Study on the photocatalytic degradation of methylene blue by La2O3/TiO2
2021-01-06HUDandanHUXiangyangYINRongcanZHANGYingCUIYumin
HU Dandan,HU Xiangyang,,YIN Rongcan,,ZHANG Ying,CUI Yumin,*
(1.School of Chemistry and Materials Engineering,Fuyang Normal University,Fuyang Anhui 236037,China;2.Anhui Provincial Key Laboratory for Degradation and Monitoring of Pollution of the Environmen,Fuyang Normal University,Fuyang Anhui 236037,China)
Abstract:La2O3/TiO2 photocatalysts were prepared by calcining with La2O3 and TiO2 as precursors,and La2O3/TiO2 photocatalysts were characterized by scanning electron microscopy,X-ray diffraction,photoluminescence spectrum,ultraviolet-visible diffuse reflection and infrared spectrum.The effects of La2O3 content,calcination temperature and catalyst amount on the photocatalytic performance of La2O3/TiO2 were investigated with methylene blue as photocatalytic degradation model compound.The results showed that the degradation rate of degraded substrates increased with the increase of catalyst dosage between 0.040 and 0.150 g.When the catalyst dosage is 0.150 g,the methylene blue concentration is 5 mg·L-1 and the volume of the methylene blue is 300 mL,the degradation rate of methylene blue can reach 90.1% after 60 minutes of light.
Key words:titanium dioxide;lanthanum trioxide;methylene blue;photocatalytic
Studies have shown that coupling between semiconductors can effectively improve the quantum efficiency of a semiconductor photocatalysis[1].Because of semiconductor coupling,heterojunction can be formed between the two interfaces,which can make full use of the complementarity of the two semiconductors’energy level structures.By using the energy level difference between the two semiconductors,the electron and hole can be separated effectively,so as to facilitate the separation,transfer and deliver of photogenerated electron and hole pairs.This prevents photogenic electrons from recombining with holes[2-3].However,there are still technical difficulties in the application of TiO2in photocatalytic technology,which can be compounded with other semiconductor materials to obtain a good solution.By combining with other materials,the spectral response range of TiO2can be widened and the visible light can be fully utilized.Moreover,it can effectively separate photoelectron-hole pairs and improve their quantum efficiency.In addition,the composite with different semiconductor materials can not only give full play to the advantages of each composite element,but also produce a synergistic effect to achieve the effect of common catalysis.The compound modification of rare earth elements provides an important way for TiO2to obtain higher catalytic activity.In order to improve the photocatalytic performance of TiO2and its utilization efficiency of visible light,the related researches focuses on the following three fields:the fixation of catalyst,the expansion of visible light wavelength response range,and the improvement of photocatalytic quantum yield and industrial application[4-6].Due to the special spectral properties and electronic structure of rare earth elements,TiO2photocatalysts can be modified in terms of light absorption performance,surface adsorption performance,crystal structure and energy band structure,etc.At the same time,many new photocatalyst systems can be constructed,which have great application prospects[7].In this study,the La2O3was added to matrix TiO2catalyst as a composite photocatalyst for methylene blue degradation,and the influence of La2O3content,calcination temperature and catalyst dosage on the photocatalytic performance of La2O3/TiO2were investigated,which would provide an efficient and economical method for the practical application of dye wastewater treatment.
1 Experiment
1.1 Reagents and instruments
La2O3,TiO2and methylene blue are all analytical pure reagents.
X-D-3 X-ray diffractometer(Beijing Puxi general instrument Co.,LTD.);SIGMA 500 scanning electron microscope(Carl Zeiss,Germany);MDX1000 fluorescence spectrometer(Beijing Puxi general instrument Co.,LTD.);WQF-501(Fourier transform infrared spectrometer);L6020046 UV-visible near infrared spectrophotometer(Perkin Elmer Co.,LTD.);OLRSK photocatalytic reactor(Hongxing science and education instrument factory,Kaifeng),etc.
1.2 Catalysts preparation
Preparation of TiO2sample:2.000 g TiO2was weighed on the analytical balance,dried and ground.Preparation of 1% La2O3/TiO2:0.020 g La2O3and 1.980 g TiO2were weighed,mixed,dried and ground.Preparation of 3% La2O3/TiO2:0.060 g La2O3and 1.940 g TiO2were weighed,mixed,dried and ground.Preparation of 9% La2O3/TiO2:0.180 g La2O3and 1.820 g TiO2were weighed,mixed,dried and ground.
Preparation of La2O3/TiO2catalysts:three parts of 3% La2O3/TiO2mixed catalyst with the mass of 2.000 g were taken and dried and ground,and two parts of La2O3/TiO2mixed catalysts were calcined for 1 h at temperatures of 550℃and 850℃,respectively.After calcining and cooling to room temperature,the samples were ground into powder.Then they were put into the sample bags.
1.3 Characterization experiment of catalyst
1.3.1 SEM characterization
Three samples of TiO2,La2O3and 3% La2O3/TiO2treated at room temperature were characterized by SEM.
1.3.2 Fourier transform infrared spectroscopy characterization
A small amount of mixed catalyst samples with different proportions(at room temperature)were taken,and an appropriate amount of KBr particles were added,ground until they were mixed evenly,pressed into thin slices,and characterized by infrared spectroscopy.
1.3.3 Photoluminescence spectral characterization
A small number of mixed catalyst samples(at room temperature)were taken and the photoluminescence properties of the catalyst samples were tested by fluorescence spectrometer.The excitation wavelength is 320 nm and the scanning range is from 360 nm to 550 nm.
1.3.4 UV-vis characterization
The catalyst samples(at room temperature)were characterized by ultraviolet-visible spectrometer.The measured wavelength is from 200 nm to 800 nm.In the experiment,try to use glass slides to make the sample compact and flat.
1.3.5 XRD characterization
The crystal phase structure of each catalyst sample(at room temperature)was analyzed by Xray diffractometer.Instrument parameters:Cu-Kα radiation,tube voltage is 36 kV,tube current is 20 mA,scanning range is from 10°to 80°and scanning speed is 8 deg/min.
1.4 Photocatalytic experiments
Four parts of 0.100 g catalysts in different proportions were weighed and prepared.The catalyst,methylene blue solution with a concentration of 300 mL of 5.00 mg·L-1and a magnetron agitator were added to the reaction vessel.After that,the above reaction containers were put into the photocatalytic reaction apparatus,the agitator and mercury lamp were turned on,and the photoreaction was conducted for 60 minutes.The catalysts of each ratio were tested once.After the reaction,the samples were centrifuged to determine the absorbance A0of the undegraded solution and the absorbance Atof the degraded solution,respectively.The degradation rate was calculated according to the formula of the degradation rateW=(A0-At)/A0×100%.
2 Results and discussion
2.1 XRD analysis
As shown in Fig.1,the positions of the diffraction peaks of TiO2and La2O3/TiO2samples are basically consistent with the 2 theta values corresponding to the XRD characteristic diffraction peaks of anatase TiO2,and correspond to the crystal surfaces of(101),(004),(200),(105),(211)and(204),respectively[8].There are no other peaks,indicating that the doping of La2O3has no destructive effect on the crystal structure of TiO2.With the increase of the amount of La2O3,the peak of(101)crystal plane first increases and then decreases.Among them,the peak strength of 1% and 3% samples is relatively strong,indicating that their crystallinity is relatively good[9].The diffraction peak of La2O3cannot be observed,because a very small amount of La2O3was dispersed on the catalyst surface,and no single phase was formed[10].

Fig.1 X-ray powder diffraction spectrum of all the samples
2.2 SEM analysis
Fig.2(a)is the scanning electron microscope image of La2O3,and the morphology of La2O3is irregular block.Fig.2(b)is the scanning electron microscope image of TiO2.It can be seen that TiO2is an irregular globule with many particles gathered together.Fig.2(c)is the scanning electron microscope image of 3% La2O3/TiO2composite catalyst.It can be seen that La2O3has adhered to the surface of TiO2,which is also the premise for the smooth expression of the modification of TiO2by La2O3[11].
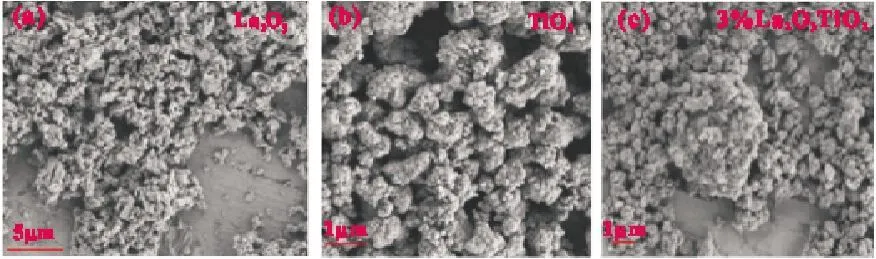
Fig.2 SEM of La2O3,TiO2and 3% La2O3/TiO2
2.3 FT-IR analysis
It can be seen from Fig.3 that the chemical structure of La2O3/TiO2composite catalyst is basically consistent with that of TiO2,indicating that the two types of samples are basically similar in structure.The absorption peak of about 543 cm-1corresponds to the stretching vibration of Ti-O-Ti bond[12],the absorption peak of about 1622 cm-1corresponds to the stretching vibration of the C=O bond in CO2,and the absorption peak of about 3 415 cm-1corresponds to the stretching vibration of the O-H bond containing trace water in the sample.The absorption peak without La-O bond,because the content of La2O3is too small for the instrument to detect.
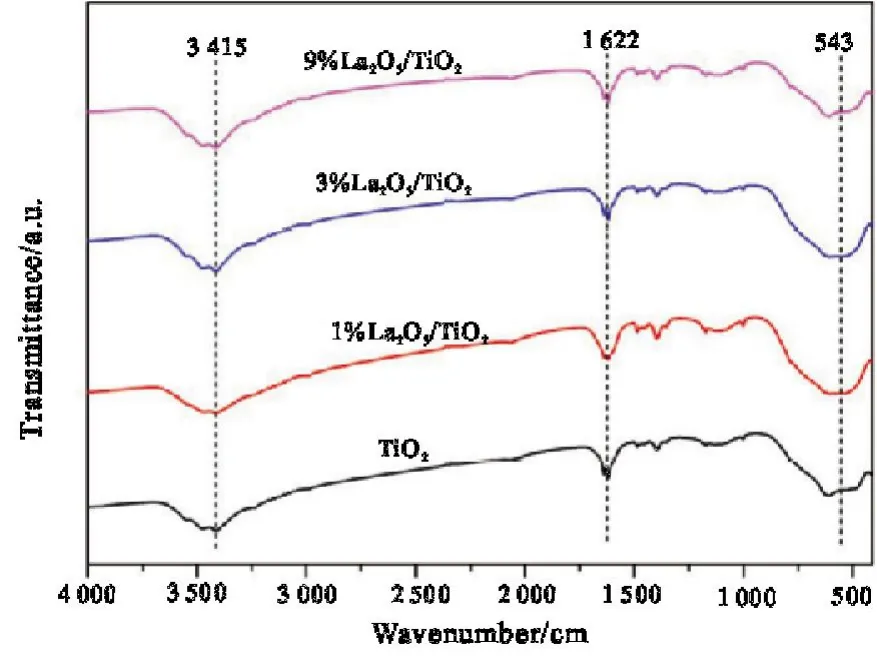
Fig.3 Fourier infrared spectra of La2O3/TiO2 series catalysts
2.4 Solid-phase UV-vis analysis
Fig.4 shows the UV-vis spectrum of La2O3/TiO2catalysts.It can be seen from the figure that the characteristic absorption wavelength of La2O3/TiO2has blue shift compared with that of TiO2.It indicates that the introduction of La2O3will increase the band gap width of the semiconductor[13],enhance the REDOX capacity of photoinduced holes and electrons[14],and it is easier to produce oxidized groups in the reaction,which is conducive to improving the photocatalytic activity.The blue shift of catalyst with 3% La2O3doping is the most obvious and the photocatalytic capacity is the strongest.
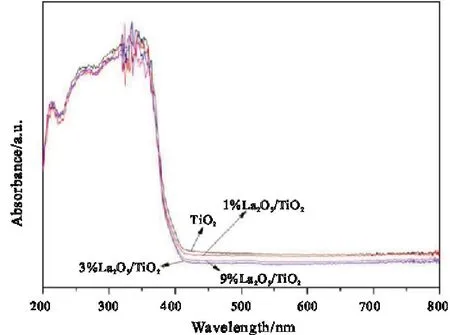
Fig.4 UV-vis spectra of La2O3/TiO2 series catalysts
2.5 Photoluminescence spectroscopy analysis
Fig.5 shows the photoluminescence spectra of La2O3/TiO2series catalysts.It can be seen from the figure that the peak intensity sequence is TiO2>1% La2O3/TiO2>9% La2O3/TiO2>3% La2O3/TiO2between 390-440 nm.The intensity of the peak is proportional to the recombination rate of the photoelectron-hole pair.The fluorescence signal of pure TiO2sample was the strongest,and its photogenerated electron-hole pair composite probability was the highest,and the photocatalytic activity was the lowest[15].Due to the modification of TiO2particles by La2O3,photogenerated electron-hole pairs of catalysts doped with La2O3have a lower composite rate,among which the photogenerated electron-hole pairs of catalysts with 3% La2O3content have the lowest composite rate,the highest separation efficiency and the best catalytic effect[16].
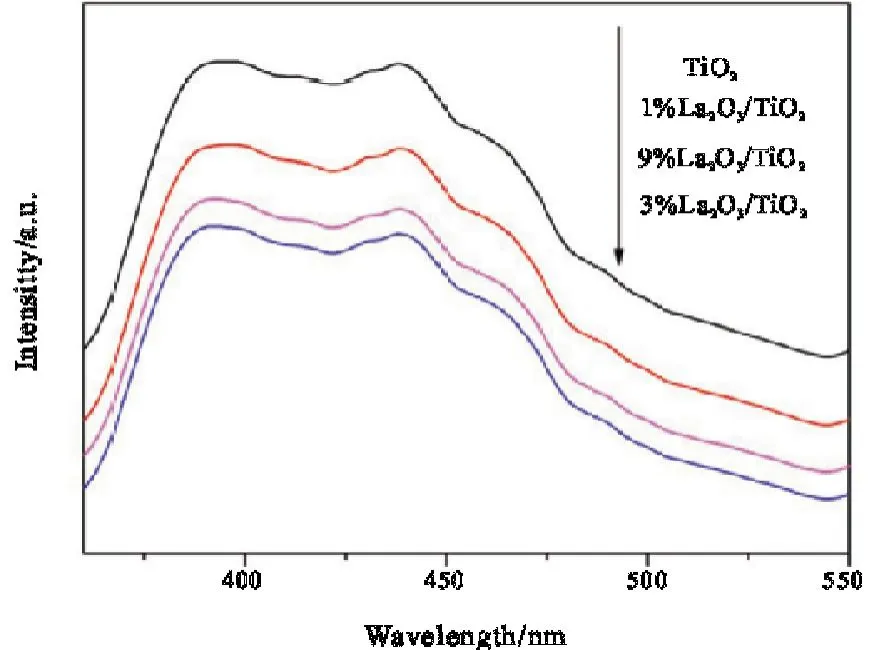
Fig.5 Photoluminescence spectra of La2O3/TiO2 series catalysts
2.6 Influence of composition of catalyst La2O3/TiO2 on its photocatalytic activity
The catalyst La2O3/TiO2was not calcined,the amount of fixed catalyst was 0.100 g,the concentration of methylene blue solution was 5.00 mg·L-1,the volume of methylene blue solution was 300 mL,the light was 1 h.The composition of the catalyst was changed,and the catalytic degradation rate of methylene blue was shown in Fig.6.

Fig.6 Influence of mass percentage of La2O3 on catalytic activity of La2O3/TiO2(0)TiO2;(1)1% La2O3/TiO2;(3)3% La2O3/TiO2and(9)9% La2O3/TiO2
As shown in Fig.6,the degradation rate of methylene blue gradually increases with the increase of mass percentage content of La2O3,and begins to decline when it exceeds 3%.Because the doping ratio of 3% of La2O3is conducive to improving the crystallinity of anatase phase of TiO2,which reduces the recombination rate of photogenerated electron-hole pairs.If La2O3is added too much,the composition probability of photogenerated electronhole pairs of TiO2will be improved.In addition,too much La2O3particles will cover the surface of TiO2molecules,impeding the photocatalytic reaction[17-18].Therefore,the optimal doping ratio of La2O3is 3%.
2.7 Influence of calcining temperature on the catalytic activity of La2O3/TiO2
The fixed catalyst was composed of 3% La2O3and TiO2,the catalyst dosage was 0.100 g,the concentration of methylene blue solution was 5.00 mg·L-1,the volume of methylene blue solution was 300 mL,the illumination time was 1 h.The calcination temperature of the catalyst was changed,and the catalytic degradation rate of methylene blue is shown in Fig.7.
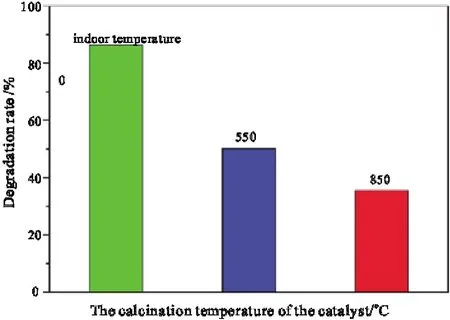
Fig.7.Influence of calcining temperature on catalytic activity of La2O3/TiO2
Fig.7 shows that as the calcining temperature increases,the activity of 3% La2O3/TiO2catalyst decreases rapidly.Because the high temperature calcining will transform anatase TiO2into rutile TiO2with poor catalytic performance[19,20],resulting in the fact that photocatalytic efficiency of 3% La2O3/TiO2decreases.
2.8 Influence of amount of catalyst on the catalytic activity of La2O3/TiO2
The fixed catalyst was composed of 3% La2O3and TiO2and it was not calcined(at room temperature).The concentration of methylene blue solution was 5.00 mg·L-1,the volume of methylene blue solution was 300 mL,the illumination time was 1 h,and the amount of catalyst was changed.The catalytic degradation rate of methylene blue is shown in Fig.8.
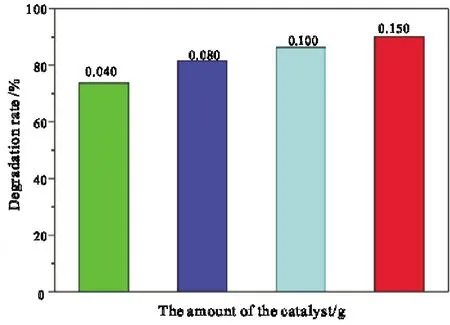
Fig.8 Influence of catalyst dosage on catalytic activity of La2O3/TiO2
As shown in Fig.8,when the amount of mixed catalyst is between 0.040-0.150 g,the degradation rate of methylene blue increases with the increase of the amount of catalyst.When the catalyst dosage is 0.150 g,the methylene blue concentration is 5 mg·L-1and the volume of methylene blue solution is 300 mL,the degradation rate of methylene blue can reach 90.1% after 60 minutes of light.
3 Conclusion
Using La2O3and TiO2as precursors,the La2O3/TiO2composite photocatalysts with different mass percentages were prepared at room temperature and under calcination conditions.Compared with pure TiO2,the La2O3/TiO2composite catalysts have higher photocatalytic activity,among which the mixed catalyst with La2O3content of 3% has the highest activity.The La2O3/TiO2mixed catalyst treated at room temperature can achieve the highest degradation rate of methylene blue,which is 86.7%.Because the addition of 3% La2O3increases the crystallinity of anatase phase of TiO2and improves the separation efficiency of photogenerated electrons and holes of TiO2.When the catalyst dosage is between 0.040 and 0.150 g,the degradation rate of methylene blue increases with the catalyst dosage.When the catalyst dosage is 0.150 g,the methylene blue concentration is 5 mg·L-1and the volume of methylene blue solution is 300 mL,the degradation rate of methylene blue can reach 90.1% after 60 minutes of light.
