Research progress in hydrate-based technologies and processes in China:A review☆
2019-12-05ChungangXuXiaosenLiKefengYanXukeRuanZhaoyangChenZhimingXia
Chungang Xu,Xiaosen Li*,Kefeng Yan,Xuke Ruan,Zhaoyang Chen,Zhiming Xia
Guangzhou Institute of Energy Conversion,Chinese Academy of Sciences,Guangzhou 510641,China
CAS Key Laboratory of Gas Hydrate,Guangzhou 510640,China
Guangdong Provincial Key Laboratory of New and Renewable Energy Research and Development,Guangzhou 510640,China
Keywords:Gas hydrate Natural gas hydrate(NGH)CO2separation Hydrate formation inhibition CH4-CO2replacement
ABSTRACT Natural gas hydrate(NGH)is considered as an alternative energy resource in the future as it is proven to contain about 2 times carbon resources of those contained in the fossil energy on Earth.Gas hydrate technology is a new technology which can be extensively used in methane production from NGH,gas separation and purification,gas transportation,sea-water desalination,pipeline safety and phase change energy storage,etc.Since the 1980s,the gas hydrate technology has become a research hotspot worldwide because of its relatively economic and environmental friendly characteristics.China is a big energy consuming country with coal as a dominant energy.With the development of the society,energy shortage and environmental pollution are becoming great obstacles to the progress of the country.Therefore,in order to ensure the sustainable development of the society,it is of great significance to develop and utilize NGH and vigorously develop the gas hydrate technology.In this paper,the research advances in hydrate-based processes in China are comprehensively reviewed from different aspects,mainly including gas separation and purification,hydrate formation inhibition,sea-water desalination and methane exploitation from NGH by CH4-CO2replacement.We are trying to show the relevant research in China,and at the same time,summarize the characteristics of the research and put forward the corresponding problems in a technical way.
1.Introduction
Energy crisis and climate deterioration are the two major problems,and as a main energy source of China,coal would be long-termly used for China's development,thus these two problems are especially prominent for China[1].It is therefore imperative to constantly explore new alternative sources of energy and develop new technologies to slow and even prevent the climate degradation.Natural gas hydrate is considered to be an alternative energy resource as it is proven to contain about 2 times carbon resource of those contained in all the fossil energy sources on Earth[2].In general,a cubic gas hydrate contains about 180 cubic gas[3].Besides,it was found that many gases can form gas hydrates under a certain temperature and pressure spontaneously.Therefore,since the 1980s,the great enthusiasm has been devoted to the research of NGH,and at the same time,the technologies related to the gas hydrate,such as hydrate-based gas separation and sea-water desalination,have also been greatly developed worldwide.And over the past 3 decades,the research fever has not diminished;on the contrary,more and more attention has been paid to the relevant research.
Gas hydrates are ice-like crystalline compounds formed by small gas molecules,e.g.,methane (CH4),nitrogen(N2),hydrogen(H2),carbon dioxide (CO2),C2H6,and water molecules.Water molecules contact each other by hydrogen bonds to constitute different cages,and the small gas molecules stably reside in the cages by van der Waals forces[4].The gas hydrates have different structures,such mainly as structure cubic I(sI),structure cubic II(sII)and structure hexagon(sH)[5].The gas hydrates can form spontaneously under a certain conditions of pressure and temperature,and different gases have their individual gas hydrate formation and decomposition equilibrium conditions.Therefore,for a gas mixture,the component with relatively moderate gas hydrate formation equilibrium condition can prior to enclose in the hydrate cages to form gas hydrates and enrich in the gas hydrate phase,leaving the balance components to be rich in the gas phase[6-7].Therefore,it is possible to separate gas based on forming gas hydrate.Gas hydrate was firstly discovered in 1810.However,it did not attract any attentions until the 1930s when it was found to plug the pipeline in the process of natural gas transport[8].Thus in order to maintain the pipeline safety,scientists put forward to different ways to prevent the hydrate formation and agglomeration in the pipeline.Due to that the diameter of the hydrate cage ranges from 0.395 to 0.571 nm,the salt ions can't squeeze into the cages,thus the hydrate-based technology is developed to the sea-water desalination [9-13].Because water can be recycled during the hydrate formation and decomposition,the technologies based on the gas hydrate is environmentally friendly relative to other traditional technologies,and because the gas hydrate can form and decompose in a relatively simple equipment under a certain temperature and pressure,thus the technology based on the gas hydrate is economical.Consequently,the hydrate-based technologies are considered to be quite promising and competitive.
In China,research on NGH and the hydrate-based technologies began in the 1990s when a research group led by Professor Chen established at the China University of Petroleum-Beijing(CUPB)to initiate the first fundamental studies on the gas hydrates[14].Then in the middle 1990s,a gas hydrate research center was established in the Guangzhou Institute of Energy Conversions(GIEC),Chinese Academy of Sciences(CAS)to study the thermal energy storage based on the gas hydrates.And since then,more and more research groups established in China.Up to date,there are more than 30 research groups working in various fields of gas hydrate,especially in the studies on CH4exploitation from the NGH,hydrate formation inhibition and hydrate-based CO2separation and capture from gas mixtures.In this paper,the latest progress in the hydrate-based processes in China is comprehensively reviewed,mainly including gas separation and purification,hydrate formation inhibition,sea-water desalination and methane exploitation from NGH by CH4-CO2replacement.We are trying to show the relevant research in China,and at the same time,summarize the characteristics of the research and put forward the corresponding problems in a technical way.
2.Hydrate-based CO2Separation and Capture
Hydrate-based CO2separation and capture is a new method which is different from those traditional technologies such as chemical adsorption,physical absorption,membrane separation and cryogenic separation,with environmental friendly and relatively low operating cost[7].In China,coal is the main energy source,accounting for more than 70%of China's energy structure.It mainly focuses on separating and capturing CO2from the emission gas of the coal-fired power plants,including post-and pre-combustion capture.Post-combustion capture refers to the treatment of flue gas(mainly composed of 15%-20%CO2,5%O2,>75%N2,etc.)before it is released directly into the atmosphere,and pre-combustion capture refers to capture CO2from the fuel gas containing about 40%CO2and 60%H2.The fuel gas is produced when the coal is pre-treated with an integrated gasification combined combustion(IGCC)technology,thus CO2/H2(40%/60%)gas mixture is also called as an IGCC syngas[7,15-21].Consequently,CO2capture and separation studies mainly involve CO2/N2and CO2/H2gas mixtures.Besides,CO2separation from CO2/CH4gas mixture is another research focus as biogas,coal-bed methane and landfill gas,which extensively distribute on Earth,are also mainly composed of CO2and CH4.The efficient CO2separation from the gas mixtures can not only reduce CO2emitted in the air but also purify CH4.
Research on CO2hydrate was initially carried out primarily in western developed countries,and since the beginning of this century,Chinese researchers and research groups have gradually carried out the relevant investigations.Among them,GIEC,a subsidiary institute of CAS,is the main research institute.
2.1.CO2separation parameters
In the process of hydrate-based CO2separation from the gas mixtures,the separation process is generally measured by the following indicators.
2.1.1.Hydrate formation induction time(t)
The induction time refers to the time from the initial state to the appearance of a visible hydrate particle under a certain temperature and pressure[22,23].However,the induction time often varies with the subjective judgement of the operator.Another definition of the induction time is the time from the initial state to the point from which the system pressure starts to dramatically decrease for a constant volume experiment or the point from which the gas starts to be supplied into the reactor for a constant pressure experiment,in a diagram of pressure vs.time,as shown in Fig.1[3].The induction time is influenced by the gas,solution,temperature and pressure.And for the same system and under the same condition,the induction time also presents different with the primary and secondary water solution.For the different system,the induction time ranges from several seconds to hundreds of hours.
2.1.2.Gas consumption or gas uptake
The gas consumption or the gas uptake refers to the sum of the gas enclosed into the hydrate slurry phase during the hydrate formation process,and its unit is generally expressed in mol/mol,meaning the molar number of the gas consumed by the hydration per mole water or water solution[7,24,25].The equation for calculating the gas consumption or the gas uptake is,

where Δngasis the mole of the gas totally consumed during the hydrate formation,and nwateris the total mole of the water in the system.
2.1.3.Hydrate formation rate
Hydrate formation rate is often expressed by gas consumption rate in the process of the hydrate formation because the hydrate quality is difficult to measure.It can be expressed as[4,6,26-29],

where N is the gas consumption and Δt is the time from the induction time point to the hydrate formation complete.The time point of the hydrate formation complete is defined as the point from which the pressure keeps constant during the hydrate formation.And,for calculating the hydrate formation rate,it need assume the hydration number firstly so that the gas consumption can be calculated accordingly.
2.1.4.CO2separation efficiency
The hydrate-based gas separation is expressed in two indictors,separation efficiency(or separation fraction(S.Fr.))and separation factor(S.F.).CO2separation efficiency is expressed as the ratio of CO2enclosed in the hydrate slurry phase to the total CO2injected into the system,and equation is[6,7,28,30,31],

where ngas,His the mole of the gas component in the hydrate slurry phase,and ngas,feedis the mole of the gas component totally injected into the system.
The separation factor reflects the selectivity of the hydrate to the gas component,and it can be calculated according to[29,32].

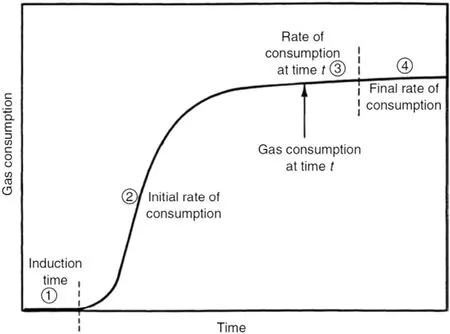
Fig.1.Diagram of the gas consumption vs.time during the hydrate formation[3].
2.2.CO2separation and capture process from gas mixtures containing CO2
2.2.1.CO2separation from CO2/N2
The studies of CO2separation is based on the investigation on the kinetics and thermodynamics of CO2hydrate formation in the pure water or solution in the presence of hydrate formation promoter.In 2009,Fan et al.[33]reported CO2capture from binary mixture via forming hydrate with the help of tetra-n-butylammonium bromide.In their study,CO2/N2(19.9%/80.1%)was used to simulate the flue gas to form hydrate in the presence of 5.0 wt% TBAB under 277.65 K and 4.30-7.30 MPa.The induction time obtained by observation was 5 min,and the CO2concentration in the residue gas phase decreased from about 19.9%to less than 10.0% and increased from 0 to 34.8%-38.2% in the hydrate phase.According to the Eqs.(3)and(4),the CO2recovery fraction and separation factor were 0.40-0.45 and 5.3-7.3,respectively.To be honest,the results could not reflect the true experiment due to that the procedure of gas sampling for composition measurement was imperfect.Besides,it was found that the gas consumption was quite small in the presence of TBAB,and the hydrate formation induction time was quite long to industrial hydrate-based CO2separation.Therefore,researchers devoted more energies to enhance the gas consumption and shorten the hydrate formation induction time.
In order to achieve the goal,the research plans were mainly embodied in promoting gas-liquid contact and improving the driving force of the gas hydrate formation,and the specific measures included developing different gas hydrate formation ways and different hydrate formation additives.Zhou et al.[34]investigated the phase equilibria and the crystallographic properties of TBAB-CO2semiclathrate hydrates.The investigation was different from the previous work.In their work,the phase equilibria of the hydrate formation and the hydrate crystallographic properties were correspondingly studied,and it was found different phase equilibria presented different crystallograph.Li et al.[6]added dodecyl trimethylammonium chloride(DTAC)into the 4.95 wt%TBAB solution to capture CO2from the simulated flue gas(CO2/N217.0%/93.0%).As a kind of surfactant,the addition of DTAC effectively promoted the dissolution of flue gas in the TBAB solution,moreover,the hydrate formation induction time was dramatically shorten to less than 10 s.With the 0.028 vol%DTAC and initial pressure of 1.66 MPa,the highest CO2recovery fraction of 0.56 and CO2separation factor of 9.6 were obtained.After the separation,the CO2concentration in the hydrate decomposition gas was about 65.0%.Thus,authors took CO2/N2(65.0%/35.0%)as feed gas to continue the CO2separation and capture,which was considered to be the second separation stage,and the CO2recovery fraction of 0.39 and CO2separation factor of 62.3 were obtained at 274.65 K.And after the second separation,the CO2concentration in the hydrate decomposition reached to more than 92.0%.Therefore,authors thought that it could achieve hydrate-based CO2separation from flue gas by a two continuous separation stages.Based on this study,Xu et al.[35]continued the study on the process of CO2separation from flue gas,and developed a set of pilot-scale separation equipment.With the equipment,the flue gas was successfully treated.However,a relatively serious problem was exposed during the CO2separation process.Due to that the density of the formed semi-clathrate TBAB hydrate is larger than water,the hydrate tended to precipitate in the reactor and eventually block the reactor and pipe,therefore there were no report on the continuous CO2separation even just last for longer than 24 h in the study.
Table 1 shows the relevant investigations on the CO2separation from gas mixtures containing CO2.In fact,the hydrate promoters of the quaternary ammonium salts are a big family,including TBAB[36-37],tetran-butyl ammonium fluoride(TBAF)[33,38-41],tetra-n-butyl ammonium chloride(TBAC)[39-40,42],tetra-n-butyl phosphonium bromide(TBPB)[39-40,43-44],tetra-n-butyl ammonium nitrate(TBANO3)[36,39-40,45-46],etc.For CO2separation,Li et al.[46]compared the effects of the quaternary ammonium salts,and they found that the solubility of the gas mixture containing CO2in TBANO3was increased or even close to that in the pure water system compared with TBAB and TBPB.Besides,it was found CO2concentration in the gas phase increased from 17.0%to 67.0%,and the CO2separation factor of 15.5 was obtained.Before the study,Fan et al.[33]compared TBAF with TBAB on hydratebased CO2separation from the simulated flue gas (CO2/N216.6%/83.4%),and they drew the conclusion that TBAF was more preferable than TBAB on separating CO2with the separation factor up to 37.0.
2.2.2.CO2separation from CO2/H2
The quaternary ammonium salt hydrate formation promoters were also used in CO2separation from CO2/H2gas mixture.Li et al.[48]investigated the effects of the concentrations of TBAB on CO2separation from the fuel gas(CO2/H239.2%/60.8%),and they confirmed the TBAB solution with the concentration of 4.95 wt%was the most suitable.Meanwhile,they reported the CO2separation efficiency varied with the pressure driving force under a certain temperature.When the driving force was lowerthan 2.5 MPa,the efficiency increased with the increase of the driving force,however,when the driving force was higher than 2.5 MPa,the efficiency decreased with the increase of the driving force because in this case,more and more H2entrapped into the hydrates,decreasing the CO2partial concentration in the hydrate phase.However,similarly,the hydrate formation rate and the gas consumption were limit.Therefore,Li et al.[7]added cyclopentane(CP)into 4.95 wt%TBAB solution to investigate the synergistic effect of CP and TBAB on CO2separation from CO2/H2.It was reported that CP of 5 vol%and ratio of solution to gas of 0.58 brought about the highest CO2separation fraction of 0.58 and factor of 30.6,and it also led to the hydrate formation induction time shorten to about 1 min.Liu et al.[20]mixed diesel/CP emulsion into TBAB solution separate CO2from IGCC syngas.In the system,the emulsion did not only effectively prevent the hydrates from agglomeration but also increase the hydrate formation rate.What's more,the formed hydrate had strong selectivity of CO2,leaving H2to enrich from the original 53.2%to 84.6% in the gas phase,and resulting in the CO2separation factor as high as 37-99.Such high S.F.,thereby,drew much attentions,because it made the hydrate-based CO2separation promising in commercial application.Yang et al.[95]added THF into TBAB solution to effectively enhance the CO2separation efficiency from the flue gas.They found that the addition of THF did not only promote the dissolving of CO2in the solution but also supply more hydrate cages for CO2molecules relative to the TBAB solution system without THF.

Table 1 List of the research of hydrate-based CO2separation from gas mixtures in China

Table 1 (continued)
Dimethyl sulfoxide(DMSO),as an important polar aprotic solvent dissolves both polar and nonpolar compounds and is miscible in a wide range of range of organic solvents as well as water,was also proved to be effective to enhance the gas consumption during the gas hydrate formation[31,74,75,83,96,97].The thermodynamic effects of DMSO and other promoters such as TBAB,THF on the hydrate-based CO2separation from fuel gas are systematically compared by Xia et al.[97]They found that DMSO,as same as THF,has the significantly positive influence on moderate the hydrate formation equilibrium condition.Further,Xia et al.[75]added DMSO into 4.95 wt%TBAB solution to investigate CO2separation from the IGCC syngas.It was found the synergic effect of DMSO and TBAB on the CO2separation were much outstanding,compared with the pure TBAB solution,the gas consumption and CO2separation efficiency increased by more than 15%.
However,no matter what additives are used,it is found that CO2can't be thoroughly separated from the gas mixture by the hydrate technology.On the one hand,with the reduction of CO2in the gas phase,the hydrate formation equilibrium condition becomes more and more severe.On the other hand,the essence of the hydrate formation and dissociation is related to the breaking and reconstruction of the balance among the gas,liquid and solid in the system.Therefore,there must be a certain amount of CO2residing in the gas phase.Thus,in order to completely separate CO2,it needs develop a combined process,such as hydrate combined membrane separation process proposed by Linga et al.[29]Actually,in Linga et al.'s work,the contribution ratio of CO2separation with the two hydrate stage separation accounted for more than 80%,leaving CO2less than 20%to separate with membrane.But it was only an experimental process,and all the experimental results were obtained with the equipments with very small capacity in lab.Not only that,there were few studies on the continuous process of hydrate-based CO2separation,and the volume of the equipments reported were quite small.Li et al.[98]developed a process of hydrate combined with chemical adsorption,as shown in Fig.2.Based on the process,a set of CO2separation apparatus was established,and more than 500 m3(STP)could be continuously treated per day.With the process,the hydrate-based CO2separation could be continuously carried out.In a continuous separation for 100 h,CO2concentration in the hydrate phase reached to up to 95.0% and H2collected after the chemical adsorption reached to 99.2%.The results showed,(a),the combined process could be promising in industrial application of CO2separation,(b),the apparatus could be continuous work for separating CO2from the fuel gas,and (c),the CO2could be thoroughly separated with the process and the apparatus.In the process of continuous separation,THF or/and CP were used as hydrate formation additives.

Fig.2.Diagram of the hydrate combined with chemical adsorption process for CO2separation and H2purification from gas mixtures.
Nevertheless,there is one problem that can't be ignored.The additives like DMSO,CP,and THF are easy to evaporate.Therefore,the application of the additives in hydrate-based CO2separation are still controversial.Chen et al.[99]thought the volatile additives should not be added in the TBAB solution or pure water in the process of hydrate-based CO2separation,therefore,they added a kind of antiagglomerant(AA)into the TBAB solution to prevent the formed hydrate from agglomeration,and finally achieved a good effect of CO2separation efficiency.On the other hand,Chen also supported to use porous sediment such as porous silica to separation CO2with hydrate technology because he thought only in this way could the hydrate-based separation be truly environmental friendly[100,101].Chen et al.[102]investigated CO2hydrate formation and dissociation properties in sand by NMR.They thought water in only the pores of the sand participated in forming hydrates and the formed hydrates were pore-filled hydrates.On the other hand,it was also found that the hydrate formation condition trended to be more harsh.Therefore,the investigation with porous sediment mainly focused on methane recovery with CO2-CH4replacement,CO2sequestration in seabed.But in the process of simulating hydrate formation,the choice of the additives could dramatically moderate the formation condition and increase the hydrate formation rate.
2.2.3.CO2separation from CO2/CH4
The gas mixtures containing CO2/CH4as the main components extensively distribute on Earth,including shale gas,bio-gas,landfilled gas,coalbed methane,etc.CO2separation from these gas mixtures are of great significance to both carbon emission reduction and methane purification.And the work is particularly important in China because CO2accounts for relatively high in these sources.In 1999,Fan and Guo co-worked and obtained CO2/CH4binary gas mixture hydrate formation equilibrium data [103].Henceforth,Sun et al.[104]investigated the sorption equilibria of CO2and CH4via hydrate.In this work,they thought the gas molecules were adsorpted into the hydrate cages,and the adsorptions between CO2and CH4distinguished dramatically,meaning that it was possible to distinguish CO2from CH4by controlling the relevant conditions.Then,Xia et al.[97]investigated the hydrate formation equilibrium conditions with landfilled gas,and confirmed it was feasible to separate CO2from landfilled gas via hydrate.Bi et al.[105]directly taken CO2/CH4as sample gas to obtain the upper-quadruplephase equilibrium data.Mao et al.[106]established a thermodynamic modeling to investigate hydrate-based CO2separation from CO2/CH4.Besides,the hydrate formation equilibrium condition data of CO2/CH4in the presence of different additives were systematically investigated also[30,107].As shown in Table 1,except for TBAB[37,77,82],TBPO[108,109],THF[110]and CP,researchers developed combined additives,i.e.,Xia et al.[31]investigated the synergic effects of TBAB+DMSO,THF+DMSO on CO2separation from landfilled gas.As mentioned above,DMSO is one kind of gas solvent,and its addition promoted the dissolution of gas in the solution,dramatically increased the gas consumption.But,it was also noted that the CO2separation efficiency was not improved.Zhang et al.[65]added active carbon into the system to investigate the hybrid method effect on CO2separation from CO2/CH4.Active carbon,as a kind of crystal seed,was proven to be effective to increase the hydrate formation rate,but no outstanding evidence proved that the gas consumption and the CO2separation efficiency were improved in the work.
However,different from those mentioned above,in the process of hydrate formation,CH4has stronger tend to entrap into the hydrate cages than N2and H2under a certain condition,and the size of CH4molecule is close to that of CO2,therefore,the CO2separation factor was found to be relatively low in the process of hydrate-based CO2separation from CO2/CH4gas mixture[30].Therefore,the focus of CO2separation from CO2/CH4is not only to improve hydrate formation rate and gas consumption but also to enhance the selectivity of hydrate to CO2during the hydrate formation.Xu et al.[86]found that CO2separation efficiencies were quite different in hydrate formation process in the presence of different additives.In their study,THF,CP and TBAB were added into the water,respectively,and the results indicated the adding of THF was helpful to enhance CH4consumption in the hydrate,while the adding of CP was beneficial to promote CO2consumption.From the point of the hydrate structure,both THF and CP could introduce to form sII hydrates and both THF and CP could occupy large 51264cages,leaving small 512cages for gas molecules.Therefore,the difference of selectivity of hydrate to CH4and CO2could only be attributed to the chemical characteristics of the additives.They considered that the polarity of the additive influenced the selectivity.The relationship of the polarity of TBAB,THF and CP is TBAB>THF >CP.Therefore,the polarization between TBAB and water made the hydrate dense,while the hydrates with THF and CP were relatively loose.Thus the denser hydrate,the lower gas consumption,because the gas diffusion was stopped in dense hydrate.Besides,the feature of miscible between CP and CH4made it difficult to get rid of the impact CP before CH4independently entering in the hydrate cages.The study was of great significance to CO2separation from CO2/CH4.
The CO2separation from CO2/CH4was also carried in the presence of porous media.Song et al.investigated the hydrate formation equilibrium condition data.The experiments were conducted at 2.10-6.80 MPa and 276.55-284.85 K with an isochoric method and CO2/CH4gas mixtures (CO2mole fractions were 0.798,0.499,and 0.199 mol·mol-1(79.8 mol%,49.9 mol%,and 19.9 mol%)).The results indicated that the gas mixtures with higher CO2,the lower hydrate equilibrium pressure under a certain temperature.And two different porous media used in the experiments proved that the porous media had a slight influence on gas mixture hydrate phase equilibrium conditions.Zhong et al.[111]further investigated the effect of the porous media on the CO2separation from CO2/CH4gas mixture(CO2/CH440.0%/60.0%)with a fixed bed of zeolite 13X(FBZ-13X).It showed,compared the FBZ-13X with the stirred tank reactor,CO2selectivity obtained in the FBZ-13X was lower than those obtained in the stirred tank reactors.
Although many studies on CO2separation(capture)from CO2/CH4(like shale gas,biogas,landfilled gas,etc.),no studies on detailed continuous separation process and scale-equipments were reported,and the reasons for the result are,on the one hand,there are no any feasible process for the continuous separation,on the other hand,the CO2separation efficiency still is too low.Therefore,it needs develop new methods,including to develop new hydrate formation additives and new processes.
3.Sea-Water Desalination
Methods of sea-water desalination include distillation,electrodialysis method and reverse osmosis.However,it is convinced that these methods have higher energy consumption.Temperature is much lower annually in alpine region of China,it provides a good condition for hydrate formation.It also provides the possibility for the desalinating process of brackish water using hydrate methods in alpine region.At a certain temperature and pressure,seawater or salt solution could forms clathrate or gas hydrate when they contacts hydrate formation agents.During the hydrate formation,the ions and salts are excluded from the hydrate crystals.Hence,it severs as a basis for separating pure water from electrolyte solution or salt solution.Fig.3 shows a simple block flow diagram of the concept of hydrate based seawater desalination[112].In general,water recovery is employed to evaluate the sea-water desalination process,and it is calculated according to Eq.(5):

where Fhis the fraction of hydrate which could be recoverable.
It is noted that water recovery relies on the kinetics and separation efficiency of hydrate formation from brine.Generally,higher water recovery means more hydrate produced and effective separation of hydrates from the brine is desirable.

Fig.3.A simple block flow diagram of the concept of hydrate based seawater desalination.
Wang et al.[113]proposed a new method of desalinating brackish water based on the model of van der Waals-Platteeuw.The phase equilibrium conditions of methane,ethane and propane hydrate in NaCl solution were calculated systematically,combined parameter equation which was used to calculate the Langmuir constant presented by DU Ya and GUO Tian-min and Pitzer equation used to water activity in the system of electrolyte solution.And the result showed that it was possible to desalinate brackish water using environment cold quantity.It also showed that propane is more suitable for brackish water desalination among methane,ethane and propane.It was significantly important to seek energy-saving and environment-friendly methods for the process of desalinating brackish water with energy shortage increasingly intensifying and with awareness of human being enhancing on energy conservation and emission reduction.
Yang et al.carried out studies to obtain the characteristics of CO2hydrate formation and dissociation by using different experimental modes with glass beads.The experiments indicated the hydrate formed rapidly at high pressure without hydrate blockage.They proposed three different modes.And it was found,considering hydrate saturation and operating pressure,5.00 MPa was a better choice for hydrate-based desalination and CO2capture.They also thought,if the initial residual solution could be completely converted to hydrate,this technology has potential for industrial applications.After then,Yang et al.took C3H8as hydrate formation promoter to investigate the effects of C3H8on the hydrate formation and dissociation for integrating CO2capture and desalination simultaneously.The investigation results indicated that the C3H8was reverse proportion to the gas consumption,and the initial solution saturation had few effects on the hydrate formation equilibrium conditions,but when the solution saturations increased from 30.0%to 50.0%,the gas consumption decreased accordingly.
Moreover,CP was also used to form hydrate for sea-water desalination because the CP hydrate formation equilibrium condition is quite moderate.Lv et al.[114]dispersed CP into water to form W/O emulsions,and then simulated CP hydrate to efficiently enhance desalination as well as hydrate formation kinetics.Besides,to make application of hydrate based desalination technology practical,the kinetic behaviors and separation capability were examined by forming cyclopentane hydrates with brine in cyclopentane dispersion systems at 274.1 K/277.1 K with initial salinity range from 3.0 wt% to 5.0 wt% and water fraction of 20.0 vol%to 90.0 vol%and rotation rate of 300 to 500 r·min-1[9].The results indicated the more CP and the more thorough dispersion of CP in the water,the more desalinized water produced with the salts removal efficiency about 80.0%.Meanwhile,the results also reminded though the higher stirring rate(rpm)could be helpful to the hydrate formation,it decreased the salt removal efficiency.Lower temperature(274.1 K)and 60.0%water cut should be optimal for higher yield of dissociated water.The ratio of washing water/dissociated water and rotation rate conditions.Zheng et al.[115]carried out series of experiments to provethat the CO2-CP guest provides a strengthened stability and moderate hydrate phase equilibrium conditions for hydrate-based desalination.And the optimal molar ratio of CP of 0.01 was recommended.The concentration was also proved in the investigation carried by Xu et al.[116]as CP hydrate could be formed at atmospheric pressure and temperatures below 280.8 K.The experiments were performed at various subcoolings for aqueous solutions with different salinities in a bubble column.With increasing subcooling and agitation,the hydrate formation decreased and the hydrate conversion increased.The most effective method involving spraying finely dispersed CP droplets(around 5 μm in diameter)into the water-filled bubble column.The latter method resulted in a 2-fold increase in seawater conversion to hydrate crystals compared with injecting millimeter-scale CP droplets.A desalination efficiency of 81.0%(the salinity decreased from 3.5 wt%to 0.67 wt%)was achieved by using a three-step separation method,including gravitational separation,filtration,and a washing step.Washing the hydrate sample using filtered water decreased the salinity from 1.5 wt%in the solid hydrates before washing to 1.05 wt%after washing.
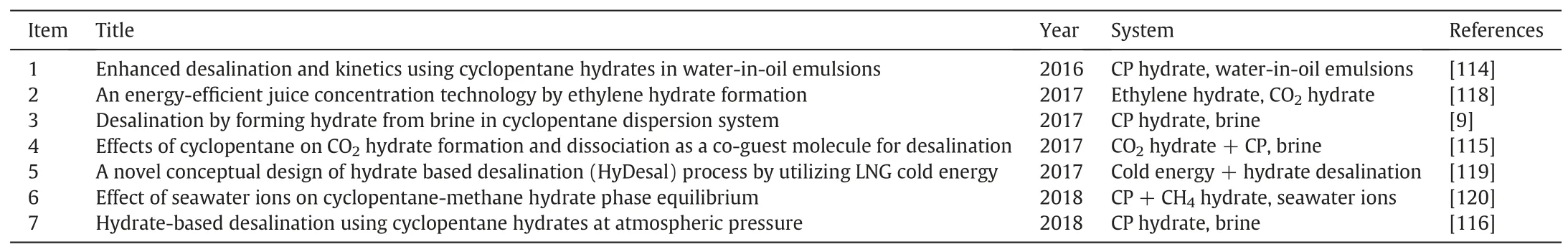
Table 2 Recent seawater desalination studies via hydrate-based technology in China
Table 2 shows the recent seawater desalination studies via hydratebased technology in China.Comprehensively,China lags behind South Korea in the work.Lee et al.[117]had developed a set of hydratedbased seawater desalination equipment for pilot-scalely producing water from brine by producing hydrate pellets and then dissociating hydrates into pure water.However,due to that the salt removal efficiency is still low and the key process of hydrate flowability is under resolution,the hydrate-based sea-water desalination is still on the lab.
4.Thermal Storage and Application
Hydrates as phase change materials(PCMs)is promising and attractive.The utilization of PCMs relies on their melting temperature(MT).In general,the PCMs with MT <15°C are used for coolness storage in air conditioning,and those with MT >90°C are used for absorption refrigeration,and those with MT in the range of 15 to 90°C are used for solar heating and the applications of heat load[121,122].Presently,the hydrates used in cold storage are formed by Chlorofluorocarbon(CFC),hydrofluorocarbon(HFC),CO2,TBAB,TBAC and THF,etc.Most refrigerant hydrates with suitable phase change temperature can form under the pressure lower than 1.0 MPa,and they have relatively larger fusion heat than eutectic salts,paraffin waxes and fatty acids.CFCs and hydro-CFCs(HCFCs)hydrates,e.g.,CFC-11,CFC-12,HCFC-21,HCFC-22,HCFC-141b,were the study objects for the hydrate energy storage.Unfortunately,due to their negative impact on the environment,the studies gradually stopped,instead of HFC hydrates[123].Besides,the heat fusion of CO2hydrate is higher than that of ice,therefore,CO2hydrates as a kind of PCM were investigated as the CO2hydrate formation and dissociation could release plentiful latent in delayed time [124].The melting point and heat latent of TBAC hydrate are 15 °C and 200.7 kJ·kg-1,while those of THF hydrate are 4.4 °C and 260.0 kJ·kg-1,respectively[125].
The most studies still focus on TBAB hydrate as TBAB hydrate has a melting point of 12°C,good fluidity and relatively large cold storage,and its storage is about 2-4 times of chilled water.Up to date,many investigations have been conducted on the TBAB hydrates and their practical application in energy storage,and the latest investigations on the hydrate-based phase change materials have been presented in Table 3.Since 2005,JEE Engineering Corporation(Japan)showed the feasibility of a set of large-scale utilization of TBAB hydrate slurry for airconditioning.By the series of experiments,the researcher demonstrated the TBAB hydrate as a secondary refrigerant for air conditioning was feasible after some technical problems were resolved.And then in 2013,Douzet et al.[126]developed a set of real size air-conditioning system using TBAB semi-clathrate hydrate slurry as secondary twophase refrigerant and carried out experimental investigation and established corresponding model[127].In their investigation,a compression cycle refrigeration unit allowed to create TBAB hydrate slurries which were thereby stored in a tank in the night.
In China,the studies on the hydrate-based energy storage are only carried out by the institutes such as the South China University ofTechnology(SCUT)[127],Tsinghua University[128],GIEC[129],and Shanghai Jiaotong University[130],etc.

Table 3 The latest investigations on the hydrate-based phase change materials
5.Gas Hydrate Inhibition
The hydrates formed in gas or oil pipelines would jeopardize the safety of the transportation since the hydrates could gradually agglomerate and stack and finally block the pipelines.Therefore,to effectively prevent the hydrate formation in the transportation pipeline is quite important and extremely essential.There are many methods for hydrate formation prevention,including system heating,depressurization,water removal and inhibition[140].But,it is considered,under many circumstances,adding inhibitor is the only feasible choice [141].Currently,there are mainly three types of inhibitors,thermodynamic inhibitor,kinetic inhibitor and antiagglomerate(AA)hydrate inhibitor.For the thermodynamic inhibitor,it shifts the equilibrium hydrate formation/dissociation curve to lower temperature or/and to higher pressure,and for the kinetic inhibitor,it slows down the hydrate nucleation or/and growth rate,and the AA inhibits the agglomeration of hydrates to form big cluster but keeps the hydrate dispersed in the system.It was proved that a combination of thermodynamic and kinetic hydrate inhibitors or/and a combination AA and kinetic inhibitor could provide better effects on the hydrate inhibition.According to the previous studies,those material with strong electrostatic charges or/and strong hydrogen-bond are effective to inhibit hydrate formation,including NaCl,methanol,poly ethylene oxide(PEO),poly N-vinylpyrrolidone(PVP),etc.[142]Also based on this concept,ionic liquid(IL)has been developed as its structure and physical properties could be fine-tuned for different applications.Currently,although it is still in its initial stage,many interesting studies have been carried out.Table 4 shows the list of Ils as kinetic hydrate inhibitors (KHIs),thermodynamic hydrate inhibitors (THIs)and the systems investigated for hydrate inhibition.
The investigation on the hydrate inhibition initiated the 1930s in the world[143].However,the work,in China,started relatively later.Moreover,the investigation focused on CO2hydrate and NGH and the characteristics of the hydrate inhibitors.In 2010,Ning et al.[144]investigated the characteristics of gas-hydrate formation,agglomeration and inhibition by an experimental system with oil-based drilling fluids(OBDF)that would be used for deep-water drilling in the South China Sea under the temperature of 4°C and pressure of 20 MPa.The results not only validated the hydrate shell formation model,but also showed that the water cut could greatly influence hydrate formation and agglomeration behaviors in the OBDF.The dominant agglomeration mechanism of hydrate particles was determined by the oleophobic effect enhanced by hydrate shell formation which weakens or destroys the interfacial films effect and the hydrophilic effect.Higher ethylene glycol concentrations could inhibit the formation of gas hydrates and acted as an anti-agglomerant to inhibit hydrates agglomeration in the OBDF.Then,Chu et al.[145]investigated inhibition effect of 1-ethyl-3-methylimidazolium chloride on methane hydrate equilibrium with a high pressure differential scanning calorimeter(DSC).And they found the most significant inhibition effect was observed at 0.4 mass fraction of EMIM-Cl in aqueous solution to lower the dissociation temperature by 12.82 K at 20.00 MPa in comparison to that of the (methane +water)system.Long et al.[146]did also conduct experimental investigation on the phase behavior of methane hydrate in the presence of imidazolium ionic liquids and their mixtures.It was observed that,for all the inhibitor systems containing[Emim][NO3]or[Emim[Cl],the inhibition effect increases progressively with the increased concentration and pressure.In addition,the mixture of[Emim][NO3]and[Emim][Cl]does not always exhibit synergetic effect on the thermodynamic inhibition of methane hydrate at higher concentrations up to 0.2 mass fraction.The higher concentrations result in the positive interactions between [Emim][NO3],and [Emim][[CI]possibly happen at higher pressures.Zhang et al.[147]investigated the inhibition effect study of carboxyl-terminated polyvinyl caprolactam(PVcap)on methane hydrate formation,and they synthesized a carboxyl-acid-group-modified PVCap (PVCSCOOH)which was proved to be a kind of effective and commercialized KHIs.It was found that the maximum subcooling degree of 2 wt%PVCSCOOH was higher than PVCap;PVCSCOOH had a better inhibition performance than PVCap of similar molecular weight.In addition,it was also noticed that PVCSCOOH could change the hydrate appearance and lower the large/small cavity ratio(L/S),to 2.00 but it had no impact on the hydrate structure.
It should say there are a lot of basic research on the hydrate inhibition,but up to date,there are very few reports on the relevant process with scale equipment.Chen et al.[189]evaluated the feasibility of anti-agglomerants from analyzing the variation of morphologies and chord length of particles using an autoclave installed with a particle video microscope and focused beam reflectance measurement probes.The experimental results from methane+water+diesel oil systems showed that the agglomeration of hydrate could be determined directly with two laser probes.The size of droplets/hydrate particles was uniform and tended to that before hydrate formation when most water converted to hydrate or the system stopped stirring for 9 h,although the agglomeration occurred when water droplets initially converted into hydrates.The evaluation method was examined under different anti-agglomerant concentrations,water cuts,and subcoolings for water+diesel oil systems.The mechanism of anti-agglomerants was thereby proposed.Shi et al.[190]conducted the viscosity investigation of NGH slurries with AA.Results indicated that the hydrates volume fraction,the continuous liquid phase viscosity and the dispersion degree of hydrates particles in the slurry were the critical factors to affect the viscosity of natural gas hydrates slurry.Yan et al.[191]developed a flow loop apparatus and investigated the flow characteristics and rheological properties of NGH slurry in the presence of AA.The investigation results indicated that the hydrate slurry could be easily and safely restarted after a long shutting-down period and exhibited obvious thixotropic behavior with the shutting-down time.
Recently,Tang et al.[192]from GIEC carried out investigation of the flow characteristics of methane hydrate slurries with low flow rate with a set of equipment with 40 m length and 3 m height(as shown in Fig.4).The results indicated that the differential pressure drop(Delta P)across two ends of the horizontal straight pipe increases with increasing hydrate concentration at the early stage of gas hydrate formation.The lower flow rates of hydrate fluid and the higher the subcooling result in the faster the transition of the hydrates macrostructures.Gas hydrates could agglomerate,and sludge hydrates appear at subcoolings of 6.5 and 8.5°C.There are three hydrate macrostructures,slurry-like,sludge-like,and their transition.When the initial pressure was 8.0 MPa,large methane hydrate blockages appeared at the gas hydrate concentration of approximately 7%.And based on the gas-liquid twophase flow model,a correlation between the gas hydrate concentration and the value of Delta P was presented.These results efficiently provided guidance for oil and gas transportation in pipelines.The investigation should be the very interesting one because the equipment used in the work was established according to the true pipeline.And more investigation with the equipment are expected,with the work parameters closer to the truth.
6.CH4Exploitation from NGH with CH4-CO2Replacement
CO4-CO2replacement in hydrate is considered to be promising as it is possible to CH4exploitation and CO2capture and sequestration simultaneously.Moreover,due to that CO2hydrate has same hydrate structure (sI)as CH4hydrate,and in the process of the replacement,the sediment stratum could be well kept instead destroyed,it is also considered to be a safe method for CH4exploitation from NGHs.The method has been proposed since the 1990s[193].In the early,the investigation focused on the kinetics or/and thermodynamics of the replacement(or swapping)with micro-or/and macro-experiments[194-202].Sun et al.[203]firstly replaced CH4in the hydrate using pressurized CO2in the presence of SDS.In 2008,Zhou et al.[204]carried out the relevant investigation on determination of appropriate condition on replacing methane from hydrate with carbon dioxide.In Zhou et al.'s investigation,the hydrate stable zones(HSZs)of NGH and CO2hydrate,both in permafrost and under deep sea,were determined by analyzing the hydration equilibrium graphs and geotherms.And it was found that the appropriate experimental condition should be in the area surrounded by four curves:the geotherm,(H-V)(CO2),(L-V)(CO2)and (H-V)(CH4)for replacing CH4from NGH with gaseous CO2,and the condition should be in the area surrounded by three curves:(L-V)(CO2),(H-L)(CO2)and(H-V)(CH4)for replacing CH4from NGH with liquid CO2.For conditions in other areas,the replacement could not occur.The investigation should be the pioneer in the field of CH4-CO2replacement
in China.And from then,the studies on CH4-CO2replacement in hydrate gradually drew more and more attentions in China.
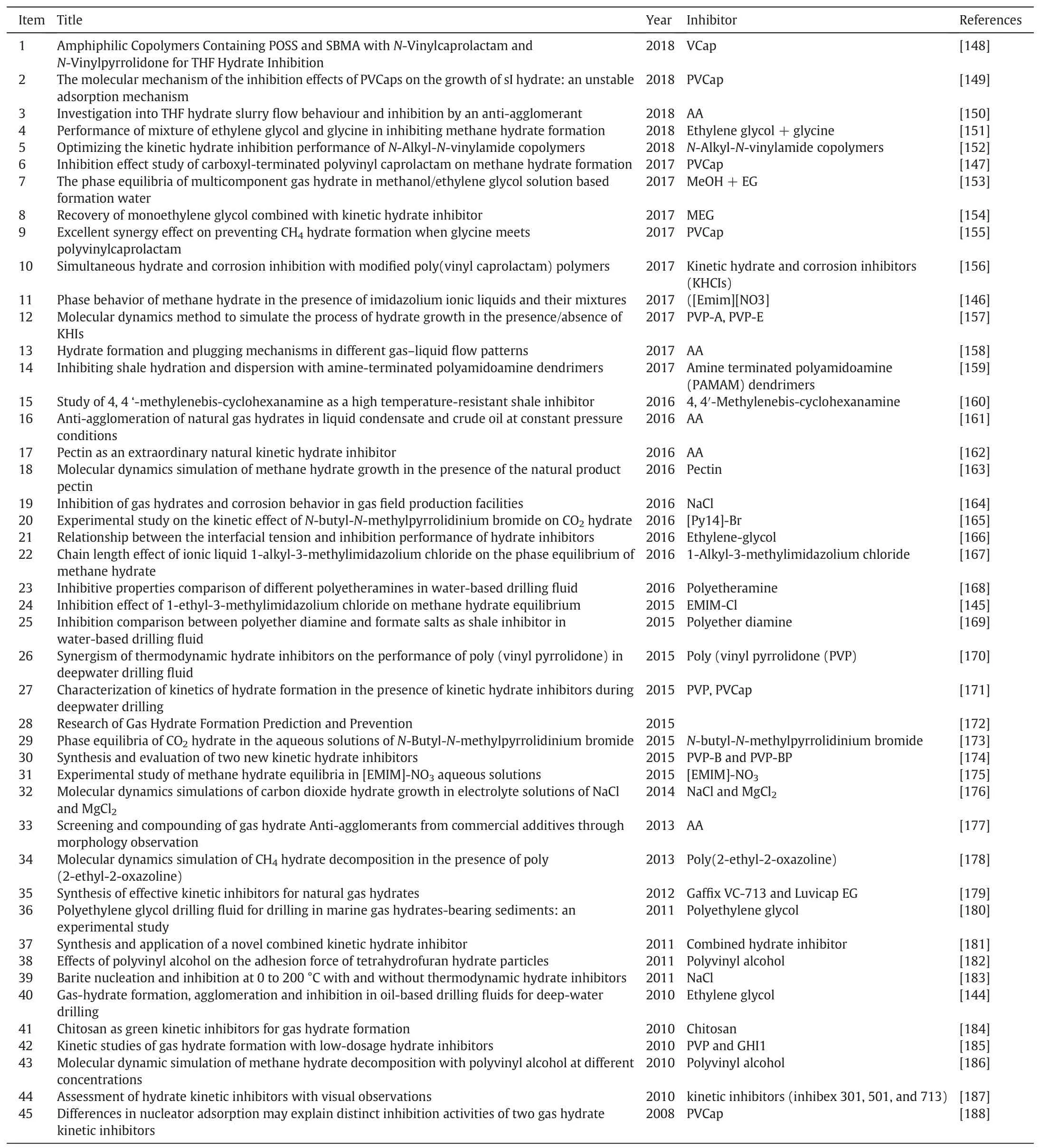
Table 4 List of investigations on hydrate formation inhibition

Fig.4.Schematic of the hydrate flow loop system.1:gas cylinder,2:visual window,3:camera,4:mass flow meter,5:gas-liquid separator,6:piston pump,7:gas compressor,8:liquid tank,V1-V7:valve,P:pressure sensor,T:thermocouple,DP:differential pressure transducer.
Geng et al.[205]investigated the potential of methane reoccupation during the replacement of methane Hydrate by CO2by molecular dynamic(MD)simulation.The MD simulation results revealed that the CH4-CO2mixed hydrate was more stable than CO2hydrate and CH4hydrate.The stabilization energy calculations of small and large cavities occupied by CH4and CO2showed that the CO2molecule was less suitable for the small cavity because of its larger size compared with the CH4molecule but was more suitable for the large cavity,which illustrating the possibility of CH4molecule in reoccupying the small cavity during the replacement of CH4hydrate by CO2,from the hydrate stability point of view.Li et al.[206]carried out experimental investigation on CH4exploitation from NGHs with CO2in simulated brine.It was found,on the one hand,the high temperature was favorable to the replacement reaction but high NaCl concentration had a negative effect on the replacement process,on the other hand,the gas diffusion in the hydrate phase was crucial to both CH4hydrate decomposition and CO2hydrate formation.According to Li et al.'s investigation,the replacement contained two processes,CH4hydrate dissociation and then CO2hydrate formation.
However,there has always been a controversy over the mechanism of the replacement.Yuan et al.[207]used a three-dimensional reactor to conduct CH4recovery with CO2.They also thought that the replacement included two processes,the CH4hydrate dissociation and CO2hydrate reformation.And then,they carried out the experimental investigations on CH4recovery with liquid CO2[208]and CO2emulsion,respectively,to further support their opinions.Zhang et al.[209]investigated the dynamics of the replacement of CH4in hydrate in porous sediments with liquid CO2using a special experimental apparatus at different temperatures and initial pressures.It was found the amount of the replaced CH4was almost the same as that of the CO2forming hydrate in the early stage and gradually become somewhat less in the later stage,and the replacement rate was not related to the region of the temperaturepressure conditions but was mainly affected by the fugacity differences of CH4hydrate decomposition and CO2hydrate formation.Song et al.[210]measured CH4-CO2replacement in hydrate using magnetic resonance imaging (MRI).The results showed that the hydrates first formed in the centre of the porous media and then grew along the axis of the vessel.The hydrate saturation and the initial water saturation were linearly correlated when the initial water saturation changed from 5%to 40%.Xu et al.[211]analyzed the replacement process using in situ Raman.And they found that the key to determine the mechanism of the replacement was the free water,i.e.,if there was free water in the process of the replacement,it meant there were the hydrate dissociation,if there was no free water,it meant the CH4-CO2replacement happened in the hydrate cages without any hydrate cage breakage.
Due to that the size of CO2molecule is larger than that of the small 512cage,it is thought that the replacement could only occur in the large 51262cages,which causing the low CH4recovery.Besides,the gas with relatively small size has stronger diffusion in the hydrate phase than the gas with big size.Therefore,researchers added smaller gas such as N2into the CO2to replace CH4in NGH,and found the CH4recovery was dramatically enhanced.Zhou et al.[212]used CO2/N2gas mixture to replace CH4,and found no structural transition in the hydrate phase.In the replacement,CO2was found to prefer replacing the CH4in the large hydrate cages,whereas N2preferred to replace the CH4in the small hydrate cages.Although the total CH4production was found to increase with addition of N2,the CH4recovery rate was low as N2captured in hydrate cages slowed the diffusion rate of CO2in hydrate phase.The results were contradicted to that reported by Liu et al.[213]Liu et al.carried MD calculation on CH4replacement with CO2/N2,and found N2exhibited a faster diffusion motion than CO2in hydrate,implying the substitution by N2was more kinetically favorable to occur.And the results highlighted that the replacement process should include the thermodynamically and simultaneously dominated CO2substitution to the kinetically dominated N2substitution.Ding et al.[214]used IGCC syngas to replace CH4from NGH,and found on one hand,the replacement consisted of two steps,CH4hydrate dissociation at the first and followed by CO2hydrate formation,on the other hand,the CH4recovery from CH4-CO2/H2replacement was more than 71%which was significantly higher than that from CH4-CO2replacement.Notably,no H2was found in the hydrate phase in the replacement process,which implying that H2did not compete with CH4molecules occupying hydrate cages but played promotion role in CO2-CH4replacement.The results offered one new idea on CO2sequestration and high quality energy recovery.
Besides,in order to increase the CH4recovery with CH4-CO2replacement,many other ways were developed.Liu et al.[215]investigated the effects of the nanobubbles on the replacement via MD simulation.It was found,one,It is found that CH4molecules were surrounded by some dense CO2molecules in the mixed bubble during the replacement,two,CO2molecules in the solution could assist the bubble formation of CH4molecules at an initial stage and prevent their further escape and reoccupation by encompassing them afterwards.Other combination methods were also developed.Zhang et al.[216]combined thermal stimulation with the replacement to enhance the CH4recovery.The results illustrated that the combination method could effectively improve CH4recovery,with the CH4replacement percentage up to 64.63%.Moreover,the total CO2stored was unequal to CH4recovered,because the replacement was sensitive to the free water in the pores of the hydrate sediments.Li et al.[217]carried out experimental investigation on CH4recovery from fracture-filled hydrate by CO2and CO2/N2replacement.The results indicated a higher replacement rate and accumulative methane recovery ratio could be obtained when the morphology of methane hydrate was well distributed thin layer,which could be obtained with the ice layer by gradually increasing its temperature.The thin layer of hydrate was the main morphology of the fracture-filled gas hydrate found in the Qilian Mountain permafrost.Therefore,the gas production from hydrate by CO2/N2(3:1)replacement might be a good choice for gas production from hydrate in Muri Basin,Qilian Mountain.Zhang et al.[218]carried out experimental studies on the effect of pressure on the replacement process of CO2-CH4hydrate below the freezing point.The investigation was also carried out based on the real hydrate condition in Qilian Mountain.The results indicated that,compared with the temperature conditions above the freezing point,the replacement rate of CO2-CH4hydrate was slow below the freezing point.These results showed the average replacement rate and efficiency increased with the increasing of injected pressure of CO2gas.Also,the average replacement rate and efficiency reached up to 0.403 mmol·h-1and 13.20%when the injected pressure of CO2was at 4.5 MPa,which providing a theoretical guidance for gas production from methane hydrate using CO2-CH4replacement method in permafrost region in the future.
It should say that the method of CH4-CO2replacement for CH4recovery is promising,but the investigations are still on the experimental stage.The main reason for the result is that the mechanism of the replacement and the control factors are still unclear,and the CH4recovery and replacement rate are still low.Presently,the investigation are extensively carried out in China,and it is expectable that the technology could be well developed in the coming future.
7.Conclusions
In this work,the research advances of the hydrate-based technologies and processes in China are comprehensively reviewed.The hydrate-based technologies are mainly applied for CO2separation and capture,sea-water desalination,thermal energy storage and conversion,gas hydrate formation inhibition and CH4exploitation from NGHs with CH4-CO2replacement,etc.In China,all these technologies have been extensively investigated,and more and more institutes take participating in the investigation.Currently,the institutes with prominent achievements include GIEC,CUPB,DLUT,SCUT.Although China started late in the investigation on the hydrate-based technologies and processes,in terms of current research level,China is synchronized with the world.Even so,all the research is still on the experimental stage because low gas consumption and low hydrate formation rate are the two crucial obstacles which limit the further development of the technologies.For the CO2separation,the hydrate combined with chemical adsorption process is experimentally proved to be effective to thoroughly separate CO2from CO2/N2or CO2/H2,but the process need further large scale experiments to testify.The research achievement of the hydrate formation inhibition seems to be most possible to be commercially applied because much research has proven that the inhibitors such as AAs,THIs,KHIs are effective to inhibit hydrate formation in the gas or oil transport in pipeline.The CH4-CO2replacement is promising to CH4recovery from NGH,but it is still on experimental stage too.And the key problem is the mechanism of the replacement is still unclear,and the CH4recovery is too low.In summary,the studies on the hydrate-based technologies and processes are on the way and promising in the future.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Decomposition behaviors of methane hydrate in porous media below the ice melting point by depressurization☆
- Fundamental mechanisms and phenomena of clathrate hydrate nucleation☆
- Methane hydrates:A future clean energy resource
- Progress and trends in hydrate based desalination(HBD)technology:A review☆
- Extraction of methane hydrate energy by carbon dioxide injection-key challenges and a paradigm shift
- A short review on natural gas hydrate,kinetic hydrate inhibitors and inhibitor synergists☆
