Evidence for pore-filling gas hydrates in the sediments through morphology observation☆
2019-12-05WenxiangZhangShuanshiFanYanhongWangXuemeiLangKaiGuoJianbiaoChen
Wenxiang Zhang,Shuanshi Fan,Yanhong Wang*,Xuemei Lang,Kai Guo,Jianbiao Chen
Key Lab of Enhanced Heat Transfer and Energy Conservation Ministry of Education,School of Chemistry and Chemical Engineering,South China University of Technology,Guangzhou 510640,China
Keywords:Gas hydrate Sediments Growth Dissociation Pore-filling
ABSTRACT To provide an evidence of natural gas hydrate occurrence state,a series of experiments on multiple growth and dissociation of 90.0% methane/10.0% propane hydrates at 1.3 MPa and 270.15 K were carried out in two sediments for morphology observation via a visible jacketed-reactor.The gas hydrate crystals were observed to form and grow on the surface of sediments at the initial growth.During the thermal decomposition,gas and liquid products had an unceasingly impact on the sediments,then gas/liquid-solid migration occurred,and a large number of cavitation appeared.In the later growth and dissociation experiments,the gas hydrate particles were in suspension or supporting states in the interstitial pore space between the sediment particles,indicating that the gas hydrate displayed a pore-filling characteristics.Through analyzing the distribution of gas hydrates and bubbles,it was found that the amount of gas hydrates distributed in the sediments was improved with multiple growth-dissociation cycle proceedings.Gas migration enhanced the sediment movement,which led to the appearance of the increasing quantity of gas bubbles in the sediments during cycles.Salts affected the growth of the gas hydrates and the migration of sediment grains,which also restricted the accumulation of gas bubbles in the sediments.According to the Raman analysis,the results showed that sII hydrates were formed for CH4and C3H8gas mixtures in different sediments and solutions with hydration number of 5.84-6.53.The Salt restricted the access of gas into the hydrate cages.
1.Introduction
Natural gas hydrates are crystalline solids composed of water and natural gas,where gas molecules are trapped as guests in hydrogen-bonded water cavities.It has received hot attention as a potential source for the future clean energy[1],which has been found to exist widely in the seafloor and permafrost[2].On May 2017,China first time succeeded in exploiting natural gas hydrate in the South China Sea,which realized continuous gas production for 60 days.The breakthrough may lead to a global energy revolution.Sediments cores recovered from the seafloor and permafrost have been reported to contain several patterns of gas hydrates:disseminated,nodular,layered,and massive[3-5].Based on the micro models of hydrate-bearing sediments,researchers proposed several kinds of ideal model,which includes grain contacting,cementing,coating,floating and load-bearing[6-8].
In recent years,it was found that natural gas hydrates in permafrost regions mainly existed as nodular and fracture fillings in consolidated sedimentary rock [9],whose distribution depended on the regional geological tectonic features,temperature,and stratum pressure [10].Gas hydrates in the seafloor mainly existed as disseminated porefillings,layered and nodular fracture fillings[11].From No.204 Voyage of International Ocean Drilling Project(ODP),gas hydrates found at the edge of Cascadia in the Oregon coast were random and non-continuous in distribution[12].Lee et al.[13]characterized the gas hydrate example from the Ulleung Basin,East Sea of Korea,where gas hydrates were identified to be fracture-filled.Salehi et al.[14]characterized gas hydrate resources in the Makran Accretionary Prism through compressional and shear wave pre-stack analysis.It reported that natural gas hydrates primarily filled the pores and fractures of their host sediments.Zhang et al.[15]reported that natural gas hydrates in the South China Sea were mainly developed into the massive and disseminated gas hydrates.The massive gas hydrate was thought to be features initially formed on the surface.While the layer of disseminated hydrates was just lying above the methane hydrate stability zone in the fine-grained sediment,which was the result of the smaller grain size of the sediments.Guan and Liang [16]reported that natural gas hydrate of Shenhu area in the north of the South China Sea existed as disseminated in the sediments,which was influenced by the low flux of methane.All of these studies indicated that the occurrence state of natural gas hydrates was complicated,which was affected and controlled by multiple factors.
Based on the occurrence state of natural gas hydrates,many researchers just studied the effect of porous media.As for porous media,researchers analyzed the influence of its composition and grain size.Bagherzadeh et al.[17]studied magnetic resonance imaging of gas hydrate formation in a bed of silica sand particles.Hydrate formation in such porous media was not uniform,and nucleation of hydrate crystals occurs inside the bed,which grew faster with smaller grain size.Uchida et al.[18]studied the decomposition of methane hydrates in sand,sandstone,clays,and glass beads.The results showed that methane hydrates not only formed in the pore spaces of the particles but also in the interlayers.The decomposition conditions were mainly affected by the pore sizes,while the surface textures and mineral components had less influence on it.Delli et al.[19]studied the permeability of porous media in the presence of gas hydrates.A progressive change in the hydrate formation morphology was observed from cementing to that of the pore-filling with increasing hydrate saturation.Zang et al.[20]studied the growth of gas hydrates in fine sand with salt water.It reported hydrate occupied the interstitial space of the partially water-saturated silica sand bed.The sediment grain size had no significant influence on the conversion rate of mixed gas hydrates.Winters et al.[21]proposed that coarser-grained reservoirs might contain high-saturation pore-filling gas hydrates,while finer-grained reservoirs might contain low-saturation disseminated or more complex gas hydrates,including nodules,layers,and high-angle planar and rotational veins.Occurrence of natural gas hydrates in stratum is very complicated,which is closely related to the mass transfer of gas,water and sediments.
Song et al.[22]in situ observed the growth of methane and carbon dioxide hydrates in porous media with magnetic resonance imaging.It proposed that gas hydrate formation preferentially started in the pore space.Ruffine et al.[23]created hydrate-bearing cores to study the morphology and formation of methane hydrate.The morphology of hydrates in sand-clay mixtures changed with the proportion of clay.Linga et al.[24]observed the growth of gas hydrates.The results showed that a small fraction of hollow silica would be displaced from the bed during the hydrate formation above the critical hollow silica to water ratio.Cook et al.[25]also studied the formation of gas hydrates.It was found that the veins or layers of hydrate typically appeared in two general orientations with fine sand and silt.Zhao et al.[26]proposed five types of hydrate deposition morphologies,which were correlated to the hydrate porosity.Yang et al.[27]reported that hydrate decomposition would result in sediment grains collapsing.It also indicated that high water saturation would impact the gas permeation [28-30].However most of these studies did not consider the multi-growth cycles and ignored the influence of sediment components,which will change the occurrence state of gas hydrates.
For providing further insight into the occurrence state of natural gas hydrates,the paper studied the occurrence of 90.0 mol%CH4/10.0 mol%C3H8gas hydrates in the sediments during three growth-dissociation cycles.The paper also analyzed the effect of salts on gas hydrate growth and dissociation processes through grid and Raman analysis.
2.Experimental
2.1.Materials
A gas mixture consisting of CH4(90.0%in volume)and C3H8(10.0%in volume)was used,which was supplied by Guangzhou Yinglai Gases Co.,Ltd.The sediments used in this study were silicate rocks(SR)and seafloor sands (SS).Silicate rocks were purchased by Guangzhou Congyuan instrument Co.,Ltd.Seafloor sands were collected by China National Offshore Oil Corporation.The deionized water used in all experimental runs was produced by an ultrapure water system with a resistivity of 18.25 mΩ·cm-1.The brine used in the experiments was the aqueous solution with 3.5 wt% NaCl.Table 1 summarized the properties of the sediments employed in this study.
2.2.Apparatus
The schematic of the experimental setup for gas hydrate growth and dissociation observation was shown in Fig.1.The observation apparatus consisted of four systems,which were reaction system,temperaturecontrol system,feeding system,and data recording system.
The jacketed reactor connected with a temperature-controlled thermostat bath(CC-K6 s,Huber,Germany).The jacketed reactor was specially modified to investigate the morphology of gas hydrate formation and dissociation in the sediments.The jacketed reactor consisted of a central and cylindrical polytetrafluoroethylene column and a pair of quartz glasses lids.The central column is a hollow cylinder with the volume of 1.2 ml,which has two sides of circular opening to connect the gas and water inlet pipes.A thermocouple was stuck on the surface of the quartz glasses to monitor the temperature of the jacketed reactor.A pressure transmitter was employed to measure the pressure insider the jacketed reactor.The data were recorded every 10 s by a data acquisition system connected to a computer.A camera was used during all experimental runs to observe the morphology of gas hydrates in the sediments.
In addition,the crystal structure of mixed CH4-C3H8gas hydrates formed in different sediments was measured by a Raman spectrometer(Renishaw in Via-Reflex).
2.3.Experimental procedure
2.3.1.Growth-dissociation observation
The amount of the sediments placed in the jacketed reactor for each experiment was 1.0 g with porosity of 0.45,and corresponding solutionsediment ratio was 30%.To eliminate air bubbles,the jacketed reactor was vacuumized for 3 min.Then,the jacketed reactor was pressurized to 1.3 MPa by the buffer tank where the valve was kept open,and the temperature decreased to 270.15 K to make the gas and water to form gas hydrates at a cooling rate of 1.0 K·min-1.During the gas hydrate formation,the camera located at the side of the jacketed reactor was used to observe the change in the sediments and was simultaneously recorded by the computer.Next,the temperature increased to 293.15 K,and gas hydrates in the sediments started to decompose,which were also observed and recorded by the camera and the computer,respectively.The same process of gas hydrates growthdissociation was repeated three times to simulate multiple disturbance of the cold and heat fluids.
2.3.2.Raman test
The Raman spectrometer with a single Mono Chromator of 1800 grooves per mm grating and a multichannel air-cooled CCD(charge coupled device)detector was employed to determine the structures of mixed CH4-C3H8hydrates formed in different sediments.After the growth of the mixed CH4-C3H8hydrates,the visual kettle was moved to a liquid nitrogen bath and depressurized.The hydrate samples were quickly taken out and preserved in liquid nitrogen.And then,the hydrate samples were quickly loaded into the temperaturecontrolled stage(Linkam THMS600)precooled to 233.15 K by liquid nitrogen.
A 532 nm incident laser beam was used.The Ar-ion laser was allowed to focus on a precise spot on the surface of the hydrate samplesby a 50×microscope objective.The spectroscopic data was detected by a CCD detector with an energy resolution of 50 mW and recorded with a 10 s integration time over 1 scan.Salient features,such as structure type,relative cage occupancy,and hydration number of the mixed CH4-C3H8hydrates in the sediments,can be delivered from spectrum analysis.

Table 1 Properties of the sediments

Fig.1.Schematic of the experimental setup for the growth-dissociation observation of gas hydrates.
3.Results and Discussion
3.1.Multiple growth-dissociation cycles for gas hydrates in the sediments
3.1.1.Morphology of gas hydrates in the sediments
The paper observed three growth-dissociation cycles of the mixed CH4-C3H8hydrates in two sediments.Fig.2 showed the morphology of gas hydrates in the sediments during multiple growth-dissociation cycles with water at the pressure of 1.3 MPa.
In the silicate rocks,gas hydrates formed in the gas phase surrounding and cementing the grains on the top of the sands during the 1st cycle with a massive form,as shown in Fig.2.Gas hydrates could not be found in the pore spaces of the sediments.When the dissociation of gas hydrates happened,the silicate rocks would shift,and some gas bubbles appeared in the sands.During the 2nd cycle,a majority of gas hydrates would be still observed in the gas phase on the top of the sands,but a few started to fill in the gas bubbles.After the 2nd dissociation of gas hydrates,a great amount of gas bubbles appeared with the transportation of the gas,which provided a better space to form disseminated porefilling gas hydrates.As expected,a large amount of gas hydrates filled in the gas bubbles as a part of the load-bearing granular frame,which displayed the pore-filling characteristics.Linga and his team[31]also reported that hydrate crystals were observed to form in the interstitial pore space between the silica sand particles.

Fig.2.Morphology of the mixed CH4-C3H8hydrates during multiple growth-dissociation cycles with water in two sediments at the pressure of 1.3 MPa.
There were some differences in the seafloor sands.Gas hydrates formed in the sediments during the 1st cycle,as shown in Fig.2,leading to the striations.It was obviously gas hydrates distributed on sediments bed surface or in the pore spaces and fractures as laminas and small veins.With the growth of gas hydrates,seafloor sediments were forced to move away over time,which agreed well with the radial shrinkage effect of hydrate decomposition reported by Li et al.[32].Gas hydrates concentrated as layers,veins,and fracture filling character in parallel orientations in the seafloor sediments.Then,the gas and water were generated in thermal decomposition,which help greatly smooth seafloor sediment bed surface.Gas hydrates reformed inside of the fracture of seafloor sediments because of local cooling caused by the dissociation of gas hydrates on the reactor wall.Both of the length and width of the gap between sediments narrowed over time.A few gas bubbles appeared in the center of seafloor sediments,which was caused by the migration of the decomposition gas.During the 2nd cycle,there were many striations in the seafloor sediments.Gas hydrate grew as layers,veins,and fracture filling character in parallel orientations in clayeysilty sediments,and the total accumulation of gas hydrates during the 2nd cycle was larger than the 1st cycle,which agreed well with the view of Cook et al.[25].Later,gas hydrates completely decomposed under continuous heating.A large amount of gas bubbles appeared in the seafloor sediments,which was affected by generated gas and water.Yeon et al.reported the interlayer distance may extend for the clay-water suspension and for the intercalated methane hydrate[33].During the 3rd cycle,there was just few striations in the seafloor sediments.Gas hydrates grew directly into the pore spaces over two previous cycles as a pore-filling form.
After adding 3.5 wt%NaCl aqueous solutions,the morphology of the mixed CH4-C3H8hydrates in the sediments changed a lot,which was shown in Fig.3.
In the silicate rocks with 3.5 wt%NaCl aqueous solutions,gas hydrates formed surrounding and cementing the grains in the 1st-3rd cycle,as shown in Fig.3.Gas hydrates grew up as granular accumulation.During the 1st cycle,the majority of gas hydrates filled in the gas phase.When gas hydrates decomposed,some gas bubbles appeared,which provide a space to form gas hydrates in the pore space of the sediment grains.After the 2nd cycle,gas bubbles extended throughout the sediment grains.It was also found that the growth pattern of surrounding could converted into the cementing or suspending.
In the seafloor sands with 3.5 wt% NaCl aqueous solutions,gas hydrates also formed in the gas phase along the top of the sediments during the 1st cycle,as shown in Fig.3.The migration of decomposition gas and water made the movement of the sediment grains,which caused the appearance of gas bubbles in the sediments.It offered a space to form disseminated gas hydrate.Gas hydrates filled in the gas bubbles during later cycles,which showed a suspending or supporting patterns to form pore-filling characteristics.
3.1.2.Growth model for pore-filling gas hydrates in the sediments
According to the morphology of gas hydrates during multiple growth-dissociation cycles in the sediments,it was found that the occurrence state of gas hydrates in the sediments varied with cycle proceedings.
Fig.4 showed the sketch map of the variation of the occurrence state of gas hydrates in the sediments with cycle proceedings.Gas hydrates gradually grew into the sediments with the multiple growth proceedings.Gas hydrate formed in the gas phase at the 1st cycle was in a massive form,which was on the top of the sediments.It could not be found in the pore spaces of the sediments.With the increasing temperature,gas and water were produced in thermal decomposition,and some gas bubbles appeared in the pore spaces of the sediments.During the second cycle,with the lower temperature,gas hydrates moved the sediments away with its growth,and the pore spaces of the sediments were widened again.The majority of gas hydrates grew as a massive form in the gas phase space,but a few were in a disseminated form(pore-filling)in the sediments.Then,gas hydrates decomposed during the 2nd cycle.The migration of generated gas bubbles and water led to a large amounts of gas pores in the sediments.Later,gas hydrates filled in the pore spaces of the sediments over two previous cycles as a disseminated pore-filling character,which also indicated that the amount of gas hydrates distributed in the sediments improved with the multiple growth-dissociation cycles.
As for micro growth pattern of gas hydrates,Sell et al.[8]proposed the characteristics of gas hydrate growth in the sediments,which included floating in the pore fluid,being a part of the loading-bearing granular frame,surrounding and cementing grains,and cementing grains contacts.
In this paper,the results showed that the structure and distribution for pore-filling gas hydrates in the sediment was affected by growth-dissociation cycles.During the first and second growth cycle,as shown in Fig.5(a)and(b),gas hydrates grew up surrounding,cementing grains and floating in the pore fluid to force sediment grains to move away,which led to the expansion of the pore spaces among sediment grains.The saturation of gas hydrates improved with the growth-dissociation cycles,which resulted in the appearance of a great number of gas bubbles after the second dissociation cycle.
Under the condition of many gas bubbles in the sediment,gas hydrates grew as a part of the load-bearing granular frame,as shown in Fig.5(c).The size of gas hydrate particles decreased a lot with a better sphericity.The number of gas hydrate particles improved a lot,which meant a lot of nucleation point appeared,stimulated by the large number of gas bubbles.Jin et al.[34]reported that the morphology of gas hydrates in the eastern Nankai trough area was a load-bearing type.In this paper,it was found that pore-filling gas hydrates might appear after the migration of thermal fluid and gas.

Fig.3.Morphology of the mixed CH4-C3H8hydrates during multiple growth-dissociation cycles with brine in two sediments at the pressure of 1.3 MPa.

Fig.4.The occurrence state of gas hydrates in the sediments during multiple growth cycles.
3.2.Distribution for gas hydrates and bubbles in the sediments
3.2.1.Distribution for gas hydrates in the sediments
With the multiple growth-dissociation of gas hydrates in the sediments,the occurrence state and the amount of gas hydrates in the sediments changed a lot,which tended to be more uniform.So as to describe the change of the shape and occurrence state of gas hydrate,the paper set the smallest hydrate particle to be a square grid,which has a side of 50 μm(seen Fig.9(a)in supporting information for details).
The total grid number of gas hydrate particles in the pore spaces of the sediments with or without salts during multiple growthdissociation cycles was summarized in Table 2 except the gas hydrate particle on the top of the sediments which extended to the gas phase.
In water,gas hydrates formed in the silicate rocks for three times,it was found 0,12 and 77 hydrate particles in the pore spaces,respectively.During the 1st cycle,gas hydrates grew as a massive form in the gas phase along the top of the grains,which did not extend to the pore spaces of the sediments.During the later cycles,the total grid number were 1776 and 7513,which showed the quantity of formed gas hydrate particles increased with the multiple growth-dissociation cycles in the silicate rocks.
After adding 3.5 wt%NaCl aqueous solutions,the total grid number of gas hydrates in the silicate rocks were 2923,3529,and 6474,respectively,during three cycles,which increased with the cycles.It increased a lot at the previous two cycles,compared to in water.While,in the 3rd cycle,it was less than in water.
As for seafloor sands,it was found 43,96,and 155 hydrate particles in the sediments during three growth processes,respectively.Gas hydrate particles occupied the square grids of 6478,9650,and 5671,respectively.Compared to the area of gas hydrates formed in the 1st cycle,it increased 49%in the 2nd formation,while decreased 12%in the 3rd cycle.The results showed that the saturation of pore-filling gas hydrates was relatively lower than in veins,layers and fracture filling characteristics.
After adding 3.5 wt%NaCl aqueous solutions,the total grid number of gas hydrates changed to 2231,3172,and 3849,respectively,during the 1st-3rd cycles.Compared to the 1st cycle,the area of gas hydrates increased 42% and 73%at the later cycles,which fitted well with the silicate rocks.All of it was less than in water,the results showed that the salt decreased the amount of the gas hydrates in the pore spaces of the sediments.

Fig.5.Growth for gas hydrates in the sediments during multiple cycles:(a)the 1st cycle,(b)the 2nd cycle,and(c)the 3rd cycle,the orange ball is the sediment grains,and the blue is hydrate crystals.

Table 2 The total grid number of gas hydrate particles in the pore spaces of the sediments
Gas hydrate particles formed in the pore spaces of the sediments became smaller with multiple growth-dissociation cycles,as shown in Fig.6.
In the silicate rocks with water(SR-water),gas hydrates became smaller with the cycle proceedings.None gas hydrate formed in the pore spaces of the sediments during the 1st cycle.Then gas hydrates grew into the pore spaces of the silicate rocks in the next two cycles with the majority of particle size less than 400 grids.The migration force of gas bubbles could bring about the movement of the sediments,compounding the appearance of more and more gas pores.It induced gas hydrate particles filled in the gas pores as a disseminated porefilling character.
In the silicate rock with 3.5 wt% NaCl solutions (SR-brine),gas hydrate particles were about 50-1000 grids during the 1st cycle,which could be caused by the porosity.During the 2nd cycle,particle size of gas hydrates was similar to the 1st cycle,which could be caused by the accumulation of decomposition gas and the dropping of the grains.During the 3rd cycle,similar to hydrates formed in water,it became scattered and smaller,whose maximum proportion was less than 20.0%.It might be induced by the uniform migration force of decomposition gas.Gas hydrates formed in NaCl aqueous solutions was relatively larger than in water.

Fig.6.Size distribution of gas hydrate particles formed in the pore spaces of the sediments during multiple growth-dissociation cycles.
In the seafloor sands with water (SS-water),the majority of gas hydrate particles was less than 100 grids.Similar to the system of SRwater,gas hydrate particle size was becoming smaller,the distribution uniformity was also improving with the multiple growth-dissociation cycles.During the 3rd growth cycles,the majority of gas hydrate particles occupied the square grids of 4-20 in the pore spaces of the seafloor sands,which was about 79.6%.Gas hydrates larger than 100 grids decreased from 30.2%to 4.5%during growth cycle proceedings.The results showed that pore-filling gas hydrates possessed an excellent dispersion.
In the seafloor sands with 3.5 wt%NaCl solutions (SS-brine),gas hydrate particles became smaller with the cycles,while it occupied 40-1000 grids,there was no smaller particles compared to hydrate formed in water.Similar to the silicate rocks,adding salt would low the amount of the smaller hydrate particles in the pore spaces of the seafloor sands.
The results showed,influenced by the temperature and natural gas flow,gas hydrates grew in the sediments initially with a lower saturation.When geothermal fluids disturbed the condition of sediments,gas hydrates might decompose to natural gas and water.After the dissipation of geothermal,natural gas and water would react again to form gas hydrates with a relatively large saturation.Gas hydrate would decompose again with another strong geothermal,similar to nucleate boiling,and migration of a large amount of natural gas resulted in the appearance of the plenty of gas bubbles in the sediments.At this time,geothermal dissipated,and gas hydrates could fill in the gas bubbles and displayed a pore-filling character.
The occurrence state of gas hydrates in the sediments could also reflect the stability of the geological environment.Gas hydrates as layers,veins,and fracture filling character in parallel or sub-parallel orientations in hydrate-bearing sediments might reflect the geological environment in this place was relatively stable,which was influenced by the geothermal fluids one or none.But for pore-filling gas hydrates in hydrate-bearing sediments,it might reflect the geothermal fluids were active,and gas migration occurred frequently.
3.2.2.Distribution for gas bubbles in the sediments
Similar to gas hydrates distribution,the paper analyzed the distribution of the gas bubbles during multiple growth-dissociation cycles through small-grid analysis.The grid was also set as a square with a side of 50 μm(seen Fig.9(b)in supporting information for details).
There were a lot of gas bubbles appeared on the surface of the sediments with the nucleate boiling-liked dissociation of gas hydrates.All gas bubbles had a good sphere shape,and the number of gas bubbles increased a lot with multiple growth-dissociation cycles.Similar to gas hydrates in the sediments,gas bubbles became smaller gradually with the cycles.The total grid number of gas bubbles in the sediments during multiple growth-dissociation cycles was summarized in Table 3.
In the silicate rocks with water,formed gas bubbles occupied 3488,7829,and 9944 grids during three cycles.Compared to the 1st cycle,the area of total gas bubbles increased 124.5%and 185.1%during the 2nd and 3rd cycles.After adding 3.5 wt%NaCl aqueous solutions,the amount of gas bubbles in the silicate rocks caused by decompositiongas decreased a lot to 978,2109,and 1050,respectively,during the 1st-3rd cycles.

Table 3 The total grid number of gas bubbles in the sediments
In the seafloor sands with water,gas bubbles occupied 1515,4786,and 4939 grids in three cycles,which increased 255.3% and 272.7%,comparing with the 1st cycle.After adding 3.5 wt%NaCl aqueous solutions,the amount of gas bubbles in the seafloor sands also decreased a lot to 1932,2572,and 3365,respectively,during three cycles.The results showed that the salt would lower the amount of the rest gas bubbles caused by migration of decomposition gas in the sediments.
With 3.5 wt%NaCl aqueous solutions,the gravity of sediments could not be hold by the migration force of gas bubbles and the surface tension between solutions and sediments,which was generated by hydrate decomposition.It resulted in less migration of sediments and less appearance of gas bubbles in the sediments.
With the dissociation of gas hydrates,some gas bubbles would appear in the sediments.Fig.7 showed size distribution of gas bubbles appeared in the sediments during multiple growth-dissociation cycles.The results showed that the size of the gas bubbles decreased gradually with the increase of recycle times.The great amount of gas bubbles provided the requisite geological condition to form pore-filling gas hydrates.In the silicate rocks with water,the size of the majorities of gas bubbles changed from 20-90 grids to 10-30 grids with the cycle proceedings.After adding 3.5 wt%NaCl aqueous solutions,it changed from 3-50 grids to 3-10 grids during three cycles.The salt restricted the accumulation of gas bubbles within the sediments.
In the seafloor sands with water,the size of the majorities of gas bubbles changed from (3-6 grids,300 grids)to 3-7 grids with the cycle proceedings.After adding 3.5 wt% NaCl aqueous solutions,it changed from 5-20 grids to 2-10 grids.Seafloor sand grains was smaller than the silicate rock grains,since migration and reversion of the seafloor sands grains was much easier during hydrates growth and dissociation.The rest gas bubbles in the seafloor sands were smaller than in the silicate rocks with the cycle proceedings,which indicated that the smaller of the sediments,the smaller of the formed gas bubbles.
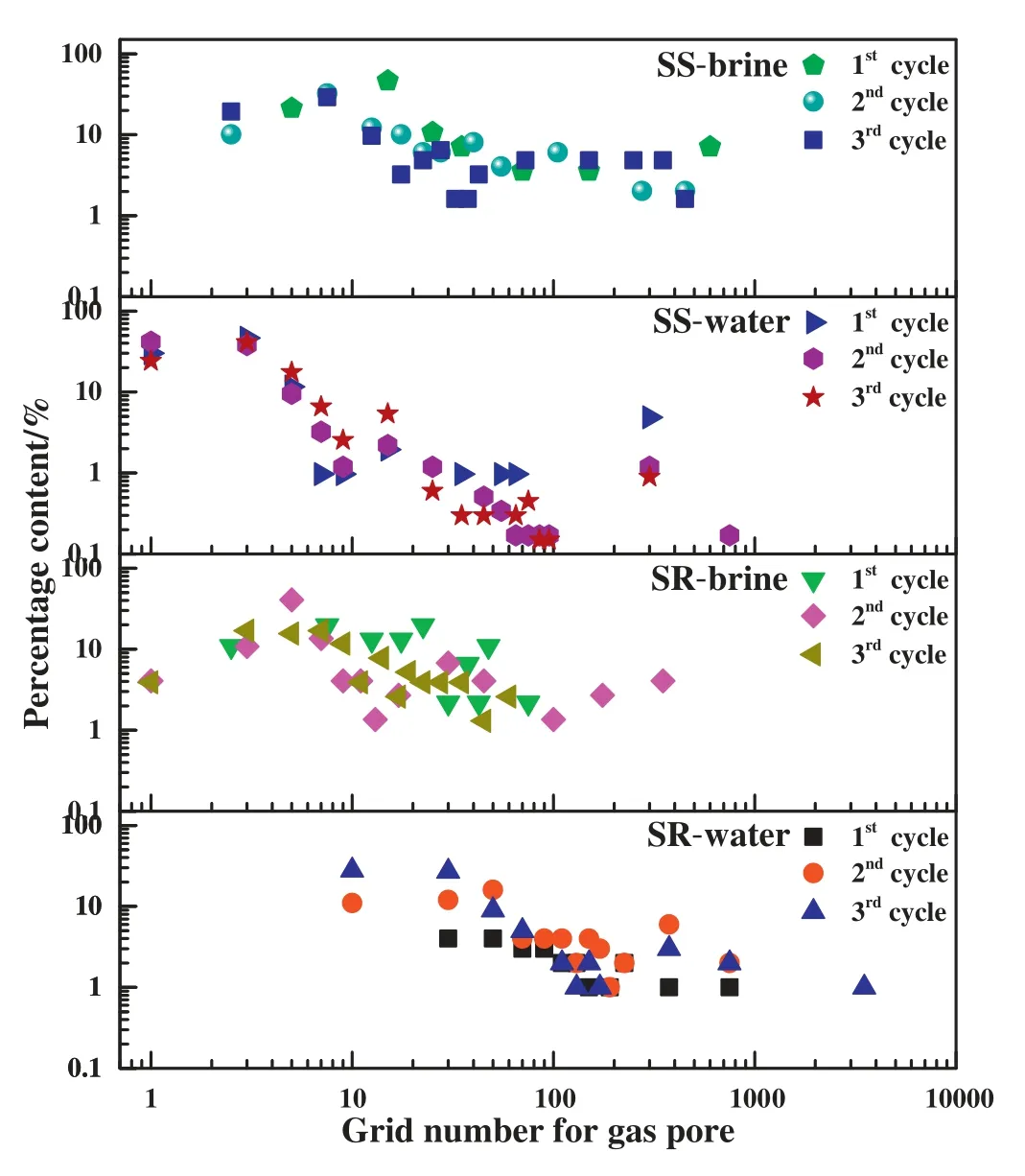
Fig.7.Size distribution of gas bubbles appeared in the sediments during multiple growth-dissociation cycles.
The growth or dissociation forces of gas hydrates were relatively easier to move the sediments with smaller size.It was found that thermal decomposition,similar to nucleate boiling,had a shock to the sediments continually.This shock enhanced the sediments movement,and finally helped facilitate many bubbles in the sediments to form pore-filling gas hydrates.
3.3.Effects of salt on hydrate crystal structure
According to the molecule size,CH4and C3H8gas mixtures could form sII hydrates,which were verified in previous studies [35,36].Fig.8 showed the Raman spectra of mixed CH4-C3H8hydrates in the sediments at 233.15 K.For the cubic sII hydrate,it consisted of 16 small cages (512)and 8 large cages (51264)in each unit crystal.The ratio of molecular diameter to cavity diameter for propane was 1.24 and 0.943,corresponding to small cages and large cages,while for methane it was 0.868 and 0.655,respectively[37].It meant propane could only occupy the large cage without distortion.While for methane molecules,both small and large cages could be occupied[38].
Moryama et al.[39]reported typical Raman spectra derived from CH4hydrates were split into two peaks,representing the incorporation of CH4into both small and large cages.The Raman peak at 2903 cm-1corresponded to CH4molecules in large cages,while the peak at 2914 cm-1was assigned to CH4in small cages.The ratio of small to large cages was 1:3 for sI hydrates and 2:1 for sII hydrates,which led to the peak intensity of CH4at around 2903 cm-1was much lower than that at around 2914 cm-1,and contrary to sI hydrates [40].It could be the method to identify the structure of gas hydrates.Liang et al.reported,for sII hydrates,the Raman spectra of CH4appeared to have a slight red shift although it was not obvious[38].
As seen in Fig.8,gas hydrate crystals formed in all systems showed structure II.The Raman bands at 2904.6 and 2915.4 cm-1were assigned to be the C--H stretching vibrations of CH4encaged in 51264and 512cages of sII hydrates,respectively.While the Raman peaks for C3H8encaged in 51264cages were much more complicated.CH3-symmetrical stretching vibrations occurred at 2871.5 cm-1and 2880 cm-1,CH3-non-symmetrical stretching vibrations were apparent at 2945.6 cm-1,and CH2-stretching vibrations occurred at 2986 cm-1.Meanwhile the broad band between 3000 and 3500 cm-1was assigned to the O--H stretching vibration of water molecules.
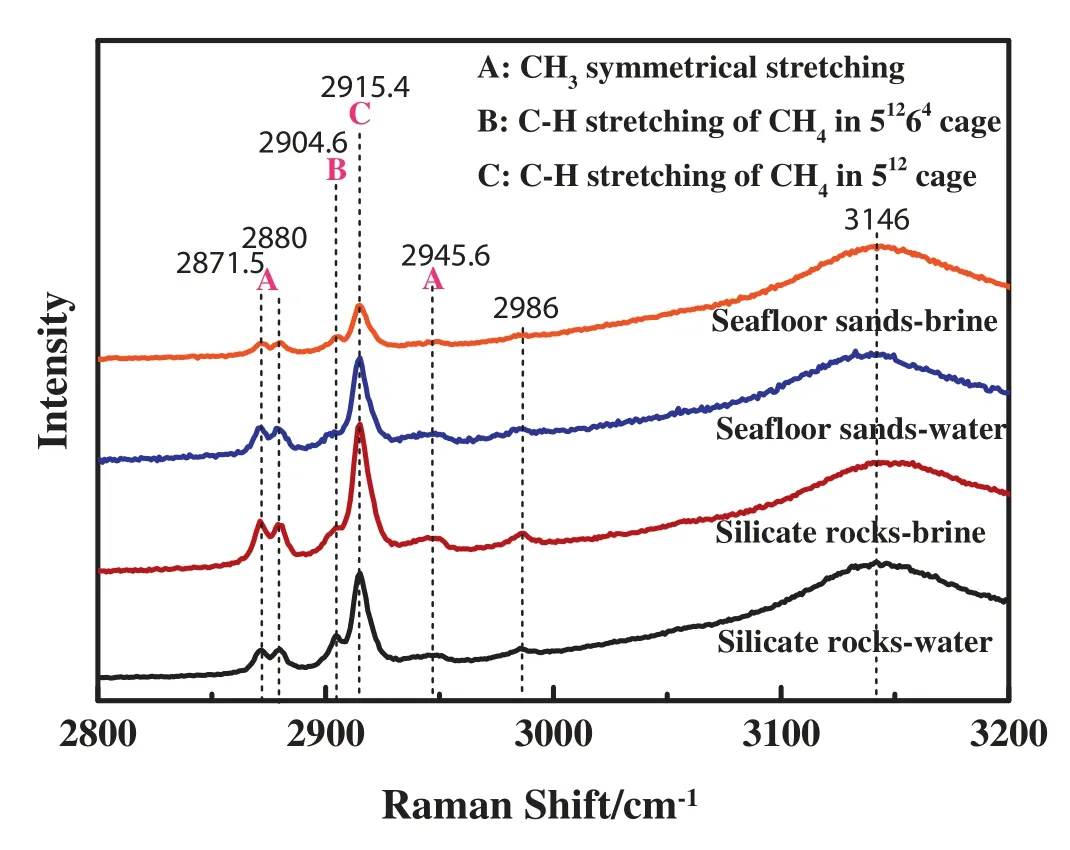
Fig.8.The Raman spectra of mixed CH4-C3H8hydrates in the sediments at 233.15 K.
Both of CH4and C3H8occupied 51264cages of sII hydrate.The integral area of each peak could be obtained by Gaussian Raman-peak-differentation-imitating analysis.The paper selected the integral area ratio of the peaks at 2904.6 cm-1and 2915.4 cm to represent the ratio of CH4molecules encaged in 51264and 512cages of sII hydrate.And the integral area ratio of the peaks at 2904.6 cm-1and 2871.5/2880 cm-1was chosen to express the ratio of CH4and C3H8molecules encaged in 51264cages of sII hydrate.
Fractional occupancy of small and large cages in sediments was listed in Table 4.In 512cages,it was filled with CH4molecules,whose fractional occupancy was 0.810-0.957.While in 51264cages,it was filled with CH4and C3H8molecules,whose fractional occupancy was 0.982-0.999,and the fractional occupancy of C3H8molecules was 1.83-3.95 times of CH4molecules.The hydration number of gas hydrates in the sediments was 5.84-6.53.
The fractional occupancy in 51264cages was larger than it in 512cages,which might be induced by the cage size and the C3H8molecules.Both of CH4and C3H8molecules can get through 51264cages,while CH4molecules can get through 512cages.
The addition of 3.5 wt%NaCl aqueous solutions affected the fraction occupancy of both of 512and 51264cages.Compared to the system of water,it decreased a little,which suggested that the salt restricted the access of gas into the cages.
4.Conclusions
Accumulation and occurrence state of gas hydrate in sediments were influenced by the cycles of growth-dissociation.The paper studied the morphology,distribution,occurrence state and structure features of CH4-C3H8mixed gas hydrates in the sediments with or without salts during three growth-dissociation cycles.Through analysis,several conclusions were summarized as follows:
(1)The amount of gas hydrates distributed in the sediments improved with multiple growth-dissociation cycles.Gas hydrates grew gradually into the sediments as pore-fillings characteristics with the cycle proceedings.The growth pattern of gas hydrates also varied during the cycles.Gas hydrates firstly grew up surrounding,cementing grains and floating in the pore fluid to force sediment grains to move away,which led to the expansion of the spaces among sediment grains.Finally,gas hydrates grew as a part of the load-bearing granular frame.
(2)Thermal decomposition of gas hydrates,similar to nucleate boiling,had a shock to the sediments continually.Gas migration enhanced the sediments movement,which resulted in the appearance of the increasing quantity of gas bubbles in the sediments during cycle proceedings.It provided the requisite geological condition further to form pore-filling gas hydrates.
(3)Salts affected the growth of the gas hydrates and the migration of grains,which also restricted the accumulation of gas bubbles within the sediments.
(4)CH4and C3H8gas mixtures all formed sII hydrates in different sediments and solutions with hydration number of 5.84-6.53.The fractional occupancy in 51264cages was larger than it in 512cages.The salt restricted the access of gas into the hydrate cages.
Supplementary Material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cjche.2019.02.007.

Table 4 Structure characteristics of mixed CH4and C3H8hydrate
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Decomposition behaviors of methane hydrate in porous media below the ice melting point by depressurization☆
- Research progress in hydrate-based technologies and processes in China:A review☆
- Fundamental mechanisms and phenomena of clathrate hydrate nucleation☆
- Methane hydrates:A future clean energy resource
- Progress and trends in hydrate based desalination(HBD)technology:A review☆
- Extraction of methane hydrate energy by carbon dioxide injection-key challenges and a paradigm shift
