甘露糖醛酸寡糖对H2O2诱导衰老心肌细胞的影响
2019-09-10牟婕冯文静毛拥军
牟婕 冯文静 毛拥军

[摘要] 目的 探討甘露糖醛酸寡糖(M3K)对过氧化氢(H2O2)诱导的心肌细胞衰老的作用及其可能机制。方法 用100 μmol/L H2O2构建H9c2心肌细胞衰老模型,应用不同浓度(10、50、100 mg/L)的M3K干预衰老心肌细胞,观察M3K对心肌细胞衰老的影响。实验分为对照组、H2O2组、H2O2+M3K低剂量组、H2O2+M3K中剂量组和H2O2+M3K高剂量组。用倒置显微镜观察各组心肌细胞数量,Western blot检测衰老标志蛋白P21和P53的表达,PT-RCR检测自噬相关基因beclin1、Atg7和LC3-Ⅱ的mRNA表达。结果 倒置显微镜观察显示,与对照组相比,H2O2组心肌细胞数量明显减少;与H2O2组相比,M3K干预各组心肌细胞数量呈剂量依赖性增多,差异有统计学意义(F=8.685,P<0.05)。Western blot检测显示,H2O2组心肌细胞P21和P53蛋白表达较对照组明显增加,M3K干预各组心肌细胞P21和P53蛋白表达较H2O2组明显降低,差异有显著意义(F=31.507、13.111,P<0.05)。PT-RCR检测显示,H2O2组心肌细胞beclin1、Atg7和LC3-Ⅱ的mRNA表达较对照组显著降低,M3K干预各组三者的mRNA表达较H2O2组显著增加(F=10.134~61.041,P<0.05)。结论 M3K可以延缓H2O2诱导的心肌细胞衰老,其机制可能与自噬的激活有关。
[关键词] 甘露糖;寡糖类;衰老;肌细胞,心脏;自噬;过氧化氢
[中图分类号] R329.25 [文献标志码] A [文章编号] 2096-5532(2019)04-0411-05
[ABSTRACT] Objective To investigate the effect of mannuronic acid oligosaccharides (M3K) on hydrogen peroxide (H2O2)-induced cardiomyocyte senescence and the possible mechanism. Methods H9c2 cardiomyocytes were treated with 100 μmol/L H2O2 to establish a model of senescence, and then the senescent H9c2 cardiomyocytes were treated with different concentrations of M3K (10, 50, and 100 mg/L) to observed the effect of M3K on cardiomyocyte senescence. The cardiomyocytes were divided into control group, H2O2 group, H2O2+low-dose M3K group, H2O2+medium-dose M3K group, and H2O2+high-dose M3K group. Inverted microscopy was used to measure the number of cardiomyocytes, Western blot was used to measure the expression of the senescence marker proteins P21 and P53, and RT-PCR was used to measure the mRNA expression of the auto-phagy-related genes beclin1, Atg7, and LC3-Ⅱ. Results Inverted microscopy showed that compared with the control group, the H2O2 group had a significant reduction in the number of cardiomyocytes, and compared with the H2O2 group, the H2O2+low-dose M3K group, the H2O2+medium-dose M3K group, and the H2O2+high-dose M3K group had a significant increase in the number of cardiomyocytes in a dose-dependent manner (F=8.685,P<0.05). Western blot showed that compared with the control group, the H2O2 group had significant increases in the protein expression of P21 and P53 in cardiomyocytes, and compared with the H2O2 group, the H2O2+low-dose M3K group, the H2O2+medium-dose M3K group, and the H2O2+high-dose M3K group had significant reductions in the protein expression of P21 and P53 (F=31.507,13.111;P<0.05). RT-PCR showed that compared with the control group, the H2O2 group had significant reductions in the mRNA expression of beclin1, Atg7, and LC3-Ⅱ, and compared with the H2O2 group, the H2O2+low-dose M3K group, the H2O2+medium-dose M3K group, and the H2O2+high-dose M3K group had significant increases in the mRNA expressions of beclin1, Atg7, and LC3-Ⅱ (F=10.134-61.041,P<0.05). Conclusion M3K can delay H2O2-induced cardiomyocyte senescence, possibly by activating autophagy.
[KEY WORDS] mannose; oligosaccharides; aging; myocytes, cardiac; autophagy; hydrogen peroxide
衰老是一个复杂的过程,随着年龄的增加,机体发生退行性改变,进而引起各种衰老相关疾病。目前,世界人口老龄化加剧,随着老龄人口的增加,衰老相关疾病逐渐增多,其中心血管疾病是导致老年人死亡的主要原因[1-2]。机体衰老后心脏会发生一系列病理性改变,如心肌纤维化、心肌肥厚、心功能不全等[3]。有研究表明,随着细胞衰老P21和P53蛋白表达增加,二者可作为衰老检测的标志性蛋白[4-6]。自噬是一种存在于细胞内的降解机制,可以清除细胞内受损的蛋白和细胞器,对细胞内稳态的调节至关重要[7-8]。衰老心肌细胞自噬调节能力降低,导致细胞内错误折叠蛋白和功能障碍的细胞器大量累积[9]。研究表明,提高心肌细胞自噬水平可以延缓心脏衰老[10]。甘露糖醛酸寡糖(M3K)是褐藻胶寡糖的一种,由来源于海洋褐藻的褐藻胶加工而成,具有抗氧化、抗炎、抗肿瘤等多种生物学活性,有重要的临床应用价值[11-13]。过氧化氢(H2O2)属于活性氧簇家族,可引起氧化应激,诱导细胞衰老,现已被广泛用于体外衰老模型的建立[14-15]。本实验采用H2O2诱导建立心肌细胞衰老模型,探讨M3K对衰老心肌细胞的保护作用及其可能机制。
1 材料和方法
1.1 实验材料
M3K由中国海洋大学医药学院实验室惠赠。心肌细胞H9c2购于中国科学院上海细胞库。胎牛血清(FBS)购于美国Gibco公司,DMEM培养液购于美国Hyclone公司,P21兔单克隆抗体和P53小鼠单克隆抗体购于美国Cell Signaling Technology公司,山羊抗兔IgG二抗和山羊抗鼠IgG二抗购于武汉伊莱瑞特公司,RIPA裂解液和BCA蛋白浓度测定试剂盒购于上海碧云天公司,ECL发光液购于美国Millipore公司,逆转录试剂盒和Mix购于瑞士Roche公司。
1.2 实验方法
1.2.1 细胞培养及处理 将H9c2心肌细胞接种于6孔板中,用含体积分数0.10 FBS和10 g/L青-链霉素的DMEM完全培养液,在37 ℃、含体积分数0.05 CO2的细胞培养箱中培养。实验分为5组:对照组(A组),H2O2组(B组),H2O2+M3K低剂量组(C组),H2O2+M3K中剂量组(D组),H2O2+M3K高剂量组(E组)。当细胞融合度达50%时,H2O2组、H2O2+M3K低剂量组、H2O2+M3K中剂量组和H2O2+M3K高剂量组细胞分别加入100 μmol/L的H2O2诱导建立衰老模型,4 h后更换新鲜DMEM完全培养液。H2O2组继续培养48 h,M3K干预各组分别加入10、50、100 mg/L的M3K培养48 h。实验过程中对照组仅进行同步换液处理。在倒置显微镜下观察,每孔随机选择3个区域进行镜下计数。收集细胞进行后续实验,所有实验均重复3次。
1.2.2 Western blot检测P21和P53蛋白表达细胞中加入RIPA裂解液提取细胞蛋白,用BCA试剂盒检测蛋白浓度。每孔加入等量的蛋白进行SDS-PAGE电泳,待溴酚蓝到达分离胶底部时转移到PVDF膜上(200 mA,90 min),用含30 g/L BSA和50 g/L脱脂奶粉的TBST溶液室温封闭2 h,加入P21和P53一抗(均1∶1 000稀释),于4 ℃摇床孵育过夜;洗脱后分别加二抗(均1∶5 000稀释)室温孵育2 h,用ECL发光液显影,凝胶成像系统成像后采用Quantity One软件分析条带灰度值。
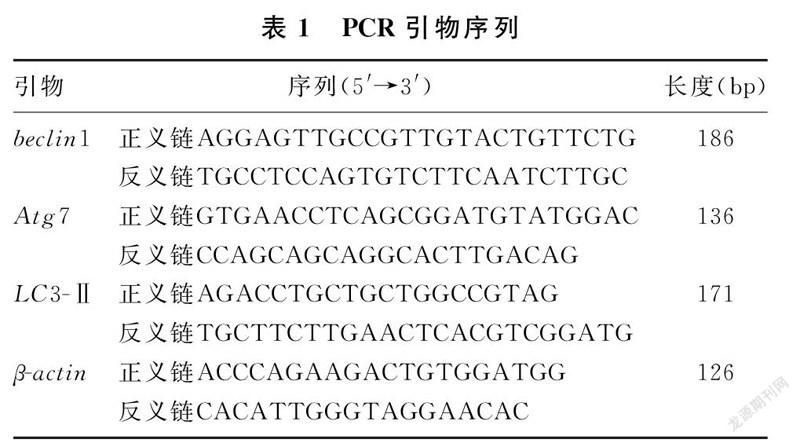
1.2.3 RT-PCR检测beclin1、Atg7和LC3-Ⅱ的mRNA表达 采用Trizol法提取细胞总RNA,Nanodrop测定RNA含量和浓度,应用逆转录试剂盒将mRNA逆转录为cDNA。采用RT-PCR法进行扩增,扩增条件:95 ℃、600 s;95 ℃、10 s,60 ℃、10 s,72 ℃、15 s,共40个循环;95 ℃、10 s,65 ℃、60 s,97 ℃、1 s。以β-actin作为内参,用2-ΔΔCt计算目的基因相对表达量。PCR引物序列见表1。
1.3 统计学方法
应用SPSS 21.0软件进行统计学分析,计量数据以±s表示,多组比较采用One-way ANOVA檢验,组间两两比较采用LSD法。
2 结 果
2.1 各组心肌细胞数量比较
培养48 h后,对照组细胞数量较多,融合度达90%;H2O2组细胞数量与对照组相比明显减少;M3K干预各组细胞数量与H2O2组相比呈剂量依赖性增加,差异均有统计学意义(F=8.685,P<0.05);H2O2+M3K高剂量组细胞数量与对照组相比差异无显著性(P>0.05)。见表2。
4期牟婕,等. 甘露糖醛酸寡糖对H2O2诱导衰老心肌细胞的影响413
2.2 各组心肌细胞P21和P53蛋白表达比较
与对照组相比,H2O2组心肌细胞P21和P53蛋白表达显著增加;与H2O2组相比较,M3K干预48 h后,H2O2+M3K低剂量组、H2O2+M3K中剂量组和H2O2+M3K高剂量组心肌细胞P21和P53蛋白表达随M3K浓度的增加而降低,差异均有显著性(F=31.507、13.111,P<0.05)。见图1、表2。
2.3 各组细胞beclin1、Atg7和LC3-Ⅱ mRNA表达比较
与对照组相比,H2O2组心肌细胞beclin1、Atg7和LC3-Ⅱ mRNA表达水平显著降低;与H2O2组相比,M3K干预48 h后,H2O2+M3K低剂量组、H2O2+M3K中剂量组和H2O2+M3K高剂量组心肌细胞beclin1、Atg7和LC3-Ⅱ mRNA表达呈浓度依赖性增加,差异均有统计学意义(F=10.134~61.041,P<0.05)。见表3。
3 讨 论
衰老是导致老年人慢性疾病发生发展的重要危险因素,并且是心血管疾病发生的独立危险因素。近年来,随着老龄人口增加,衰老相关疾病给社会带来了巨大的医疗和经济负担。因此,探讨衰老相关机制、延缓衰老成为目前的研究热点[16]。H2O2诱导的氧化损伤可以较好地模拟老年人体内氧化应激,现已被广泛用于衰老模型的建立[17-18]。本实验用H2O2成功诱导了心肌细胞内氧化应激,引起细胞衰老。细胞衰老后表现为细胞周期阻滯,增殖减少。M3K属于海藻寡糖,具有多种生物学活性[11]。本研究结果显示,用M3K干预衰老心肌细胞后,细胞周期阻滞延缓,增殖加快。
P21作为细胞周期蛋白依赖性激酶抑制因子在衰老中起重要作用,它通过与细胞周期蛋白结合,导致细胞周期阻滞,阻断细胞增殖过程。细胞衰老后,P21蛋白表达增加,因此,P21可作为衰老标志物之一[19-20]。本实验采用Western blot方法检测P21蛋白的表达,观察M3K对衰老心肌细胞的影响。结果显示,H2O2诱导心肌细胞衰老后P21表达增加,而用M3K干预衰老细胞48 h可使P21蛋白表达下调,缓解衰老心肌细胞的细胞周期阻滞,进而延缓心肌细胞衰老。P53是氧化应激的关键调控分子之一[21]。P53作为“基因监护人”,可通过调节细胞周期阻滞、细胞凋亡和衰老发挥肿瘤抑制作用[22]。有研究表明,p53基因过表达小鼠可以出现早衰表型[23]。P53的表达与机体衰老呈正相关,机体衰老后,P53表达增加[24-25]。本研究结果显示,P53在H2O2诱导的衰老心肌细胞中表达增加,而M3K干预衰老细胞可使P53蛋白表达下调,表明M3K可能延缓了H2O2诱导的心肌细胞衰老。
自噬可分为巨自噬、微自噬和分子伴侣介导的自噬3种类型,其中巨自噬是最常见的一种,其自噬过程依赖于溶酶体。LC3-Ⅱ、beclin1和Atg7是自噬溶酶体形成的关键蛋白,参与溶酶体膜的形成,自噬被激活后,三者的表达增加[7,26]。机体衰老后自噬调节能力也随之下降,细胞内受损蛋白质和细胞器大量积累,进一步加速衰老[27-28]。已有大量的研究表明,激活自噬可能延缓组织和细胞衰老[29]。为了探讨M3K延缓心肌细胞衰老是否与自噬的激活有关,本研究采用RT-PCR方法检测了自噬相关基因LC3-Ⅱ、beclin1和Atg7 mRNA的表达。结果显示,H2O2诱导的衰老细胞中LC3-Ⅱ、beclin1和Atg7 mRNA表达减少,而给予M3K处理后,细胞中LC3-Ⅱ、beclin1和Atg7 mRNA表达增加,表明M3K延缓心肌细胞衰老可能与自噬激活有关。
综上所述,M3K可以延缓H2O2诱导的H9c2心肌细胞衰老,其作用机制可能与细胞内自噬的激活有关,但其具体的分子机制还需要进一步研究。
[参考文献]
[1] SKIBSKA B, GORACA A. The protective effect of lipoic acid on selected cardiovascular diseases caused by age-related oxidative stress[J]. Oxidative Medicine and Cellular Longevity, 2015,2015:313021.
[2] EZZATI M, OBERMEYER Z, TZOULAKI I, et al. Contributions of risk factors and medical care to cardiovascular mortality trends[J]. Nature Reviews Cardiology, 2015,12(9):508-530.
[3] ALFARAS I, DI GERMANIO C, BERNIER M, et al. Pharmacological strategies to retard cardiovascular aging[J]. Circulation Research, 2016,118(10):1626-1642.
[4] WANG Ziling, CHEN Linbo, QIU Zhu, et al. Ginsenoside Rg1 ameliorates testicular senescence changes in Dgalinduced aging mice via antiinflammatory and antioxidative mechanisms[J]. Molecular Medicine Reports, 2018,17(5):6269-6276.
[5] CARRASCO G E, MORENO M, MORENO C L, et al. Increased Arf/p53 activity in stem cells, aging and cancer[J]. Aging Cell, 2017,16(2):219-225.
[6] JIANG C, LIU G, LUCKHARDT T, et al. Serpine 1 induces alveolar type Ⅱ cell senescence through activating p53-p21-Rb pathway in fibrotic lung disease[J]. Aging Cell, 2017,16(5):1114-1124.
[7] LAPIERRE L R, KUMSTA C, SANDRI M, et al. Transcriptional and epigenetic regulation of autophagy in aging[J]. Autophagy, 2015,11(6):867-880.
[8] KIM S N, KWON H J, AKINDEHIN S, et al. Effects of epigallocatechin-3-gallate on autophagic lipolysis in adipocytes[J]. Nutrients, 2017,9(7):680-694.
[9] SHIRAKABE A, IKEDA Y, SCIARRETTA S A, et al. Aging and autophagy in the heart[J]. Circulation Research, 2016,118(10):1563-1576.
[10] REN Jun, YANG Lifang, ZHU Li, et al. Akt2 ablation prolongs life span and improves myocardial contractile function with adaptive cardiac remodeling:role of Sirt1-mediated autophagy regulation[J]. Aging Cell, 2017,16(5):976-987.
[11] ZHU Benwei, CHEN Meijuan, YIN Heng, et al. Enzymatic hydrolysis of alginate to produce oligosaccharides by a new purified endo-type alginate lyase[J]. Marine Drugs, 2016,14(6):108-119.
[12] GUO Junjie, MA Leilei, SHI Hongtao, et al. Alginate oligosaccharide prevents acute doxorubicin cardiotoxicity by suppressing oxidative stress and endoplasmic reticulum-mediated apoptosis[J]. Marine Drugs, 2016,14(12):231-244.
[13] Guo Junjie, XU Fengqiang, LI Yonghong, et al. Alginate oligosaccharide alleviates myocardial reperfusion injury by inhibiting nitrative and oxidative stress and endoplasmic reticulum stress-mediated apoptosis[J]. Drug Des Devel Ther, 2017,11:2387-2397.
[14] ZHU Wei, WU Yan, MENG Yifang, et al. Effect of curcumin on aging retinal pigment epithelial cells[J]. Drug Des Devel Ther, 2015,9:5337-5344.
[15] CHEUNG T M, GANATRA M P, FU J J, et al. The effect of stress-induced senescence on aging human cord blood-derived endothelial cells[J]. Cardiovascular Engineering and Technology, 2013,4(2):220-230.
[16] PERRIDON B W, LEUVENINK H G, HILLEBRANDS J L, et al. The role of hydrogen sulfide in aging and age-related pathologies[J]. Aging, 2016,8(10):2264-2289.
[17] LI Ruilin, LU Zhaoyang, HUANG Jingjuan, et al. SRT1720, a SIRT1 specific activator, protected H2O2-induced senescent endothelium[J]. American Journal of Translational Research, 2016,8(7):2876-2888.
[18] SONG Zhiming, LIU Yong, HAO Baoshun, et al. Ginse-noside Rb1 prevents H2O2-induced HUVEC senescence by stimulating sirtuin-1 pathway[J]. PLoS One, 2014,9(11):e112699.
[19] HSU Y M, YIN M C. EPA or DHA enhanced oxidative stress and aging protein expression in brain of D-galactose treated mice[J]. Biomedicine (Taipei), 2016,6(3):23-30.
[20] JI Musi, SU Xiaohua, LIU Jizhen, et al. Comparison of naturally aging and D-galactose induced aging model in beagle dogs[J]. Experimental and Therapeutic Medicine, 2017,14(6):5881-5888.
[21] GAMBINO V, DE MICHELE G, VENEZIA O A, et al. Oxidative stress activates a specific p53 transcriptional response that regulates cellular senescence and aging[J]. Aging Cell, 2013,12(3):435-445.
[22] QIAN Yingjuan, CHEN Xinbin. Senescence regulation by the p53 protein family[J]. Methods Mol Biol, 2013,965:37-61.
[23] GUEST I, ILIC Z, SCRABLE H, et al. Survival of irradiated recipient mice after transplantation of bone marrow from young, old and“early aging”mice[J]. Aging, 2015,7(12):1212-1222.
[24] LESSEL D, WU D Y, TRUJILLO C, et al. Dysfunction of the MDM2/p53 axis is linked to premature aging[J]. Journal of Clinical Investigation, 2017,127(10):3598-3608.
[25] BU H, WEDEL S, CAVINATO M, et al. MicroRNA regulation of oxidative stress-induced cellular senescence[J]. Oxid Med Cell Longev, 2017,2017:1-12.
[26] WU Yingying, CHEN Chao, YU Xiao, et al. Effects of tiaozhi granule on regulation of autophagy levels in HUVECs[J]. Evidence-Based Complementary and Alternative Medicine, 2018,2018:1-10.
[27] GHOSH A K, MAU T, O’BRIEN M, et al. Impaired autophagy activity is linked to elevated ER-stress and inflammation in aging adipose tissue[J]. Aging, 2016,8(10):2525-2537.
[28] CARAMES B, OLMER M, KIOSSES W B, et al. The relationship of autophagy defects to cartilage damage during joint aging in a mouse model[J]. Arthritis Rheumatol, 2015,67(6):1568-1576.
[29] PAPP D, KOVACS T, BILLES V, et al. AUTEN-67, an autophagy-enhancing drug candidate with potent antiaging and neuroprotective effects[J]. Autophagy, 2016,12(2):273-286.
(本文編辑 马伟平)
