Hepatocellular carcinoma with tumor thrombus in bile duct: A proposal of new classification according to resectability of primary lesion
2021-01-13DiZhouGangFengHuWeiChenGaoXiaoYuZhangWenBinGuanJianDongWangFeiMa
Di Zhou, Gang-Feng Hu, Wei-.Chen Gao, Xiao-Yu Zhang, Wen-Bin Guan, Jian-Dong Wang, Fei Ma
Abstract
Key Words: Hepatocellular carcinoma; Tumor thrombus; Bile duct; Diagnosis; Treatment
INTRODUCTION
Hepatocellular carcinoma (HCC) with tumor thrombus in the bile duct (BDTT), a special pathological morphology in HCC, was gradually discovered and has attracted attention since the middle of the last century. BDTT was first reported by Malloryet al[1]in 1947. In 1975, Linet al[2]named BDTT “Icteric-Type Hepatocarcinoma” on the basis of the symptoms of jaundice. Prior to the 1990s, the incidence of HCC combined with BDTT was quite low, accounting for only 0.53% to 2% of all HCC cases. Recently, some studies have reported that 12.9% of all HCC patients have BDTT, and many among them do not have gross jaundice at first diagnosis[3-5]. In terms of prognosis, the median tumor-free survival of patients with BDTT is 8 mo and that of patients without BDTT is 33 mo. The 1-, 3-, and 5-yr cumulative tumor-free survival rates of HCC patients with and without BDTT are 37.2%, 11.5%, and 0% and 59.4%, 47.9%, and 24.5%, respectively. The 5-yr survival of HCC patients with BDTT is 20 mo less on average than those without BDTT[6,7].
Pathologically, BDTT is mostly formed in the background liver of nodular cirrhosis caused by hepatitis B virus (HBV) or hepatitis C virus(HCV). In general, BDTT is a purple-black, soft thrombus without dense adhesion to the bile duct wall, which is the pathological basis of the commonly used bile duct thrombus extraction in clinical practice[8,9]. There are two pathological types of BDTT. The first is mainly composed of cancer cells, which are yellow-gray in color after fixation. The other type, called ”cancerous thrombosis,” is composed of blood clots and cancer cells caused by invasive hemorrhage of the bile duct wall. Regarding the features of primary HCC lesions that cause BDTT, the tumors are mostly diffuse or invasive with moderate or poor differentiation, no capsule or only partial capsule, and strong invasiveness, which causes BDTT formation. It is worth noting that some primary HCC lesions are too small to be detected by preoperative imaging examinations.
Notably, there are two kinds of BDTT for which the alert should be triggered: (1) BDTT without a macroscopically visible first-occurring HCC mass; and (2) BDTT as the first and only manifestation of recurrent HCC with no liver-occupying lesion. Nonspecific clinical and laboratory findings, such as slight cholangitis without jaundice, normal alpha-fetoprotein (AFP) value and localized bile duct dilatation, are not uncommon among patients with the abovementioned two kinds of HCC with BDTT[10]. Because of insufficient vigilance in this disease, BDTT easily tends to be misdiagnosed as cholangitis or choledocholithiasis, especially in patients with a history of radical hepatectomy with no abnormal clinical manifestations during their follow-up period.
To date, most of the published papers regarding HCC with BDTT have focused on its surgical procedures, but very few have discussed the relationships between the primary tumors and thrombus from the etiological and pathological perspective, which might be more critical for the diagnosis and treatment. In the present retrospective study, we reviewed and analyzed our diagnosis and treatment experience regarding seven HCC patients with BDTT, including misdiagnosed cases. We also propose a new classification for HCC with BDTT based on its clinicpathological features.
MATERIALS AND METHODS
Patients
Seven patients diagnosed with HCC with BDTT were admitted to our department between January 2010 and December 2019 (Table 1). Among them, only three were referred to the outpatient clinic due to visible jaundice. Three patients received hepatectomy for first-occurring HCC lesions, and BDTT was treated by liver resection, extrahepatic bile duct resection, and choledocholithotomy with thrombus extraction. Then, the BDTT was extracted through choledocholithotomy without further tumor resection in three patients due to extensive intra- and extrahepatic metastatic tumors or undetectable HCC lesions. The last remaining case was clinically confirmed as HCC with BDTT by the history of multiple recurrent HCC, the rising value of AFP > 700 ng/mL, and distal intrahepatic bile duct dilation.
Ethical issues
The study was performed in accordance with the Declaration of Helsinki. Ethical approval for the present study was granted by Xinhua Hospital Affiliated to Shanghai Jiao Tong University, School of Medicine (Shanghai, China). The study was strictly in accordance with the Declaration of Helsinki and International Ethical Guidelines for Health-related Research Involving Humans. All the included patients signed an informed consent form. A multidisciplinary team made up of hepatobiliary surgeons, radiologists, oncologists, gastroenterologists, and pathologists selected candidates for the treatment together.
Inclusion and exclusion criteria
Inclusion criteria: (1) Patients diagnosed as HCC with BDTT based on pathology or typical imaging findings; and (2) Patients with well-defined outcomes including total hospital stay and follow-up results of survival related data.
Exclusion criteria: (1) Patients diagnosed as non-HCC tumors or other diseases causing thrombus in biliary ducts; (2) Patients diagnosed as primary biliary tumors; (3) Patients diagnosed as mixed tumors of hepatobiliary systems with thrombus in the bile ducts; and (4) Patients who did not have well-defined outcomes or follow-up results.
Methods
All patients received serum AFP detection and imaging tests, including multidetector computed tomography scans and magnetic resonance cholangiopancreatography (MRCP). Positron emission tomography-computed tomography (PET-CT) was performed for patients who were suspected to have metastasis. The imaging data (images and diagnostic reports) were reviewed independently by two experienced radiologists, and a consensus was reached upon confirmation of the main findings.
Endoscopic retrograde cholangiopancreatography (ERCP) or percutaneous transhepatic cholangiography (PTC) was performed when patients had difficulty in diagnosis of BDTT or biliary drainage was indicated. ERCP: Side-viewingduodenoscope (JF-260V, Olympus Medical System, Tokyo, Japan) was inserted through the mouth and passed all the way to the duodenum. After the ampulla of Vater was confirmed by camera, catheters and cannulas were passed through the duodenoscope and then into the biliary tree at the ampulla of Vater. Radiographic contrast material (Diatrizoate meglumine, Xudong Haipu Pharmaceutical Co., LTD, Shanghai, China) was injected so the biliary tree was visualized under X-ray. PTC: The right hepatic duct (RHD) was punctured by a 22G needle (Neff Percutaneous Access Set, Cook Medical LLC, Bloomington, IN, United States). The biliary tree was shown by injecting diatrizoate meglumine (Xudong Haipu Pharmaceutical Co., LTD, Shanghai, China)viathe needle. After radiography, an 8.5F external drainage tube (Biliary Drainage Catheter, Cook Medical LLC, Bloomington, IN, United States) was placed in the common bile duct (CBD).

Table 1 Basic clinical data of the patients of hepatocellular carcinoma with tumor thrombus in bile duct
Pathological reports were also performed in a similar way by pathologists. The anatomical location of the BDTT was confirmed and categorized according to the Satoh classification system: Type 1 [BDTT located in the first branch of the hepatic duct but not reaching the confluence of the RHD and left hepatic duct (LHD)]; type 2 (BDTT extending across the confluence of the RHD and LHD); type 3 (BDTT separate from the primary HCC lesion and growth in the CBD)[11].
Follow-up
The patients were requested to be re-examined at outpatient visits every 1-2 mo after surgical treatment by receiving physical examinations, routine full blood counts, liver function tests, tumor marker sets, computed tomography (CT), and magnetic resonance imaging (MRI)/MRCP with data recorded in the electronic medical record. All of the living patients were followed up until December 2019.
Literature review
A systematic search was performed for the causes of misdiagnosis and mistreatment of HCC with BDTT using PubMed, Embase, Ovid, and Web of Science independently by two researchers. The relevant studies published from January 2000 until January 2020 were searched and gathered through the following terms: Hepatocellular carcinoma with tumor thrombus in the bile duct, BDTT OR BDT OR bile duct tumor thrombus. Studies that focused on nonprimary hepatocellular carcinoma, did not provide enough information on misdiagnosis or mistreatment of HCC with BDTT, and papers published in languages other than English were excluded.
RESULTS
Background of liver cirrhosis
Patients in this study included 6 men and 1 woman who ranged in age from 58 to 75 years. The clinicopathological characteristics of the patients are summarized in Table 1. Four patients (No. 1, 4, 5, 6) were positive for HBsAg, HBeAb, and HBcAb, and one patient was positive for HCV-AB-IgG (No. 3). Among them, four patients (No. 1, 4, 5, 7) were pathologically diagnosed with cirrhosis. HBV-DNA was detected by means of fluorescent quantitative PCR for patients with positive HBsAg, and the results showed < 0.5 × 103cps/mL for all of the patients (Table 2).
Initial diagnosis
The results of CT or MRI suggested HCC with BDTT for 4 patients (No. 2, 4, 6, 7) as the initial diagnosis. However, the primary HCC lesion of patient No. 1 was misdiagnosed as a gastrointestinal stromal tumor of the stomach. For patient No. 5, BDTT was mistreated as CBD stones by ERCP without a pathological examination at his first admission. Missed diagnosis occurred in one patient (No. 3) who had a history of multiple recurrent HCC.
Clinicopathological characteristics of BDTT and primary HCC lesions
Patient No. 1 was diagnosed with poor differentiation of massive HCC (8 cm × 6 cm × 6 cm) with microscopic BDTT, portal vein tumor thrombus and gastric invasion (Figure 1, Table 3).
For patient No. 2, recurrence in the form of BDTT in CBD (Satoh’s type 3) without visible intrahepatic HCC mass was diagnosed by PET-CT and percutaneous transhepatic cholangioscopy (PTC) 6 years after his first hepatectomy. A 5.5 cm-long BDTT was extracted from the CBD, and moderately differentiated HCC was confirmed by pathology (Figure 2, Table 3).
Notably, patient No. 3, at 19 mo and 9 mo before BDTT was diagnosed, received hepatectomy for poorly differentiated HCC and resection for a single recurrent tumor located in the abdominal cavity, respectively. Although this patient had a history of multiple recurrent HCC, BDTT located in the left intrahepatic bile duct without a mass-like tumor in the liver was finally diagnosed when the AFP level climbed over 700 ng/mL and obvious biliary dilation and metastatic lesions in the omentum majus and lung could be detected (Figure 3).
Two patients (No. 5 and 6) were diagnosed with BDTT with invisible first-occurring HCC in the liver. Their thrombi were both located in the CBD (Satoh’s type 3). The AFP values of patients No. 5 and 6 were both within the normal range. This might be the main factor that led to a delay in diagnosis in the above two cases. The extraction of BDTT (moderately differentiated HCC) from CBD was the only choice of surgical treatment for patient No. 6 because recurrent HCC in the celiac lymph nodes andperitoneal metastases had already existed. It was also regrettable to note that BDTT was misdiagnosed as a CBD stone in patient No. 5, whose CT images and AFP did not suggest HCC at his first admission. However, although closely observed during follow-up after ERCP by testing serum tumor markers and imaging detection, BDTT was finally diagnosed as poorly differentiated HCC during CBD exploration at his readmission. At that time, an arterially enhanced mass in the CBD could be detected in CT images (Figure 4). Unfortunately, intrahepatic lesions could not be detected during the operation, and consequently, the patient only received transhepatic arterial chemotherapy and embolization (TACE) after BDTT extraction.

Table 2 Results of antigens and antibodies tests of hepatitis B virus and hepatitis C virus
The HCC lesions and BDTTs were both resectable for patients No. 4 and No. 7. The HCC lesion in patient No. 4 was a 9 cm × 4 cm × 9 cm massive tumor occupying the right lobe with a connected thrombus extending across the confluence of the RHD and left hepatic duct (Satoh’s type 2). Radical right hepatectomy combined with extrahepatic bile duct resection was chosen for this patient because the cancerous thrombus infiltrated the outer bile duct wall (Figure 5, Table 3). Patient No. 7, who had obvious signs including elevated AFP levels, a large liver mass (7 cm × 4 cm × 1 cm) on CT scans and a thrombus in the CBD (Satoh’s type 3) confirmed by ERCP, was successfully cured by hepatectomy with thrombus extraction (Figure 6).
Prognosis
Only one patient (No. 7) had disease-free survival after surgery for 60 mo. Four patients (No. 1, 2, 4, 6) died due to multiple recurrent or metastatic tumors in the liverand systemic metastases other than BDTT. Among these cases, multiple intrahepatic metastases occurred in patient No. 1 with microscopic BDTT, while recurrences in a patient with a thrombus infiltrating the CBD (patient No. 4) occurred in the hepatoduodenal ligament as well as the omentum. Patient No. 5 received TACE, while for patient No. 3, lenvatinib + sintilizumab was utilized for adjuvant therapy. The above 2 patients were still alive with tumors during follow-up periods of 1.5 mo and 5 mo, respectively.

Table 3 Pathological characteristics of bile duct tumor thrombi and primary or recurrent hepatocellular carcinoma lesions when bile duct tumor thrombi was diagnosed
Literature review
Only five retrospective studies met our search criteria (Table 4). Notably, all of the studies were from China[12-16]. The studies included 151 patients with HCC with BDTT who received various treatments from 1984 to 2018. The preoperative diagnostic methods only included ultrasound, CT, MRI, and ERCP. The retrieved data showed that BDTT was most commonly misdiagnosed as choledocholithiasis or cholangiocarcinoma, which occurred in 5.7%-66.7% of the patients. However, none of the included studies mentioned the effect of misdiagnosis on the prognosis of the patients.

Table 4 Summary of studies focused on misdiagnosis of hepatocellular carcinoma with bile duct tumor thrombi
DISCUSSION
To date, the greatest challenge of handling HCC with BDTT is how to avoid misdiagnosis of the tumor thrombus rather than the choice of surgical approaches, especially in cases of BDTT without obvious intrahepatic HCC mass. According to our experience and literature review, the major obstacles and loopholes for making correct diagnoses might exist because surgeons are not familiar with the pathophysiological features of HCC with BDTT and because there is relatively low sensitivity of imaging examinations. Unfortunately, in the present study, the initial diagnoses of the 7 included patients were achieved based on imaging examinations, and 3 patients (42.86%) were misdiagnosed and mistreated. Only one patient received ERCP and clarified the range of the thrombus before surgery, but no patient received pathological evidence until the operation was performed. Therefore, to pursue correct diagnosis and R0 resection, a more comprehensive set of diagnostic modalities should be implemented. The key points of making a definite preoperative diagnosis for HCC with BDTT might include: (1) Confirming the location and size of the primary tumor and whether the primary tumor invades the bile duct; (2) Clarifying the extent of BDTT; (3) Acquiring a pathological diagnosis of BDTT before surgical treatment if possible; and (4) Paying more attention to the suggestive role of tumor markers when intrahepatic HCC lesions are hard to detect.

Figure 1 Computed tomography images of patient No. 1 who was diagnosed as hepatocellular carcinoma with microscopic tumor thrombus in bile duct. A, B: Xxxx; C-F: The blue arrows indicate the portal vein tumor thrombus (C and E) and the yellow arrows indicated the large hepatocellular carcinoma tumor which invaded the full thickness of the gastric wall (D and F); G: Bile duct tumor thrombus was only detectable under the microscope. The green arrows indicate bile duct tumor thrombus, and the black stars indicate the intrahepatic bile duct muscularis.
First, understanding the pathophysiological features of HCC with BDTT was helpful to assess clinical signs properly. The mechanisms and hypothesis of the formation of a BDTT are as follows: (1) The primary tumor directly invades the bile duct, and the BDTT is connected with the primary tumor; (2) The primary tumor metastasizes into the biliary system through microvessels and lymphatic vessels; (3) The primary tumor metastasizes through the arteriovenous shunt to the bile duct system; and (4) The primary tumor metastasizes through the nerves around the bile duct[17]. In the latter three mechanisms, there might be a certain distance between the primary tumor and the BDTT,i.e.the bile duct is not directly infiltrated by the tumor. Notably, the primary tumor can invade the bile duct and form a tumor thrombus even when the tumor itself is still minuscule[18].
Second, combined applications of imaging examinations, endoscopy, and pathology might contribute to improving the diagnostic rate of HCC with BDTT. Theoretically, in CT scans, BDTT shows a slightly lower or equal-density soft tissue shadow in the bile duct, which is similar to the original lesion after enhancement. High-medium differentiated tumors may exhibit light-moderate enhancement. For MRI, BDTT shows a heterogeneous or intermittent increased signal on T2-weighted phase imaging or diffusion-weighted imaging compared to that of the surrounding liver parenchyma. There is no significant attenuation of signals in the short time inversion recovery phase. However, in practical use, CT and MRI are not effective enough in diagnosing BDTT and assessing the extent of tumor thrombus progression. This might be due to the imaging findings of tumor thrombi are often intermittent or the density commonly varies greatly. A retrospective study of 392 patients conducted from 1993 to 2011 by Tanet al[19]revealed that HCC with BDTT was easily misdiagnosed by CT or MRI as HCC compressing the hepatic hilum, hilar cholangiocarcinoma, metastatic hepatic cancer with tumor thrombus, bile duct stones,etc. The total misdiagnosis rates of the above diseases were 4.1%, 4.3%, 2.3%, 1.0%, and 3.8%, respectively.

Figure 2 Positron emission tomography-computed tomography (A), computed tomography (B and C), and positron emission tomography (D and E) images of patient No. 2 who was diagnosed as recurrent hepatocellular carcinoma with bile duct tumor thrombus located in the common bile duct but did not have visible metastatic lesions in the liver. The blue arrow indicates the portal vein, the red arrow indicates the hepatic artery, and the green arrow indicates the bile duct tumor thrombus in common hepatic duct and common bile duct.

Figure 3 Variation trend of serum alpha-fetoprotein value and computed tomography images of patient No. 3. A, B: The yellow arrow indicated the first recurrent hepatocellular carcinoma lesion in the abdominal cavity in September 2018; C, D: Computed tomography images taken in May 2018 did not show any tumor lesion or dilated bile ducts in the liver parenchyma; G, H: The black arrows indicate the dilated intrahepatic bile ducts in June 2019. AFP: Alpha-fetoprotein.
Intraductal ultrasonography (IDUS) might be a good candidate when BDTT is difficult to diagnose by CT or MRI. IDUS can help to determine whether stenosis in the bile duct is caused by intraductally originated neoplasms or extraductal tumor invasion. In patients with BDTT, IDUS showed signs of a ‘polypoid tumor with a narrow base’ and a ‘nodule within a nodule’ with the absence of a ‘papillary-surface pattern’ that were more strongly associated with HCC than with intraductal polypoidtype cholangiocarcinoma[20].
To date, only a few studies have evaluated the effectiveness of preoperative pathological diagnosis of HCC combined with BDTT due to its relatively low incidence rate. As a type of malignant biliary stenosis (cholangiocarcinoma, metastatic liver cancers invading the bile duct and HCC combined with BDTT,etc.), there are three methods for pathological diagnosis of BDTT before surgery: (1) Tissue biopsy, including oral choledochoscopy, peroral cholangioscopy-guided forceps biopsy (POCFB) and endoscopic ultrasound-guided fine needle aspiration biopsy (EUS-FNAB); (2) Biliary tract cytology brushes; and (3) Direct biopsy under cholangiography. In recent years, with clinical application of the SpyGlass system, the ability of pathological diagnosis in the biliary tract has been significantly improved through its three subsystems: Optical probe (SpyGlass); access catheter (SpyScope); and biopsy forceps (SpyBite). A meta-analysis published in 2015 by Navaneethanet al[21]suggested that the sensitivity of SpyBite-based POC-FB is 60.1% for the diagnosis of biliary malignant stenosis, which is much higher than that of cytoplasmic brush (5.8%) and direct biopsy (29.4%). The sensitivity and specificity of SpyBite for diagnosing biliary malignant stenosis were 74.7% and 93.3%, respectively. Regarding the choice of POC-FB and EUS-FNAB, Leeet al[22]recommended EUS-FNAB for BDTT located in the distal part of the CBD, which is closer to the duodenum, while SpyGlass with POC-FB is more advantageous for thrombi in the proximal bile duct.
Third, the sensitivity of AFP for diagnosing HCC is only 33%-65%, and it is also significantly elevated in active hepatitis and cirrhosis. Protein induced by Vitamin K Absence or Antagonist-II (PIVKA II) and hepatoma-specific alpha-fetoprotein (AFPL3) potentially play a significant role in indicating HCC with BDTT, especially when intrahepatic lesions cannot be detected. PIVKA-II, also known as des gamma-carboxy prothrombin, was first recognized as a tumor marker for HCC in 1984. When compared with AFP, the advantages of PIVKA-II are as follows: (1) PIVKA-II has no correlation but a complementary effect with AFP; (2) PIVKA-II is also not expressed in other malignant tumors but is expressed in up to 91% of HCCs; and (3) The expression level of PIVKA-II is significantly correlated with differentiation, intrahepatic metastasis, tumor metastasis node stage, and recurrence of HCC[23-25]. AFP-L3 is defined as a specific subtype of AFP that binds to Lens culinaris agglutinin with a normal ratio ranging from 0% to 10%. AFP-L3 is effective in differentiating HCC from hepatitis or cirrhosis when the AFP value of the patient is > 50 ng/mL and AFP-L3 accounts for more than 20%[26,27]. For HCC patients with AFP < 20 ng/mL, Choi JYet al[27]reported that the combination of AFP-L3 and PIVKA-II with sensitivity and specificity up to 92.1% and 79.7%, respectively, successfully detected 81.8% of stage I HCC and 86.7% of tumors < 2 cm.
Ueda and Satoh classifications are the two most commonly used typing methods for HCC with BDTT, which are based on the location of the thrombus in the biliary system. However, they did not reflect the whole field of clinicopathological features of HCC with BDTT, which is critical for the diagnosis and treatment. According to our study and literature review, the resectability of intrahepatic HCC lesions and whether the bile duct wall was infiltrated by tumor thrombus might be the two influences on the choice of surgical treatment. In the present study, the 7 cases of HCC with BDTT took many forms but easily fell into 4 types that we identified (Figure 7): Type I: HCC with microscopic BDTT (patient No. 1); Type II: Resectable first-occurring or recurrent HCC mass in the liver with BDTT (patients No. 4, 7); Type III: BDTT without an obvious HCC mass in the liver (patients No. 5, 6); Type IV: BDTT accompanied with unresectable intra- or extrahepatic HCC lesions (patient No. 2), which should be considered as a form of systemic metastases. BDTT can be further divided into two subtypes according to whether tumor cells of the thrombus invade the extrahepatic bile duct wall (e.g., patients No. 4 and No. 7 were categorized as having Type IIIa and Type IIIb, respectively).

Figure 4 Computed tomography and magnetic resonance cholangiopancreatography images of patient No. 5. A-D: Computed tomography images of suspected bile duct tumor thrombus (without obvious enhancement in arterial phase) taken before it was misdiagnosed and mistreated as common bile duct stone by endoscopic retrograde cholangiopancreatography during the patient’s first admission; E-H: Computed tomography images of the bile duct tumor thrombus (green arrow) taken in outpatient follow-up 3 mo after last endoscopic retrograde cholangiopancreatography treatment; I-L: Computed tomography images of the bile duct tumor thrombus (green arrows) taken before receiving thrombus extraction during the patient’s second admission.
For treating BDTT, the controversy mainly lies in the choice of thrombus extraction or choledochotomy for patients without macroscopic bile duct wall invasion. Advocates for choledochotomy suggested that although BDTT could be easily detached, some remaining HCC cells were microscopically observed to infiltrate the full thickness of the bile duct wall and scatter into its peripheral plexus[17]. However, most surgeons believe that it is feasible to perform thrombus extraction if R0 resection of primary HCC could be achieved because metastasis and recurrence rather than BDTT are more important prognostic factors[28,29]. In addition, it is also advantageous to preserve the extrahepatic bile duct so that recurrent tumors can be treated by TACE. Therefore, thrombus extraction might be reasonable, but choledochoscopy should be performed to confirm that there are no tumor residues.
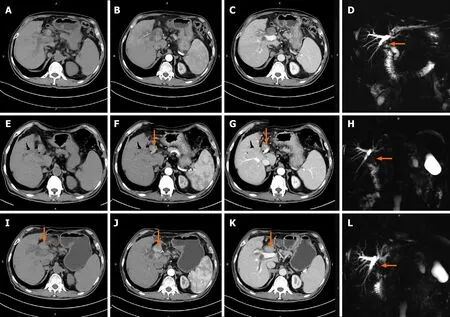
Figure 5 Computed tomography images of patient No. 4. A-F: The yellow arrows indicate hepatocellular carcinoma lesion in hepatic arterial phase (A-C) and portal-vessel phase (D-F). The green arrows indicate bile duct tumor thrombus in the common bile duct.
The treatment for patients with BDTT without an obvious HCC mass (abovementioned proposal classification of type III) was another pain point due to a recurrence rate up to 80% after the first treatment with a survival period ranging from only 6 mo to 22 mo[10]. Comprehending the history of recurrent cholangiolithiasis, combined applications of IDUS, endoscopy with preoperative pathology, and intraoperative ultrasound might help to improve its diagnostic rate, but there has been very little related experience until now. Successful early detection of recurrence depends on the implementation of close follow-up after the first treatment. Adjuvant therapies, such as TACE, radiofrequency ablation, and targeted therapy, can improve the prognosis to a certain degree.
CONCLUSION
In conclusion, BDTT is a special pathological morphology of HCC that is easily misdiagnosed as various benign and malignant hepatobiliary diseases. Routine imaging examinations such as CT or MRI are sometimes insufficient to provide diagnostic evidence. We proposed a new classification for HCC with BDTT in order to reflect its pathological characteristics and emphasize the significance of primary tumor resectability in its treatment. More attention should be paid to different clinical patterns of this disease, especially those without visible HCC mass in the liver.

Figure 6 Computed tomography and endoscopic retrograde cholangiopancreatography images of patient No. 7. A-F: The yellow arrows indicate hepatocellular carcinoma lesions in the arterial phase (A-C) and portal-vessel phase (D-F) in computed tomography images; G-I: The green arrows indicate the location of the bile duct tumor thrombus in the common bile duct, and endoscopic nasobiliary drainage was placed through endoscopic retrograde cholangiopancreatography.

Figure 7 New proposal of classification for hepatocellular carcinoma with bile duct tumor thrombus. Type I: Hepatocellular carcinoma (HCC) with microscopic bile duct tumor thrombus (BDTT); Type II: Resectable first-occurring or recurrent HCC mass in the liver with BDTT; Type III: BDTT without obvious HCC mass in the liver; Type IV: BDTT accompanied with unresectable intra- or extrahepatic HCC lesions. BDTT can be further divided into two subtypes according to whether tumor cells of thrombus invades into the extrahepatic bile duct wall (subtype a) or not (subtype b). BDTT: Bile duct tumor thrombus.
ARTICLE HIGHLIGHTS


Research objectives
In the present retrospective study, we reviewed and analyzed our diagnosis and treatment experience regarding seven HCC patients with BDTT, including misdiagnosed cases. We also propose a new classification for HCC with BDTT based on its clinico-pathological features.
Research methods
A retrospective review of the diagnosis and treatment experience regarding seven typical HCC patients with BDTT between January 2010 and December 2019 was conducted.
Research results
BDTT was preoperatively confirmed by computed tomography/magnetic resonance imaging in only four patients. Three patients with recurrent HCC and one patient with first-occurring HCC had no visible intrahepatic tumors; of these, misdiagnosis occurred in two patients, and three patients died. One patient was mistreated as having common bile duct stones, and another patient with a history of multiple recurrent HCC was misdiagnosed until obvious biliary dilation could be detected. Only one patient who received hepatectomy accompanied by BDTT extraction exhibited disease-free survival during the follow-up period. A new classification was proposed for HCC with BDTT as follows: HCC with microscopic BDTT (Type I); resectable primary or recurrent HCC mass in the liver with BDTT (Type II); BDTT without an obvious HCC mass in the liver (Type III); and BDTT accompanied with unresectable intra- or extrahepatic HCC lesions (Type IV).
Research conclusions
We herein propose a new classification system for HCC with BDTT to reflect its pathological characteristics and emphasize the significance of primary tumor resectability in its treatment.
Research perspectives
The classification system and its guiding significance in the treatment of HCC with BDTT.
杂志排行
World Journal of Gastroenterology的其它文章
- Prognostic value of changes in serum carcinoembryonic antigen levels for preoperative chemoradiotherapy response in locally advanced rectal cancer
- Development and validation of a three-long noncoding RNA signature for predicting prognosis of patients with gastric cancer
- Use of the alkaline phosphatase to prealbumin ratio as an independent predictive factor for the prognosis of gastric cancer
- Active tuberculosis in inflammatory bowel disease patients under treatment from an endemic area in Latin America
- Prevalence and predictors of nonalcoholic fatty liver disease in South Asian women with polycystic ovary syndrome
- Associations between serum uric acid and hepatobiliary-pancreatic cancer: A cohort study
