Role of oxysterol-binding protein-related proteins in malignant human tumours
2020-04-22HaoLiuShuaiHuang
Hao Liu,Shuai Huang
Hao Liu,Shuai Huang,Department of Hepatobiliary and Pancreatic Surgery,The First Affiliated Hospital of Zhengzhou University,Zhengzhou 450052,Henan Province,China
Abstract
Key words: Oxysterol-binding protein family;Oxysterol-binding protein-related protein;Malignant tumour;Role;Human tumour;Tumour proliferation,migration,and invasion
INTRODUCTION
Oxysterols are oxygenated derivatives of cholesterol molecules that are formed in our human body or ingestedviathe diet and oxygenated forms of plant sterols,which are phytosterols.Moreover,cholesterol precursors could also be named oxysterols[1].Under different circumstances,oxysterols exert opposite effects depending on the oxysterol concentration.For example,oxysterols are natural components found at low concentrations in the human body and can intervene in many physiological capabilities[2,3]by not only taking part in cholesterol metabolism regulation but also being involved in respective signalling pathways,membrane fluidity,and the activity of many membrane proteins[1].Additionally,oxysterols are involved in certain human clinical pathologies and even influence the carcinogenesis and progression of malignant tumours,such as breast,prostate,colon,and bile duct cancers[1].However,the role of oxysterols in carcinogenesis and cancer progression needs to be further elucidated.Oxysterols exert a very sophisticated effect on miscellaneous cell lines.Various studies have noted that oxysterols can adjust inflammatory and signalling pathways or exert pro-cancerous and pro-proliferative activities through oxysterolbinding proteins (OSBPs)[1].
The OSBP family is a group of proteins that mediate oxysterol metabolism and bioactivity in cells.In human malignant tumour cells,12 genes can encode members of the OSBP-related protein (ORP) family and produce 15 proteins through alternative splicing and transcription[1].The ORP family consists of at least 12 members characterized by a conserved domain that might bind oxysterols,cholesterol,or phospholipids and strongly affects lipid metabolism,cellular signal transduction,or vesicle transport in response to binding to the corresponding ligand[1,4].OSBPs and ORPs form a very large number of lipid transfer proteins.The ORP family participates in the regulation of metabolism and the trafficking of cholesterol and lipid molecules,particularly in the endoplasmic reticulum (ER) and Golgi apparatus[4].These proteins also affect other cellular processes,such as vesicular trafficking,by acting as scaffolds in cell signalling pathways and being involved in cell cytoskeleton formation and cell adhesion[4].It is believed that OSBPs are associated with cell proliferation,cell migration,and carcinogenesis.In human cancer cells,ORPs are differentially expressed at the mRNA or protein level,which suggests that they play a role in tumourigenesis and progression[1,4].Many studies have revealed the putative role of OSBPs in various cancer types[1].ORPs have the capacity to accelerate human tumour cell proliferation,migration,and invasion.Oncogenic signalling can reprogram metabolic pathways,and this mechanism has been proposed as a hallmark of cancer cells and could offer attractive targets for anticancer strategies.Many studies have revealed the putative role of OSBPs in various human cancer types[5-7].The present review focuses on the biological role of ORP family members,particularly in malignant human tumours (Table 1).
Substantial lines of evidence indicate that certain members of the OSBP/ORP family can result in cancer development.However,the exact mechanism that OSBP/ORP family members play in cancer cell initiation and progression and their effects in these processes are currently unclear.Complete elucidation of the exact roles that OSBP and ORP family members play in human tumour cells will allow the conquering of malignant tumours and significant improvements in the overall prognosis of cancer patients.This is a grand plan;thus,the ORP family has a very high potential research value and awaits further exploration.The ORP family will definitely provide potential and important clinical therapeutic targets in cancer patients.
ORPS AS SCAFFOLDS FOR SIGNALLING PROTEIN COMPLEXES
Eighteen years ago,it was revealed that ORPs participate in the management of cell signalling and development[8].TheCaenorhabditis elegansORPs determine the functions of bone morphogenetic protein receptor-associated molecule-interacting protein in transforming growth factor-β signalling and body length regulation.A study conducted in 2005 showed that human ORPs work as scaffolds for two proteinphosphatases,PP2A and HePTP,and thus regulate the activity of ERK in a sterolspecific manner[9].Moreover,cholesterol-bound ORPs bind with active phosphatases and induce the migration of cholesterol and the dissociation of oxysterol 25-hydroxycholesterol (25-OHC) from the complex,and these effects are concomitant with the targeting of ORPs to the Golgi complex.In contrast,Romeoet al[10]found that the upregulation of 7-ketocholesterol (7KC)viaORPs regulates profilin-1,which is an actin-binding protein associated with endothelial dysfunction and atherosclerosis.This signalling transparently involves the reciprocity of the ORP-7KC complex with tyrosine kinase JAK-2,which phosphorylates Tyr394 on ORPs to induce STAT3 activation and profilin expression.Interestingly,a previous study[11]revealed that ORP9,whose phosphorylation targets contain phosphorylated protein kinase 2,is dependent on mammalian target of protein kinase C-b or rapamycin.ORP9 interacts with these kinases and negatively regulates the phosphorylation of PDK-2 in Akt/protein kinase B,which is a major regulator of cell survival,cell cycle progression,and glucose metabolism.A key implication of these findings is that ORPs can also function as scaffold-like lipid sensors in tumour cell signalling.Whether these signalling capabilities of ORPs are based on their localization at membrane contact sites is unclear,and we used OSBPs and ERK as examples and found that the above-described regulatory function of OSBPs appears to represent a behaviour mode that is independent of membrane contact sites (MCS).The location of OSBPs in the Golgi apparatus is closely related to several phenotypic effects of OSBP manipulation.When located in the Golgi complex,ORPs activate the ceramide transporter CERT in a sterol-dependent manner,and this effect enhances the non-vesicular flux of ceramide to trans-Golgi and promotes sphingomyelin synthesis.ORPs therefore act in cooperation with CERT and the PC transfer protein Nir2 to regulate the lipid composition of Golgi membranes[13].Protein kinase D-mediated phosphorylation can negatively regulate the ORPs in the Golgi apparatus[14].Based on a previous study[15],it is known that ORP overexpression in a human neuroglioma cell line and human HEK293 cells inhibits the processing of amyloid precursor protein to b-amyloid,whereas the knockdown of OSBPs exerts the opposite effect.ORPs induce the sequestration of APP-Notch2 heterodimers in the Golgi complex,and this effect is reversed by the addition of the high-affinity OSBP ligand 25-OHC,which is consistent with the distribution of ORPs[16].
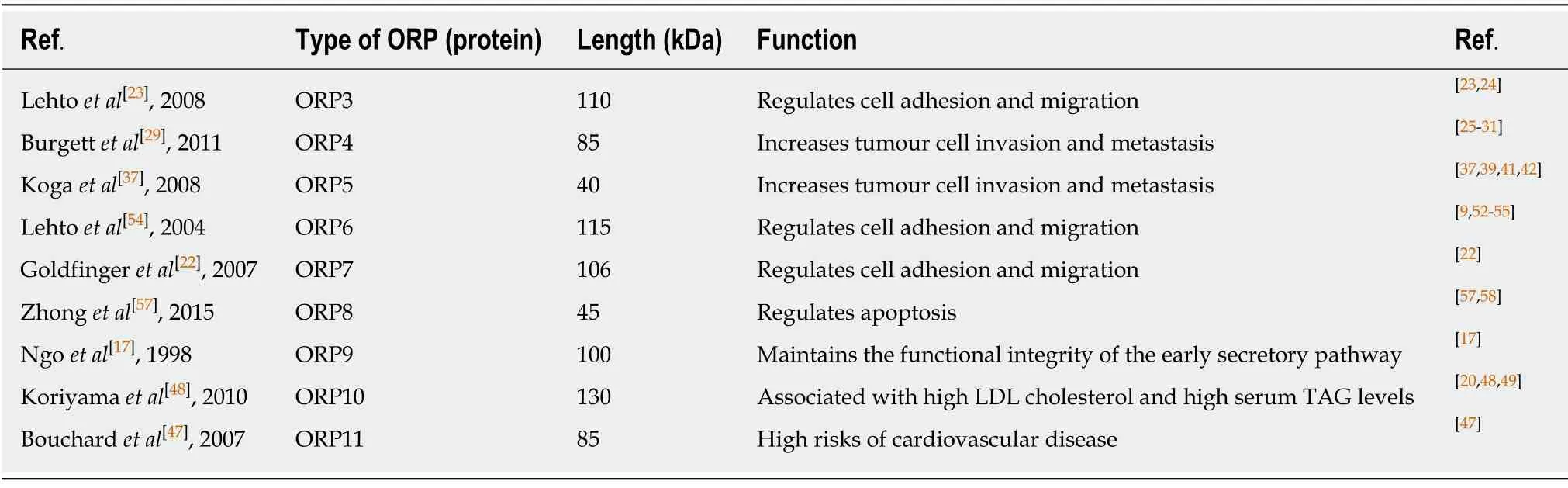
Table1 Specific types and functions of different oxysterol binding protein-related proteins and their associations with malignant human tumours
ROLE OF MAMMALIAN ORPS IN INTRACELLULAR MEMBRANE TRAFFICKING
Substantial lines of evidence indicate that ORPs can manage human intracellular membrane trafficking.In human cells,the overexpression of ORP9S could tamper ERGolgi protein transport,and deletion of the long variant ORP9L,which is a protein localized in the ER and trans-Golgi membrane,results in fragmentation of the Golgi apparatus,inhibition of the transfer of proteins from the ER to the Golgi apparatus,and the accumulation of cholesterol in the endoplasm[17].These results suggest that ORP9 can maintain the functional integrity of the early secretory pathway by controlling the lipid composition and organization at the ER and trans-Golgi[18].The significant findings revealed that an excess of the PH domain can potentially interferes with the small GTPase ARF,resulting in Golgi PI4P sequestration[19].ORP10 can control the secretion of hepatocyte apoB100-containing lipoproteins,which localize to not only microtubules but also Golgi membranes[20].ORP9 management is considered to reflect an imbalance in the sterol distribution in membranes associated with the early secretory mechanism[17].However,ORP10 can function as a specific carrier in microtubule-dependent dynamics.ORP7 interacts with GATE-16,which is a small ubiquitin-like protein that functions as a chaperone for the attachment of soluble NSF with the protein receptor GS28 in the Golgi complex[21].ORP7 hurts the ability of GATE-16 to escort GS28,which can enhance GS28 proteasomal degradation.ORP7 has a function in mediating the sterol regulation of Golgi membrane traffickingviathis function.
ORP3 IS A PHOSPHOPROTEIN THAT REGULATES CELL ADHESION
ORP7,a small GTPase that regulates cell adhesion and migration[22],interacts physically with R-Ras,and ORP7 and ORP3 play a role in Ras signalling.Previous studies[23]have revealed that ORP3 can control cell adhesion and spreading on a fibronectin substratum,organization of the actin cytoskeleton,β1-integrin activity,and macrophage phagocytic function,and these cellular processes are also regulated by R-Ras.Based on the latest results,we know that the interaction between ORP3 and its ER membrane anchors depends on the phosphorylation of ORP3,and the targeting of ORP3 to the plasma membrane is changed by the PH domain,which leads to the localization of some proteins at the putative ER-plasma membrane contact site.This finding reveals a functional interplay between ORP3 and protein kinase signalling cascades and suggests that this protein could mediate communication between the ER and the plasma membrane in response to specific signals;for instance,ORP3 could have a function similar to that of yeast Osh3p[24].ORP3 is expressed abundantly in leukocytes and in several types of epithelia,and elevated expression of this protein has been detected in certain forms of leukaemia and solid tumours,which suggests that this protein might regulate cell signalling and adhesion in a manner that facilitates malignant growth (Figure 1).
ORP4 IS A PREREQUISITE FOR TUMOUR CELL GROWTH
ORP4 is expressed as three important variants:ORP4L,ORP4M,and ORP4S[25].ORP4 is constitutively expressed in the human brain,heart,and testis but is absent in other human and mouse tissues[26,27].ORP4-knockout mice exhibit teratozoospermia due to the death of developing spermatozoa,which indicates that ORP4 is essential for the liveness of specific cell populations[26].Early studies discovered ORP4L in leukocytes from patients with chronic myeloid leukaemia but not in those from healthy donors[27].Recent studies have shown that ORP4L is involved in human malignant tumour cell proliferation and survival[28]and is a target of the natural anti-proliferative steroidal saponin OSW-1[29],and these findings reveal the participation of ORP4L in the control of oncogenic cell growth.
ORP4 has a human cell growth regulatory activity[28].Similar to other ORPs,ORP4 binds to sterols and PI(4)P.ORP4 requires sterol-binding activity and PI(4)P-binding activity to boost human malignant cell survival and proliferation[26].In human nontransformed intestinal epithelial cells-18,ORP4 silencing can rapidly activate the apoptotic cell death pathway,including nuclear PARP proteolysis,fragmentation of DNA,caspase-3 and JNK phosphorylation,and nucleosome release[28].ORP4 is highly expressed in IEC-18 cells transformed with mammalian oncogenic H-Ras.ORP4 is not involved in silencing-mediated apoptotic responses,partly because oncogenic H-Ras conversion activates tumour proliferative pathways and suppresses tumour apoptotic pathways.In contrast,the overexpression of ORP4 in IEC-RAS clones can promote tumour transformation,but ORP4 cannot be silenced by gene knockdown and leads to apoptosis.The silencing of ORP4 in HEK293 and HeLa cells can inhibit cell proliferation to result in growth arrest but not cell death[28].A recent study showed that ORP4L boosts cervical carcinoma cell (including HeLa,C33A,and CaSki)proliferation[30].ORP4L contains human tumour cell growth by engaging intracellular Ca2+signalling.ORP4 expression has continuously been implicated in breast tumours,lung tumours,and leukaemia[27,31-33].A previous study[33]revealed that T-cell acute lymphoblastic leukaemia (T-ALL) cells are characterized by increased levels of oxidative phosphorylation and a higher ATP content.The cell number is proportional to the content of cellular ATP[31].ORP4L is primarily expressed in most T-ALL cells but not in normal T-cells.ORP4L acts as an adaptor responsible for the assembly of CD3e,Gaq/11,and PLCβ3 into a complex that activates PLCβ3[31].Moreover,ORP4L overexpression has the opposite outcomes:The silencing of ORP4L can inhibit PLC3 activation,reduce the production of IP3,and suppress the release of Ca2+from the ER.In addition,ORP4L promotes the transfer of Ca2+to the mitochondrion,and pyruvate dehydrogenase is dephosphorylated by Ca2+-dependent phosphatase[30].ORP4L silencing can cause cell death,which might be due to defects in the transmission of Ca2+from the ER to the mitochondrion,leading to activation of the cell energy sensor AMP kinase and the induction of autophagy[31].The important role of ORP4L in Ca2+signalling and T-ALL cell survival appears to be separate from intracellular cholesterol transport.The silencing of ORP4L has no effect on cholesterol distribution in the ER,plasma membrane,and mitochondrion[31].Therefore,whether other lipids are bound to and carried by ORP4 to promote prosurvival activity in human Tlymphocyte cells remains unclear.
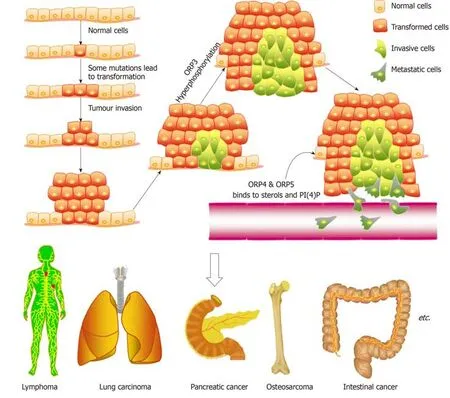
Figure1 Oxysterol binding-related protein family members promote tumour transformation,invasion,migration,and metastasis at the cellular,tissue,and organ levels.
ORP5 IS ENGAGED IN HUMAN TUMOUR CELL INVASION AND TUMOUR PROGRESSION
ORP5 is a tail-anchored ER membrane protein that functions as a lipid transporter amidst intracellular membranes[34].ER-anchored ORP5 serves as a phosphatidylserine transporter due to its ORD[35].Through a countertransport process occurring at membrane link sites,ORP5 transfers PS from the ER to the cell plasma membrane and PI(4)P to the ER[19].Previous studies have investigated the role of ORP5 in conveying PS from the ER to the mitochondrion and in sustaining appropriate mitochondrial action[36].
ORP5 expression has been connected to increases in human tumour cell invasion or metastasis.ORP5 is generally expressed in human pancreatic tumour cells.The invasion rate of both murine and human pancreatic tumour cells is increased by ORP5 overexpression and decreased by ORP5 consumption[37].Moreover,through analyses of clinical samples,we revealed that in our cohort of patients with pancreatic tumours,ORP5 overexpression was connected with a poor prognosis[37].Previous studies have revealed that ORP5 is overexpressed in human lung tumour tissues,particularly in human lung tissues of metastatic individuals[38].The specific correlation of high ORP5 expression with human tumour cell invasion or tumour progression is currently unexplained.The ORD of ORP5 carries a charge that gives it the ability to extract,bind,and transport lipid substances between the ER and other cell membranes[34,39,40].The mutation of some residues in the ORD of ORP5 that can bind to PS[35]or PI(4)P[39,41]obviously reduces the capacity of ORP5 to accelerate human malignant tumour cell invasion,proliferation,and migration.These results reveal that ORP5 regulates the distribution of PS and other lipids and thus plays an important role in tumour cell survival.For example,PS is essential for Akt signalling activation[40].The ORP5-mediated transport of PS might actually activate the Akt signalling pathway,causing reinforced tumour cell movement.Consistent with this possibility,we found that in human HepG2 cells,ORP5 depletes insulin-induced Akt activation or the mTORC1 signalling pathway.Moreover,PS is also responsible for the tissue distribution of cholesterol in the cytoplasmic leaflets of the plasma membrane[42].However,the ORP5-mediated function of PS might be related to intracellular cholesterol transport[34].Because proper intracellular cholesterol transport plays an important role in mTOR transduction[43],the ORP5-mediated transport of PS might promote mTORC1 activation and cell proliferation in some tumour cells.
ORP10 IS A MEMBER OF THE OSBP FAMILY,AND GENETIC VARIATIONS IN OSBPL10 ARE ASSOCIATED WITH DYSLIPIDAEMIAS AND PERIPHERAL ARTERY DISEASE
Increasing lines of evidence suggest that OSBPs and ORPs are involved in dyslipidaemias.OSBPs were isolated as high-affinity cytoplasmic receptors for several oxysterols[44,45].In mammals,including humans,the OSBP-like (OSBPL) gene family consists of 12 members,and these members display different expression patterns,subcellular localizations,and substrate specificities.
ORPs are divided into six subfamilies,and subfamily VI includes the closely related RP10 and ORP11.Single-nucleotide polymorphisms (SNPs) in genes encoding subfamily VI proteins (OSBPL10 and 11) have been associated with dyslipidaemic diseases.ORP11 expression is upregulated in the visceral adipose tissue of obese men at a high risk for cardiovascular disease[46].Further studies associated a number of SNPs in the OSBPL11 gene with risk factors for cardiovascular disease,including hypertension and hyperglycaemia[47].Some studies have shown that polymorphisms in the OSBPL10 gene are associated with high serum TAG levels in Finnish patients with dyslipidaemia.In addition,polymorphisms in the OSBPL10 gene are reportedly associated with high LDL cholesterol levels[48]and peripheral arterial disease[49]in Japanese subjects.
OTHER ORPS AND TUMOURS
In our latest study,ORP4,ORP5,and many other members of the ORP family were also linked to tumours.For instance,all types of malignancies,including colorectal adenocarcinoma,lymphoma,testicular tumour,and osteosarcoma,are strongly associated with elevated ORP3 expression levels[9,50-54].The highest expression of ORP6 is found in the brain and skeletal muscle.In F9 cells,endogenous ORP6 is predominantly associated with the nuclear envelope.The expression data demonstrate that ORP3,ORP6,and ORP7 are not merely redundant gene products but show marked quantitative differences in tissue expression,which suggests that their functions have tissue-specific aspects.Moreover,cholangiocarcinoma patients exhibit high levels of several ORPs,which indicates that oxysterols are involved in cancer progression through OSBPs or that OSBPs might serve as molecular markers for cholangiocarcinoma[55].ORP3 reportedly activates the small GTPase R-Ras[23].Overphosphorylated ORP3 interacts with the ER membrane protein VAPA to stimulate R-Ras signalling[56].The mutual effect of ORP3-VAPA improves Akt signalling and the activity of 1-integrin[56].ORP3 can alter human tumour cells and cell signalling pathway adhesion functions to promote tumour cell proliferation,migration,and invasion[23,56].More importantly,similar to ORP5,ORP8 is primarily expressed in many tumour cells.However,ORP8 is expressed at a very low level in human haematoma cells,whereas the microRNA miR-143,which downregulates ORP8 expression,is reportedly upregulated in these cells[57,58].The apoptosis of human haematoma cells requires the expression of ORP8.Although ORP8 can affect Fas translocation,the precise mechanism through which ORP8 regulates apoptosis remains to be discovered[57].
Many studies have demonstrated a substantial relationship of apoptosis with oxysterols,the suppression of proliferation,and cell cycle arrest.A previous study[59]explained this phenomenon using bile duct cancer formation as an example.The lesion caused by oxysterols,which mediate oxidative DNA,needs to be repaired.The mutations associated with absent or incorrect DNA repair cause either carcinogenesis or cell death[60].In contrast,oxysterol receptors,such as LXRs,might regulate antiproliferative function.However,different oxysterols play different roles in many cell types.For instance,the Ames test showed a CT mutagenic effect.The CT treatment of Chinese mouse ovarian epithelial cells increases the formation of reactive oxygen species and chromosome aberrations[61].In contrast,the CT precursor cholestane-5α,6α-epoxy-3β-ol exerts anti-proliferative effects,resulting in cell cycle arrest in the human malignant leukaemia cell lines HL-60 and Molt-4[62].Moreover,the cytotoxicity of oxysterols differs in various cell lines,e.g.,the IC50values of 25-HC and 7β-HC are variable in many human leukaemia cell lineages[63].Oxysterols induce all types of effects when used alone or in admixtures.For instance,the application of a mixture of 7β-HC and 25-HC yields lower pro-apoptotic outcomes than the usage of 7β-HC alone[64].Other studies have reported varying effects of oxysterols administered alone or in admixtures[65].In addition,representative combinations of oxysterol compounds in diets should be further investigated (Figure 2).
CONCLUSION
We can conclude that ORP3 has the ability to regulate cell adhesion and migration.ORP4 can increase tumour cell invasion and metastasis,and ORP5 has the ability to increase tumour cell invasion and metastasis.In addition,ORP6 can regulate cell adhesion and migration,ORP7 has the ability to regulate cell adhesion and migration,and ORP8 can regulate apoptosis.ORP9 has the ability to maintain the functional integrity of the early secretory pathway,and ORP10 might cause high LDL cholesterol and high serum TAG levels.Moreover,ORP11 might be associated with high risks of cardiovascular disease.
Further studies on oxysterols are required to confirm their important functions in tumour prevention,prognosis,or treatment and in carcinogenesis.Therefore,the extraction of various samples from the human circulation and target tissues at different pathological states for genomic,proteomic,and metabolomic analyses is required for the clinical application of oxysterol in diagnosis and treatment.It is believed that the application of the ORP family in the treatment of malignant tumours will be spectacular,and we believe that all human malignant tumours and related challenges will be conquered in the future.
In the past few years,accumulating lines of evidence from studies conducted by different research groups in the field of malignant tumours have revealed that ORP family members are linked to tumours and have the capacity to accelerate human tumour cell proliferation,migration,and invasion.It is believed that future research will provide more detailed information on the mechanism through which ORPs regulate the growth of tumour cells and will help us establish ORPs as new targets for the treatment of various cancers.
In conclusion,the ORP family plays an essential role in malignant tumour diseases.Many mechanistic questions remain,and much research work needs to be performed.Specifically,the establishment of new bioanalytical technologies and novel experimental animal models could help researchers uncover the secrets of the ORP family.
The ORP family members can accelerate human tumour cell proliferation,migration,and invasion.In this review,the mechanisms and functions of various ORPs are introduced in detail.We also attempt to identify the roles these proteins play in malignant tumours with the ultimate aim of determining the exact role of the OSBP/ORP family in human tumour cells.
The following are the remaining questions regarding the relationship between sterol signalling and sensory genes:Can we find their genetic variation or sporadic mutations in target tissues,and do these mutations exhibit individual differences based on oxysterol levels? Are there invasive or non-invasive markers that can be used to predict pathological risk? Are there any signs showing that a potential preventive measure,namely,the use of oxysterols,could be used to reduce disease risk? Finally,can certain genes or proteins in the oxysterol pathway be used as targets in personalized treatment?
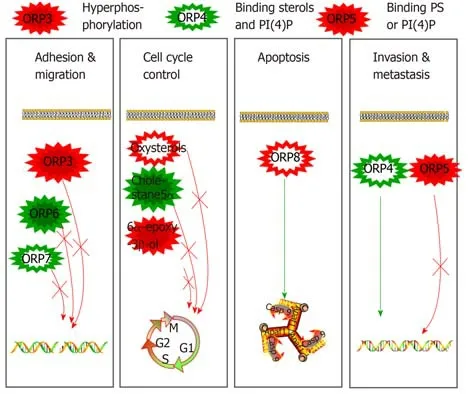
Figure2 Mechanism through which oxysterol binding-related protein family members promote tumour migration,invasion,and metastasis via hyperphosphorylation or binding to the sterols PS and PI(4)P.
杂志排行
World Journal of Clinical Cases的其它文章
- Oncogenic role of Tc17 cells in cervical cancer development
- Acute distal common bile duct angle is risk factor for postendoscopic retrograde cholangiopancreatography pancreatitis in beginner endoscopist
- Three-dimensional computed tomography mapping of posterior malleolar fractures
- Application of a modified surgical position in anterior approach for total cervical artificial disc replacement
- Potential role of the compound Eucommia bone tonic granules in patients with osteoarthritis and osteonecrosis:A retrospective study
- Prognostic factors for overall survival in prostate cancer patients with different site-specific visceral metastases:A study of 1358 patients
