鲜枣中糖精的快速、无标签检测
2020-12-28吴昊邵菲郭小玉吴一萍杨海峰
吴昊 邵菲 郭小玉 吴一萍 杨海峰


摘要:糖精是食品工業中最古老的人造甜味剂之一,因为没有卡路里而被广泛使用,但其滥用是非法的,食品中最大允许添加量为8.189xl0-4mol·L-l.介绍了以六磷酸肌醇(IP6)为保护剂合成的银(Ag)纳米粒子(Ag NPs),即Ag NPs@IP6,并提出了一种基于表面增强拉曼散射(SERS)的快速方法.探讨了食品中糖精的测定,用最佳SERS法测定水中糖精的最低可检测浓度可达50 nmol·L-1,符合食品添加剂耐受性水平的国家食品安全标准.提出了基于便携式拉曼的AgNPs@IP6的SERS方法,可用于现场检测食品中的糖精,如新鲜枣果.
关键词:糖精;银(Ag)纳米粒子(Ag NPs);表面增强拉曼散射光谱(SERS);快速检测
1 Introduction
The abuse of the additives is a current problem in the field of food safety, which is a major- issue ofconcem to the people ' s healthcare[1].Saccharin is one of the oldest artificial sweeteners used in food industrieshecause it has no calories.ln the 1970s, PRICE et al 2_found that saccharin had close correlation with bladdercancer in rodents. Consequently, foods containing saccharin must be laheled with a warning to match therequirement of the “Saccharin Study and Labeling Aet”of 1977.However, in the year 2000, due to somereports exploring the different rodent ' s cellular microenvironment ,involving high pH , high calcium phosphate ,and high protein levels.[3-4], frmn human situation, the United States removed the warning labels from theexternal packing of food containing saccharin.Next, some researches showed saccharin might give rise to therelease of insulin in humans and rats , which has not heen confirmed by the later control studies[5-7 ].In 2012,Qin[8-9] found the close, relationship be,tween inflammatory bowel dise,ase and the intake amount of saccharin,meaning that the saccharin is health risk for human as food additives.ln China, the acceptable daily intake(ADI) value of the saccharin is in the range from 8.189xl0-4 to 2.729xl0-2mol.L-1 for different foods.Thereupon, even if it remains controversy over the safety for saccharin as the food additive , some methods todetect saccharin have been developed.WANG et al[10] proposed a competitve enzyme-linked immunosorbentassay to determine the sodium saccharin in food samples.This immunosorbent method showed an excellentspeeifieity for sodium saeeharin with the limit of detection ( LOD ) of l.146xl0-8 mol·L-l by the diazo-reaction ,but it needed more than l.5 h for a whole test process and even 12 h in a preparatory process.Bergamo et aldemonstrated an accurate analytical technique for simultaneous determination of different artificial sweetenersby using capillary electrophoresis with capacitively coupled contactless conductivity with the 30 kV separationvoltage,s and 450 kHz operating condition , but it was not easy to actualize an on-site strategy.GREMBECKA etal[12]reported a HPLC-CAD-UV/DAD protocol to analyze the mixture of artifieial sweeteners with the LODless than 3xl0-6mol.L-l and relative standard deviation (RSD) less than 2% but it has to perform the tediouspre-treatments, control exorbitant operating conditions, and require large-sized instrument. Therefore, it isnecessary to explore some fast approaches for pre-screening in food on field or market.
The surface-enhanced Raman scattering (SERS) technique has become one of the most potentialspectroscopic tools for label-free determination of the metal ions , bio-analytes or food additivesl[13-16] , due to itsextraordinary capability for signal enhancement and inherent narrow width of Raman pe,ak.The amplifiedRaman intensities could be attributed to the contributions of electromagnetic (EM) field enhancementi[17-18] andchemical enhancement ( CE) [19].With the huge electromagnetic field from "hot spots" behveen neighbor noblemetal nanoparticles by laser inducing [20] , Raman signal for some especial molecules could be dramaticallyelevated even down to single molecule level[21-22].The additional merits of SERS technique such as rapidness,no interference hy water, and simple pre-treatment of sample have aroused great interests of many analysts invarious disciplines.As ahove-mentioned, SERS-based methods have lots of applications in life science[23-25] ,biotherapy[26] , and chemical analysis[27].Also , SERS spectroscopy provides fingerprint vibrational information ofmolecule moieties adsorbed on a metallic surface,bringing; an intrinsic selectwity.lt is perspective that with thedevelopment of reasonably active and stahle SERS suhstrates, Raman spectroscopy will play the key role inquality control application for goods and foods.
According to our previous work [28-30 ],inositol hexakisphosphate (IP6) as a naturally non-toxic substance,which has the strong interaction with metallic ions,could be used to synthesize and stabilize, the SERS substrates.In this work, tuning the ratio of IP6 and AgNO3 amounts for obtaining silver (Ag) nanoparticles (Ag NPs)( designated as Ag NPs@IP6)with optimal sensitivity was explored.Herein,we proposed the Ag NPs@IP6-basedSERS method to determine saccharin in the food product of fresh jujuhe fruit.The lowest detectableconcentration for saccharin was 50 nmol·L1,which meets the requirement of National Food Safety Standard fortolerance level of food additives.This SERS protocol with good reproducibility can be employed for on-marketmonitoring the food quality by using the portable Raman system.
2 Materials and methods
2.1
Chemicals and materials
Silver nitrate ( AgN03) ,sodium salt of IP6 and saccharin 98% (mass fraction) were obtained from Sigma-Aldrich (USA).Crystal violet, perchloric acid, acetic anhydride, sodium hydroxide ( NaOH) , Rhodamine 6G(R6G) . hydroxyl-ammomum chloride (NH20H-HCI) and acetic acid were purchased from SinopharmChemical Reagent ( Shanghai, China).Ethanol was obtained from Shanghai Titan Scientific Co., Ltd.Raw freshjujube fruit (Raw-J) was purchased from a local supermarket, and retail jujuhe fruit (Retail-J) was boughtfrom a local agricultural trade market. All reagents were of anal}'tical grade and used without furtherpurification.Deionized water ( 18 MQ·cm) was produced using a Millipore water purification system.
The morphology of the Ag NPs@IP6 was measured with a JEOL JEM-2000 FX transmission electronmicroscopy (TEM) operating at 200 kV by dropping the colloids onto a carbon coated Cu grid.The surfaceplasmon resonance spectra were collected with 7504 UV-visible spectroscopy (Shanghai Xinmao InstrumentCo., Ltd.) .Raman experune,nt was conducted by using a Portable Stabilized R.Laser Analyzer ( Enwave , USA ) ,equipped with diode laser at 785 nm and an adjustable power of the maximum at 300 mW.The acquisition timeand accumulations for e,ach Raman spectrum were set at 5 s and 3 tunes,respective,ly.
2.2
Preparation ofAg NPs
Ag NPs were synthesized according to Leopold' s method[31].Briefly, 1 mL of 0.3 mol·L 1 NaOH solutionwas added int0 89 mL of l.5 mmol-L 1 NH20H-HCl solution under magnetic stirring at room temperature.Then, different volumes of 10 mmoI-L 1 AgN03 solution were added into the above solution by dropwise.AgNPs were successfully produced while the color of solution turned brownish.Then,the as-obtained Ag NPs werestored in the dark at 20℃ and 60% relative humidity before use.
For the synthesis of Ag NPs@IP6,different volumes of l mmol·L 1 IP6 were mixed with 5 mL of 10 mmol·L 1AgN03 solution under vigorously stirring, then boiled for 15 min.Every mixture was gradually added into thesame reducing solution (1 mL of 0.3 mol·L 1 NaOH solution mixed with 89 mL of l.5 mmoI·L I NH2OH ·HC1solution) and kept stirring at 60℃ until the color of solution tumed yellow brown.The Ag NPs@IP6 colloid wasalso stored in the dark at 20 'C and 60% relative humidity.
2.3
SERS determination of saccharin
The SERS substrate was washed twice by using deionized water and then collected by centrifugation at8 000 r·min l for 5 min.The supematant was removed carefully before it was mixed with target molecules.Saccharin standard solutions in the concentration range from lxl0 1 to lxlo s moI·L1 were prepared by serialdilutions.Then, 5uL of saccharin solution with different concentrations was mixed with 5 uL of the as-prepared substrate ( Ag NPs or Ag NPs@IP6) and then dropped onto the eleaned aluminum foil paper.
For the detection of real samples, firstly, the fresh jujube fruit was umnerse.d into saccharin solution(5.Oxl0 2 mol·L1) for 10 min.The treated Retail-J and Raw-J were both extracted by the 20% ( volumefraction) ethanol aqueous solutions,respectively and then 5 uL of extract was mixed with 5 uL of the as-preparedAg NPs@IP6.
The SERS experiment was carried after the mixture totally dried at the condition of 20℃ and 60%relative humidity.
2.4
Titration determination of saccharin
According to suggestion by the National Standard of China ,the titration method was a routine technique toanalyze the saccharin in foods.ln detail, the sample containing saccharin \~'as added into the 20 mL of aceticacid and 5 mL of acetic anhydride.Then, two drops of crystal violet (1.23xl0 2 moI-L ') were injected as anindicator.Finally,the above solution was titrated by 0.1 mol·L1 of perchloric acid until the color of the solutionturned cyan and the experiment was re,peated for three tunes.
3
Results and discussion
3.1
Preparation and characterizations ofAg NPs@IP6
Silver substrates have their inherent surface plasmon resonance (SPR) phenomenon to enhance theRaman scattering of adsorbed species , and the enhancement factor is related to their geometry[32-33], chemicalcomposition[34], and size distribution[35].In this work , silver nanoparticles were picked out as SERS suhstratesto carry out the detection of saccharin residue on the surface of foods because of Raman signal-enhancingpeculiar-ity of Ag NPs 36'.Unfortunately , silver nanoparticles are unstable under ambient condition.Normally,the citrate salt was used as reducing agent to obtain Ag NPs and citrate salt residue on the Ag NPs resultedin the Raman spectroscopic interference to the trace detection.We used NH20H - HCl with no Raman activityas the reduction reagent to prepare Ag NPs.IP6 molecules were introduced into the synthesis procedure t。stabilize Ag NPs for real application requirement.lnterestingly, tuning the ratio of AgN03 and IP6, the as-obtained Ag NPs@IP6 products showed different SERS effects ( R6G as Raman probe).As shown in figure l( a) ,Raman intensity of R6G rises with the increase of the dosage of AgN03.However, the stability of Ag NPsbecomes worse and results in a serious aggregation in the case of amount of AgN03 increased from 5.0 t0 7.0 mL.As a result , the optimal volume of lX10 2 mol·L 1 AgN03 solution is fixed at 5.0 mL.Additionally , the usageof IP6 is also carefully examined for the, long-tenn stability of Ag NPs.As shown in figure l (b) , 3.0 mL of1.OX10 3 mol·L1 IP6 may be the best one for constructing SERS substrate.The excessive amount of IP6 willincrease the thickness of IP6 at the surface of Ag NPs ,which suppresses the Raman signals of target sample.
Figure 2 depicts the TEM images of the Ag NPs and Ag NPs@IP6.1t is found that their average diametersare around 50 nm.The thickness ofIP6 shell for Ag NPs@IP6is about 6+2 nm.ln figure 2(e) ,the SPR bands forAg NPs and Ag NPs@IP6 are observed at 403 nm and 408 nm,respectively.Closely investigating the TEM imagein figure 3 shows that the gap between Ag NPs @IP6 is less than 10 nm and the distribution of Ag NPs@IP6exhih.its more uniform than that of Ag NPs ,which agrees with the narrow band in SPR spectrum of Ag NPs@IP6.
3.2
The SERS performance
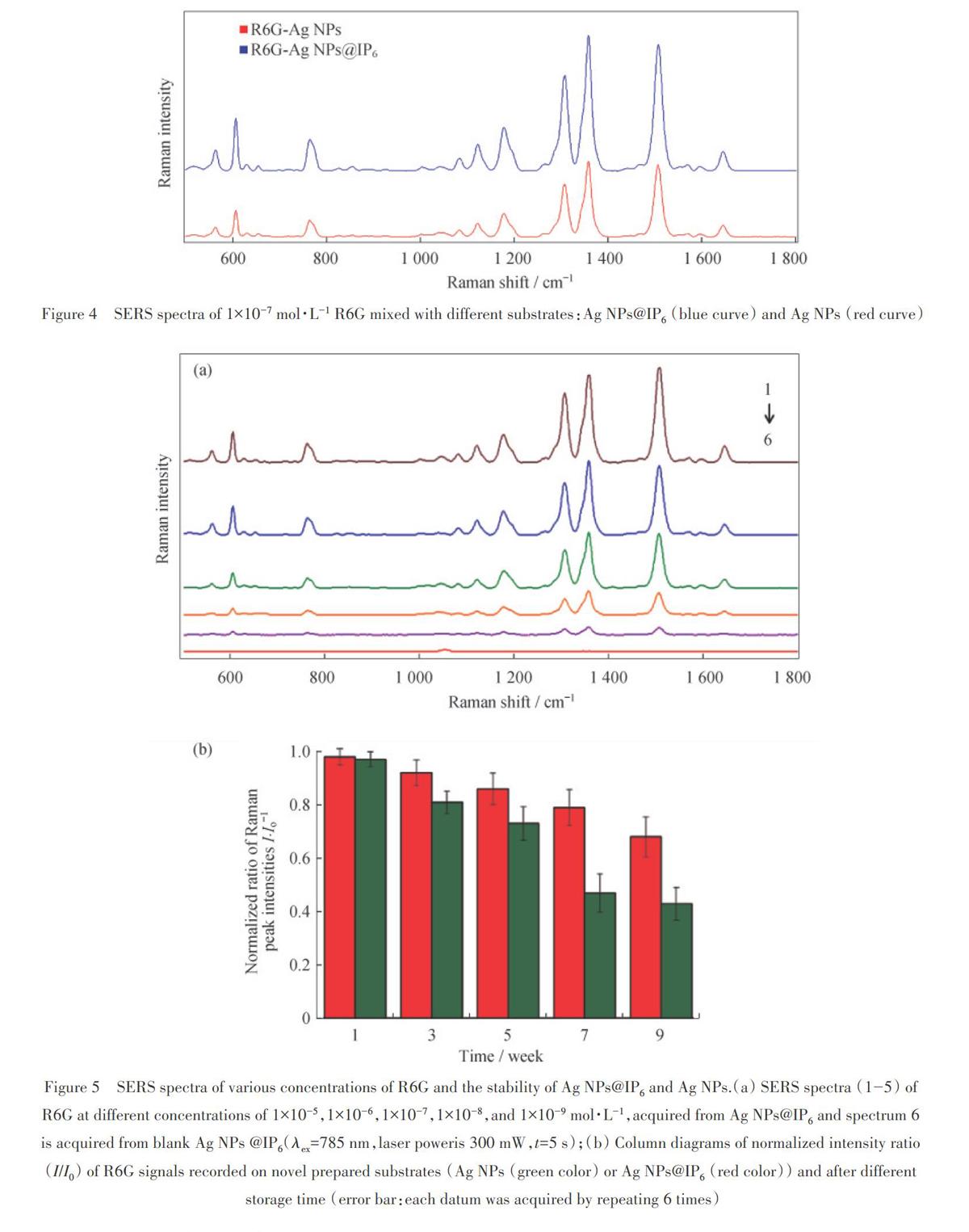
For the evaluation of the SERS effects of the Ag NPs@IP6and Ag NPs,l.Oxl0 mol·L1R6G solution wasused as the Raman prohe.lt was found from figure, 4 that the SERS signal of R6G from Ag NPs@IP6 is strongerthan that from Ag NPs.The enhancement should arise from “hot-spot" formation via Ag NPs@IP6 bridging eachother.ln figure 5 ( a) ,in case of R6G, the lowest detectable concentration could be down to l.Oxl0 9 mol·L1.Asa consequence ,the Ag NPs@IP6colloid is regarded as a promising substrate to elevate the SERS se,nsitwity foranalyzing saccharin in real samples.
The stability of the as-made Ag NPs@IP6 was also monitored by the time-de,pendent SERS experiments.The statistical results as column diagrams in comparison with Ag NPs are given in figure 5 (b).Obviously,witha storage time within 3 weeks , as compared with the original signal intensity from newly prepared suhstrate , theSERS signal of Ag NPs@IP6could keep about 90% even under ambient condition (20℃ and 60% relativehumidity) and it exhibits a long-tenn stability,which is be,neficial to the real application.
3.3
Detection of saccharin with portable Raman system
For the interpretation of the SERS bands,normal Raman speetrum of powder saeeharin and SERS speetrumattrihuted to vas(C-S-N) and vs( C-C) and a band at 1 175 cm l could he due t0 8(C-C-H) and p(N-H).SERS h. and at 1 287 cm I belongs to co-contributions of p ( C-H) and v( C-N-O) .The strongest characteristichand at 1 385 cm-l in SERS spectrum is from the asymmetric stretching modes of O=S=O group while it is avery weak band in normal Raman spectrum.This indicates that the saccharin molecules are anchored on theAg NPs@IP6 surface via lone pairs of oxygen atom.
As shown in figure 7, concentration dependent SERS spectra of saccharin in the aqueous solution arerecorded with the, Ag NPs@IP6substrates.The linear relationship was plotted by the, intensities of the, Ramanband at 1 385 cm l versus the varying concentrations of saccharin in the range from l.Oxl0 4 t0 1.OX10 3 mol·L1.The SERS substrate of hlank Ag NPs@IP6 was also recorded with no interference on the spectrum of targetmolecule (figure 8).The error hars given by six independent measurements are for the indication of thestandard deviation.The, lowest detectable concentration reaches 5.Oxl0 8 mol·L1,indicating that this optimizedAg NPs@IP6-based SERS method could be performed for a practical analysis.
The investigation of SERS reproducibility of saccharin on the Ag NPs@IP6 was given in figure 9 and theRSD is about 10.4%.The RSD value of Ag NPs@IP6 is less than 20.0%, remarking the good reproducibility[37]of such Ag NPs@IP6 substrates.
3.4
Detection of saccharin in real sample
The SERS-based protocol to determine saccharin might have interference of food matrix, such asacesulfame potassium, sucrose and so on. Especially, in food production proeess, acesulfame potassium isroutinely mixed with saccharin to offer sweeter tastes by some enterprises[38]. Herein, SERS spectra ofsaccharin, acesulfame potassium, sorbitol, sucrose, and glucose together with the mixture of ahove 5 speeieswere recorded,whieh were shown in figure lO.It is confirmed that the charaeteristie Raman band of saccharinat 1 385 cm-1 without interfering could be distinguished from the others.
Pitifully in news report, some. fresh jujube fruits were added with excessive saccharin by bad vendors toobtain illegal economic benefits.For conducting SERS detection, the pre-treatment approach for sample has animportant process[39-42].We used 20%( volume fraction)ethanol aqueous solution to extract the raw jujubes ( Raw-J) , the retail jujubes ( Retail-J) , and the spiked jujubes ( Spiked-J).AIl of the SERS signals were recorded fromthe ethanol extract solution after they were mixed with Ag NPs@IP6 substrates.ln figure ll(line l) , no obviousRaman signal from the extract solution of Raw-J is visible,while in figure 11 (line 2) , the Raman spectrum of theextract solution from Spiked-J shows the character-istic bands of saccharin.The SERS spectrum of the extractfrom Retail-J presents the characteristic bands at 779 and 1 385 cm l of saccharin as shown in figure ll(line 3) ,meaning that Retail-J might be added by saccharin.We also tested the same sample of Retail-J according; tothe, titration method recommended by GB 4578-2008.As demonstrated in table 2, the results obtained by thetitration method are not exactly same as those from the SERS method, but the SERS results are still inacceptable values ,especially for quick on-market analysis with the help of the portahle Raman system.
4 Conclusion
Through preparing an optimal Ag NPs@IP6with an IP6 shell of around 6 mn in thickness,the Ag NPs@IP6based SERS approach for the rapid determination of saeeharin in fresh jujube fruit was developed.The Ag NPs@IP6showed a much stronger Raman scattering enhancement factor and long-term stability.The lowest detectableconcentration of saccharin down t0 50 nmol·L-1 was achie,ved.For real application, by using the ethanolsolution, after a facile pre-treatment method was done to extract the saccharin from the food products , the SERStest could be conducted.Although the SERS result had about 25% RSD in comparison with the result from thetitration method recommended by the National Standard of China, as a perspectve, this Ag NPs@IP6-basedSERS approach with the aid of portahle Raman spectrometer could realize rapid , sensitive and on-site detectionof saccharin for food quality control.
References:
[1] JACKSON L S.Chemical food safety issues in the United States : past, present, and future [J].Journal of Agricultural andFood Chemistry ,2009 ,57( 18) : 8161-8170.
[2] PRICE J M, BIAVA C G. OSER B L, et aI.Bladder tumors in rats fed eyclohexylamine or high closes of a mixture ofcyclamate and saccharin[J ].Science , 1970, 167 ( 3921 ) : 1131- 1132.
[3]
WHYSNER J, WILLIAMS G M.Saccharin mechanistic data ancl risk assessment: urine composition, enhanced cellproliferation , and tumor promotion [J].Pharmacology and 'rherapeutics , 1996 ,7 1 ( 1/2 ) : 225-252.
[4] DY BING E.Development and implementation of the IPCS conceptual framework for evaluating< mode of action of chemicalcareinogens [J].Toxicology , 2002 , 181/182 ( 1/2/3 ) : 121-125.
[5]
JUST T, PAU HW, ENGEL U, et al.Cephalie phase insulin release in healthy humans after taste stimulation?
[J].Appetite,2008(3)51:622-627.
[6] IONESCU E, ROHNER-JEANRENAUD F'. PROIETTO J, et aI.Taste-induced changes in plasma insulin and glucoseturnover in lean and genetic.ally ohese rats [J ].Diahetes , 1988 ,37 ( 6) : 773-779.
[7]BERTHOUD H R,'rRIMBLE E R, SIEGEL E G,et aI.Cephalicphase insulin secretion in normal and pancreatic islet-transplanted rats [J].American Journal of Physiology, 1980,238(4 ) : E336-E340.
[8] QIN X F.lmpaired inactivation of digestive proteases by deconjugated hiliruhin :the possihle mechanism for inflammatoryhowel disease [J].Medical Hypotheses ,2002,59( 2 ) : 159-163.
[9] QIN X F.Etiology of inflammatory howel disease: a unified hypothesis [J].World Journal of Gastroenterology, 2012, 18( 15 ) :1708-1722.
[10]WANG Y, XU Z L, XIE Y Y, et al. Development of polyclonal antibody-based indirect competitive enzyme-linkedimmunosorhent assay for sodium saecharin residue in food samples [J ] .Food Chemistry , 2011 , 126( 2 ) : 815-820.
[11] BERGAMO A B , FRACASSI D S J A , DE JESUS D P.Simultaneous determination of aspartame , cyclamate , saccharin andacesulfame-K in soft drinks and tahletop sweetener formulations by capillary electrophoresis with capacitively coupledcontactless conductivity detection [J].Food Chemistry ,2011 .124 ( 4 ) : 1714-1717.
[12]
GREMBECKA M , BARAN P, BLAZEWICZ A , et aI.Simultaneous determination of aspartame , acesulfame-K, saccharin ,eitric acid and sodium benzoate in various food proclucts using HPLC-CAD-UV/DAD [J].European Food Researeh andTechnology ,2013 , 238( 3 ) : 357-365.
[13]CHEN Y ,WULH, CHEN Y H.et aI.Determination of mercury (II) by surface-enhanced Raman scattering spectroscopybased on thiol-funetionalized silver nanoparticles [J] .Microchimica Aeta , 2012 , 177 ( 3/4 ) : 341-348.
[14]SANLES-SOBRIDO M , RODRIGUEZ-LORENZO L,LORENZO-ABALDE S,et al.Lahel-free SERS detection of relevantbioanalytes on silver-coated earhon nanotubes :the case of cocaine [J] .Nanoseale ,2009,1 ( 1 ) : 153-158.
[15] LI M H, CUO X Y, WANG H, et al.Rapicl and lahel-free Raman detection of azodiearbonamide with asthma risk [J].Sensors and Actuators B ,2015 ,216 :535-541.
[16] ZHEW G J K. HE L L.Surface-enhanced Raman spectroscopy for the chemical analysis of food[J].Comprehensive Reviewsin Food Science and Food Safety,2014 , 13( 3) :317-328.
[17] JEANMAIRE D L, VAN DUYNE R P. Surface Raman spectroelectroehemistry: Part I. Heterocyclie, aromatic, andaliphatie amines adsorbed on the anodized silver electrode [J].Journal of Electroanalytical Chemistry and InterfacialElectrochemistry , 1977 , 84 ( 1) : 1-20.
[18] MOSKovi'rs M.Surface-enhanced spectroscopy [J].Reviews of Modern Physics , 1985 ,57 ( 3 ) :783-826.
[19]ALBRECHT M G, CREIGHrrON J A.Anomalously intense Raman spectra of pyricline at a silver electrode [J].Journal ofthe American Chemical Society, 1977,99( 15 ) : 5215-5217.
[20]
WANC Y, YAN B, CHEN L.SERS tags : novel optical nanoprobes for hioanalysis [J].Chemical Review, 2013 , 113 ( 3 ) :1391-1428.
[21]NIE S M,EMORY S R.Prohing single molecules and single nanoparticles by surface-enhanced Raman scattering [J].Seience , 1997 ,275 ( 5303 ) : 1102-1106.
[22 ] KNEIPP K,WANG Y ,KNEIPP H,et aI.Single molecule detection using surface-enhanced Raman scattering (SERS) [J].Physical Review Letters , 1997 ,78( 9 ) : 1667-1670.
[23]
LIU T Y ,TSAI K T, WANG, H H , et aI.Functionalized arrays of Raman-enhancing nanoparticles for capture and culture-free analysis of hacteria in human blood [J].Nature Communication ,201 1 ,2 :538-545.
[24]LI Y T, LI D W, CAO Y,et al.Lahel-free in-situ monitoring of protein tyrosine nitration in blood by surface-enhancedRaman spectroscopy [J].Biosensors & Bioelectronics ,2015 ,69: 1-7.
[25]QIAN X, PENC X H, ANSARI D 0, et al./n, vivo turuor targeting and spec.troscopic detec.tion with surface-enhanc.edRaman nanoparticle tags [J ].Nature Biotechnology,2008 ,26( 1 ) : 83-90.
[26]MOHS A M, MANCINI M C, SINCHAL S, et al.Hand-held spectroscopic device for in, vivo and intraoperative tumordetection : contrast enhancement, detection sensitvity, and tissue penetration [J].Analytical Chemistry, 2010, 82 ( 21 ) :9058-9065.
[27]WILLErrS K A, VAN DUYNE R P.Localized surface plasmon resonance spectroscopy and sensing [J].Annual Review ofPhysical Chemistry , 2007 , 58( 1 ) :267-297.
[28]
WANC N, YANG H F.ZHU X,et aI.Synthesis of anti-aggregation silver nanoparticles based on inositol hexakisphosphoricmicelles for a stahle surface enhanced Raman scattering substrate [J ].Nanotechnology ,2009 ,20(3 1) :315603.
[29] FOX C H, EBERL M. Phytic acicl (lP6) , novel broad spectrum anti-neoplastic agent: a systematic review [J].Complementary Therapies in Medicine ,2002 , 10( 4 ) : 229-234.
[30] LIU J R, GUO Y N, HUANG W D.Study on the corrosion resistance of phytic acid conversion coating for magnesiumalloys [J ].Surface & Coatings Technology , 2006 , 201 ( 3/4 ) : 1536-1541.
[31]
LEOPOLD N,LENDL B.A new method for fast preparation of h~hly surface-enhancecl Raman sc.attering (SERS) activesilver colloicls at room temperature hy recluetion of silver nitrate with hydroxylamine hydrochloride [J ].Journal of PhysicalChemistry B ,2003 ,107 ( 24) :5723-5727.
[32] RODRICUEZ L L,ALVAREZ P R A ,PASTORIZA S I,et al.Zeptomol detection through controlled ultrasensitive surface-enhanced Raman scatteringl; [J] .Journal of the American Chemical Society ,2009 , 131 ( 13 ) :4616-161 8.
[33] HUANG X, EL-SAYED I H , QIAN W , et al.Cancer cell imaging ancl photothermal therapy in the near-infrared region byusingl; gold nanorods [J].Journal of the American Chemical Society, 2006 , 128( 6 ) : 21 15-2120.
[34] LIU B H. HANG M, ZHANC Z P, et al.Shell thickness-dependent Raman enhancement for rapid identification anddetection of pesticide residues at fruit peels [J ].Analytical Chemistry , 2012,84: 255-26 1.
[35]LlNK S, EL-SAYED M A.Size and temperature dependence of the plasmon ahsorption of colloidal gold nanoparticles [J].Jouranl of Physical Chemistry B , 1999 , 103 ( 21 ) :4212-4217.
[36] ABALDE-CELA S, ALDEAN UEVA-POTEL P, MATEO_MArrEO C , et aI.Surface-enhanced Raman sc,attering hiomedicalapplications of plasmonic colloiclal particles [J].Journal of the Royal Society Interface ,2010, 7 (4 ) : S435-S450.
[37] SANTOS E D B, SIGOLI F A, MAZALI I O.Surface-enhanced Raman scattering of 4-aminohenzenethiol on silvernanoparticles suhstrate [J].Vihratioanl Spectroscopy , 2013 , 68 : 246-250.
[38]ALLEN A L, MCCEARY J E , KNOPIK V S, et aI.Bitterness of the non-nutritive sweetener acesulfame potassium varieswith polymorphisms in TAS2R9 and TAS2R31 [J].Chemical Senses ,2013 ,38( 5 ) :379-389.
[39] ZHANG H,ZHAI S D,LI Y M,et aI.Effect of different sample pretreatment methods on the concentrations of excitatoryamino acids in cerehrospinal fluid determined by high-performance liquid chromatography [J].Journal of ChromatographyB,2003,784(1):131-135.
[40]GONG W B, LIU C,MU X D, et aI.Hydrogen peroxide-assisted sodium carbonate pretreatment for the enhancement ofenzymatic saccharification of cornstover [J ].ACS Sustainahle Chemistry and Engineering,2015 ,3 ( 12) :3477 - 3485.
[41]LIU H, PANG B, WANG H S, et al. Optimization of alkaline sulfite pretreatment and comparatwe study with sodiumhydroxide pretreatment for improving enzymatic digestihility of corn stover [J].Journal of Agricultural and Food Chemistry ,2015,63(12):3229-3234.
[42]
ZHANG H D, WU S B.Efficient sugar release hy acetic acid ethanol-hased organosolv pretreatment and enzymaticsaccharification [J] .Journal of Agricultural and Food Chemistry ,2014 ,62(48) : 1 1681-11687.
(責任编辑:郁慧,顾浩然)
