B(C6F5)3催化合成二吲哚甲烷类化合物的研究
2020-10-16马静雨刘双磊张振国金俊阳贾振华
马静雨,刘双磊,张振国,金俊阳,贾振华
(1.南京工业大学化学与分子工程学院,先进化学制造研究院,南京211816;2.南京工业大学先进轻质高性能材料研究中心,南京211816)
1 Introduction
Indole skeletons are ubiquitous in many natural and synthetic organic compounds that possess a broad spectrum of biological activities[1].In particular,3,3′-bisindolylmethanes(BIMs)units are important constitutions among a class of indole alkaloids,which are widely found in various bioactive natural products,agrochemicals,fine chemicals,pharmaceuticals and functional materials(Fig.1).Since Fischer first synthesized 3,3′-BIMs in 1886,the general method to prepare 3,3′-BIMs is condensation of indoles and carbonyl compounds in the presence of acids following Friedel-Crafts pathway[2].However,most of these protocols demonstrated low efficiency and suffered from several limitations such as long reaction time,involving expensive and toxic metallic reagents,utilizing stoichiometric amount of acids,high temperatures as well as harsh conditions[3].

Fig.1 Representative 3,3′-bisindolylmethanes(BIMs)and corresponding biological activities
Since Masseyet al.prepared tris(penta-fluorophenyl)-borane[B(C6F5)3]in the 1960s[4,5],the major interest of its utilization was focused on the catalysis of polymerization as an initiator[6-11].In 1996,Pierset al.discovered the first B(C6F5)3-catalyzed hydrosilylation of carbonyls[12].Until 2006,Stephanet al.discovered the complex of B(C6F5)3substituted with bulky dimesitylphosphine atpara-position was able to activate the hydrogen reversibly,which was thereafter termed as‘frustrated Lewis pairs’(FLPs)[13,14].During last fifteen years,the interest not only in the field of FLPs but the catalysis involving B(C6F5)3was gathered.In 2018,Zhonget al.[15]first reported a strategy of B(C6F5)3-catalyzed double Markovnikov addition to indoles with aryl alkynes under neat conditions to obtain the symmetrical 3,3′-BIMs.In the same year,Heet al.[16]described a similar protocol of B(C6F5)3-catalyzed synthesis of the symmetrical 3,3′-BIMs under metal-free conditions,in which carboxylic acids was treated as alkylating reagents in the presence of Et3SiH.Herein,we report a simple and direct protocol for preparing the 3,3′-bisindolylmethane derivatives from the indoles and aryl aldehydes as the substratesviaa B(C6F5)3-catalyzed process of dehydration.
2 Experimental
2.1 Reagents and Instruments
Unless otherwise stated,all reagents were purchased from commercial suppliers and used without further purification.All reactions were carried out under atmosphere using undistilled solvent.1H and13C nuclear magnetic resonance(NMR)spectra were recorded in CDCl3on Bruker Avance or JOEL 400 MHz spectrometers.Column chromatography was generally performed on silica gel(300—400 mesh)and all reactions were monitored by thin layer chromatography(TLC)using UV light to visualize the course of the reactions.
2.2 General Procedure
To a 4 mL glass vial equipped with a magnetic stir bar was added B(C6F5)3(12.8 mg,5%,molar fraction)followed by the addition of benzaldehyde(51 μL,0.5 mmol)and 1,2-dichloroethane(DCE,1 mol/L,1 mL).Subsequently,1-methyl indole(137 μL,1.1 mmol)was added into the solution in air.The mixture was stirred for indicated time at room temperature until benzaldehyde was consumed completely monitored by TLC.Once the reaction was completed,the resulting residue was concentratedin vacuoandn-hexane(3 mL)was added.After the mixture was stirred for 12 h,n-hexane was filtered undervacuoto obtain the 3,3′-(phenylmethylene)bis(1-methyl-1H-indole)as a white solid(163 mg,93%).
3 Results and Discussion
3.1 Optimization of Reaction Conditions
To continue our recent interest in the main group catalysis,we began the optimization of reaction conditions with benzaldehyde(1a)and 1-methyl-indole(2a)as the model substrates,5%(molar fraction)B(C6F5)3as catalyst to screen the solvents(Table 1,Entries 1-5).To our delight,at certain concentration of 1a(0.4 mol/L)with different solvents,the model reaction proceeded rapidly and the desired product,3,3′-(phenylmethylene)bis(1-methyl-1H-indole)(3a),was generated in only 30 min with the yield from 18%in DMSO to 98%in DCE.Under catalyst-free conditions,compound 3a was not detected(Table 1,entry 6).When the catalyst loading(molar fraction)was lowered down from 5%to 1%,the yield of 3a was decreased to 84%(Table 1,entry 7).Under neat conditions,compound 3a was formed in a yield of 83%(Table 1,Entry 10).Moreover,the higher concentration would result in the higher yield of compound 3a within short reaction time(Table 1,entries 11-15).Eventually,this transformation was accomplished quantitatively in 15 min under optimal conditions and the target product was isolated in a yield of 93%(Table 1,entry 15).

Table 1 Optimization results of reaction conditionsa
3.2 Scope of B(C6F5)3-catalyzed Efficient Synthesis of 3,3′-Bisindolylmethane Derivatives
With optimized reaction conditions in hand,the scope of B(C6F5)3-catalyzed efficient synthesis of 3,3′-bisindolylmethane derivatives was explored.In this work,aryl aldehydes were examined and the core structure of the products contained multiple aryl groups.Moreover,because the solubility of most compounds was poor inn-hexane,all the target products were simply purified at 0.5 mmol scale without column chromatography.As such,a series of benzaldehyde and its derivatives with electron-donating groups and electron-withdrawing groups at diverse positions were employed in the process of dehydration with 1-methyl-indole(2a)to afford the corresponding products in excellent yields(Scheme 1,3a-3r).Notably,4-cyan substitution could accelerate the process to achieve this transformation in 10 min(3l).Polycyclic aryl aldehydes,for instance,4-phenylbenzaldehyde and 2-naphthaldehyde were converted to the privileged 3,3′-bisindolylmethane derivatives in 89%and 91%respectively(3s and 3t).To be delighted,indole was comparable with benzaldehyde to generate 3,3′-(phenylmethylene)bis(1H-indole) in 77%yield(3u).Similarly,when the derivatives of 1-methyl-indole with different substitutes were subjected to optimal conditions,the reactions went smoothly in indicated reaction time with full conversion of benzaldehyde(3v-3y,the spectra and data of 3,3′-bisindolylmethane derivatives see the Supporting information of this paper).
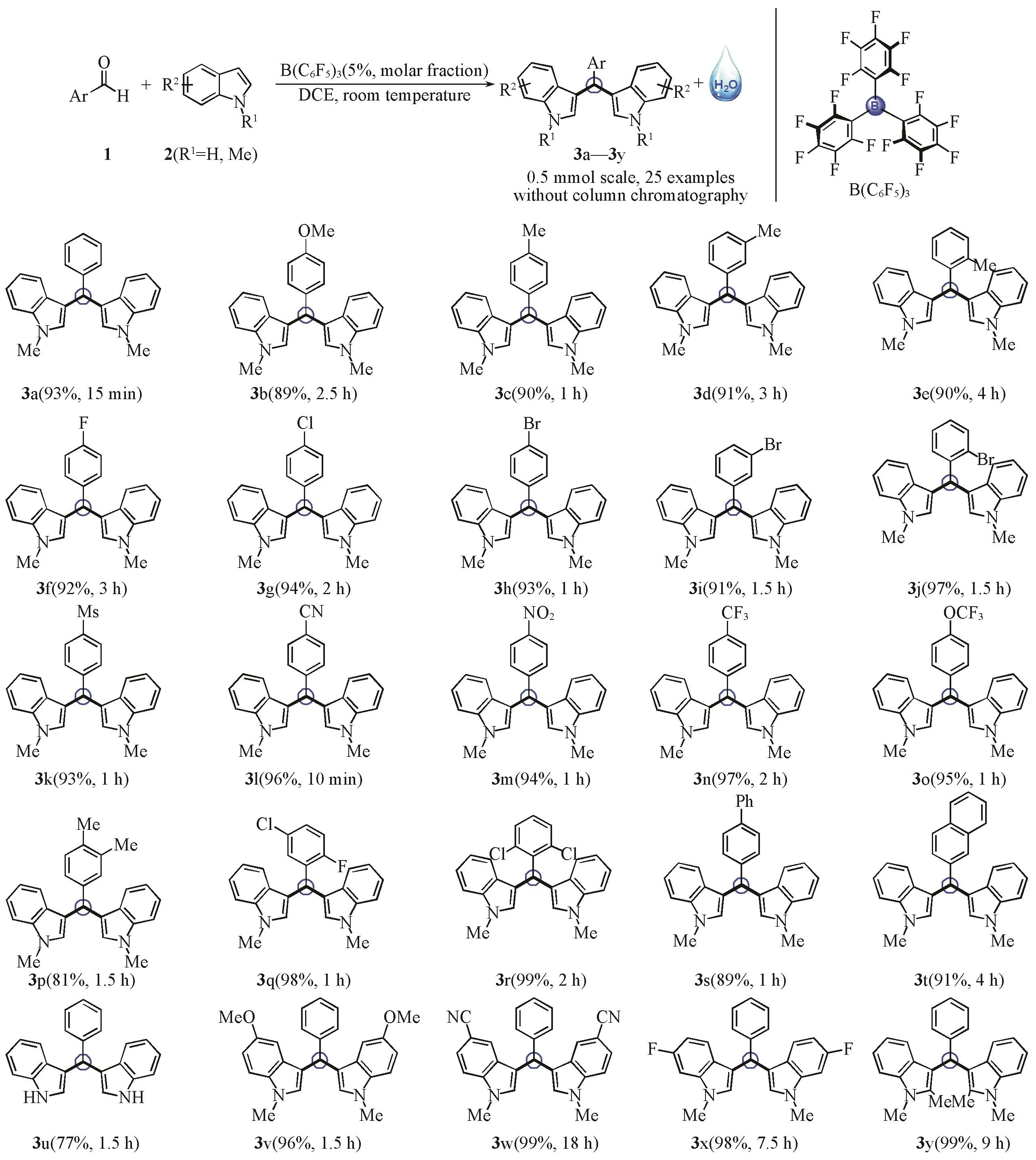
Scheme 1 Scope of B(C6F5)3-catalyzed efficient synthesis of 3,3′-bisindolylmethane derivatives
In 2004,Resconi et al.[17]investigated the synthesis of zwitterionic species with 1-methyl-indole(2a)and B(C6F5)3.To gain more insight into the mechanism,1H NMR techniques were utilized to analyze the in-situ formed mixture.When the molar ratio of 2a to B(C6F5)3was 1∶1,the signal of proton of C3 decreased obviously.Based on these evidences(see the Surpporting information of this paper),the mechanism was proposed and the pathway is depicted in Scheme 2.Initially,β-attack of the boron by compound 2a at C3 position occurred to form the zwitterionic intermediate Ⅰ,which subsequently attacked the benzaldehyde(1a)to generate the intermediate Ⅱ.Followed by β-elimination to release the boron hydroxyl anion,the key intermediate Ⅲwas achieved,which was tended to tautomerize with intermediateⅣ.With the assist of boron hydroxyl anion,the second molecular 2a was involved to form the intermediateⅤ.Finally,the dehydration process was proceeded to yield the 3,3′-bisindolylmethanes(BIMs)and regenerate B(C6F5)3.

Scheme 2 Proposed mechanism of B(C6F5)3-catalyzed dehydration process
4 Conclusions
In summary,we have developed a simple and direct protocol to synthesize 3,3′-bisindolylmethanes(BIMs)derivatives.Under B(C6F5)3catalyzed conditions,aryl aldehydes and indole derivatives were efficiently converted to BIMS in excellent yields.During the dehydration process,varies of substituted groups were well tolerated and BIMs were isolated without further column chromatography due to their poor solubility inn-hexane.Our strategy would provide a mild,efficient,metal-free and environmentally friendly route toward syntheses of BIMs,which possess potential bioactivities in medicinal chemistry.
This paper is supported by the Start-up Grant of Nanjing Technogy University(No.38037037)and the SICAM Fellowship from Jiangsu National Synergetic Innovation Center for Advanced Materials.