS2greatly enhanced the catalytic activity of HER with an overpotential of 49 mV at 10 mA/cm2and a Tafel slope of 43.2 mV/dec.

Fig.8 Schematic representation of the disordered structure in oxygen⁃incorporated MoS2 ultrathin nanosheets(A),constructed model of an individual oxygen⁃incorporated MoS2 nanodomain and the illustration of the HER process at the active sites(B),polarization curves of the oxygen⁃incor⁃porated MoS2 ultrathin nanosheets(C)[81],TEM image of Ni⁃doped nanoporous graphene(G)(D),hydrogen adsorption sites and configuration of the interstitial atoms in the hollow centers of the benzene rings(Nisub))/G model withΔGH*=-0.10 eV(left)and calculated Gibbs free energy dia⁃gram(right)of the HER at equilibrium potential for a Pt catalyst and Ni⁃doped graphene(substi⁃tutional dopants occupying C sites in the graphene lattice(Niab)/G,Nisub/G,and anchoring atoms on defect sites(Nidef)/G)samples(E)[82]and schematic illustration for the synthesis of multi⁃hetero⁃atoms⁃doped MoS2(M⁃MoS2)(F)[83]
4 Conclusions and Perspective
This review summarized the recent progress in 2D materials for HER.We introduced the synthesis methods of 2D materials,including top-down and bottom-up methods.Among them,exfoliation method,CVD method,hydrothermal method,and template method,which are commonly used for synthesizing 2D materials were elaborated in detail.At the same time,modification strategies to improve HER activity of 2D materials have also been reviewed,such as edge engineering,defect engineering,and doping engineering.Edge engi⁃neering and defect engineering could create a large number of HER active sites,while the intrinsic activity was significantly improvedviadoping engineering.
Although there has been great progress in achieving high HER catalytic performance for 2D materials,we still face some challenges and opportunities in this field.First of all,the HER mechanism catalyzed by these 2D nanomaterials was still unclear,and further exploration was required.Exploring advancedin situcharac⁃terization and developing individual nanosheet-based electrocatalytic device were urgently needed.Then,there was still a lack of work dedicated to systematically studying the contributions of defects,strains,vacan⁃cies,and phase transitions.Thirdly,the corrosion of the catalyst in the electrolysis process still plagued peo⁃ple,and the development of a robust catalyst was urgently needed.It is a good choice to develop new synthetic methods and modification strategies.Besides,coupling with oxidative alternative reactions could also boost H2evolution reaction.Finally,it was extremely important to find cheap,efficient,and stable alternatives for HER.We believe that the fascinating structure and electronic properties of 2D nanomaterials,as well as the strategies for controlling their morphology and electronic structure,will open up important technological oppor⁃tunities for future applications in the hydrogen production industry.
This work is supported by the National Natural Science Foundation of China(Nos.22071173 and 21701122)and the Natural Science Foundation of Tianjin City,China(No.17JCJQJC44700).
