Review on structural control and modification of graphene oxide-based membranes in water treatment:From separation performance to robust operation☆
2019-08-19NingZhangWenxuQiLiliHuangEnJiangJunjiangBaoXiaopengZhangBaigangAnGaohongHe
Ning Zhang,Wenxu Qi,Lili Huang,En Jiang,Junjiang Bao,Xiaopeng Zhang,Baigang An*,Gaohong He,*
1 State Key Laboratory of Fine Chemicals,School of Petroleum and Chemical Engineering,Dalian University of Technology,Panjin 124221,China
2 School of Chemical Engineering,University of Science and Technology Liaoning,Anshan 114051,China
Keywords:Membranes Graphene oxide Water flux Selectivity Stability Fouling
A B S T R A C T Membrane separation has become an important technology to deal with the global water crisis.The polymerbased membrane technology is currently in the forefront of water purification and desalination but is plagued with some bottlenecks.Laminated graphene oxide(GO)membranes exhibit excellent advantages in water purification and desalination due to the single atomic layer structure,hydrophilic property,rich oxygen-containing groups for modification, mechanical and chemical robust, anti-fouling properties, facile and large-scale production,etc.Thus the GO-based membrane technology is believed to offer huge opportunities for efficient and practical water treatment.This review systematically summarizes the current progress on the water flux and selectivity intensification,stability improvement,anti-fouling and anti-biofouling ability enhancement by structural control and modification.To improve the performance of the laminated GO membrane,interlayer spacing tunability and surface modification are mainly used to enhance its water flux and selectivity. It is found that the stability and biofouling also block the service life of the GO membrane.The crosslinking method is found to effectively solve the stability of GO membrane in aqueous environment.Introducing nanoparticles is a widely used method to improve the membrane biofouling ability. Overall, we believe that this review could provide benefit to researchers in the area of GO-based membrane technology for water treatment.
1.Introduction
The increasing population growth,urbanization and industrialization have led to the growing water consumption and uncontrollable wastewater discharge[1,2].In order to address the global water crisis,membranes for water purification and desalination are becoming more and more popular due to the its advantages of low energy consumption,low investment cost,ease of operation and possibility for continuous operation[3,4].High selectivity and permeability are the key properties determining the membrane performance. A tremendous amount of effort has been paid to develop new membranes with better selectivity and improved permeability in recent years[5].
The commonly used separation membranes for water treatment can be classified into organic,inorganic and organic/inorganic membranes.Undoubtedly, organic or polymeric membranes are widely used in desalination and wastewater treatment due to their excellent costeffective and defect-free production.Inorganic membranes have excellent anti-pollution ability,high tunability and reusability,and good chemical stability [6,7]. The organic/inorganic membranes combine the unique properties of both polymeric matrix and inorganic filters,making a great progress in dealing with the critical issue of trade-off relationship between permeability and electivity as well as membrane fouling and scaling[8,9].One of the most remarkable achievements is the development of mixed matrix membranes(MMMs)and thin film composite(TFC)membranes[10,11].
The recent rise of nanotechnology has opened up a new way to facilitate the incorporation of nanomaterials into the field of membrane fabrication, particularly with two-dimensional (2D) inorganic nanomaterials[12,13].The 2D inorganic material has a single atomic layer thickness and it is easy to form a membrane.The laminated 2D material can form a separation film of several-atom thickness,and the stack can form micro/nano-scale channel for molecular permeation.Furthermore,these channels provide a critical energy barrier for repelling the solute [14]. The separation mechanism can be explained by the size screening mechanism, the diameter of the solute is larger than the pore size of the membrane, and the diameter of the water molecule is smaller than the pore size of the membrane, thereby achieving the separation effect[15].It is possible to prepare a separation membrane with excellent performance of water flux and rejection.The 2D inorganic nanomaterials can be produced by several approaches,which are divided into two categories [16]:top-down methods such as micromechanical cleavage [17], mechanical force-assisted liquid exfoliation[18],ion intercalation-assisted liquid exfoliation[19],ion exchange-assisted liquid exfoliation [20], oxidation-assisted liquid exfoliation[21],selective etching-assisted liquid exfoliation[22],and bottom-up methods such as chemical vapor deposition [23], wetchemical syntheses[24].
Graphene holds immense potential to act as the new generation of reverse osmosis(RO)membrane due to its thinner,more mechanically and chemically robust and more ion-selective properties than the commonly used polyamide(PA)active layers of the TFC membranes[25].Nanoporous graphene material has been reported to possess high flux and excellent ion/molecule-sieving properties [26]. Subnanometer pores could be created on the graphene lattice by several methods including oxidative etching[27],hydrogen plasma[28]and ion bombardment[29]etc.Albeit the achievements of subnanopores with desired sizes,there remain challenging tasks on how to control the pore size distribution and synthesize large-area single-atom nanoporous graphene membranes. Compared with the nanoporous graphene, laminated graphene oxide(GO)membrane exhibits greater potential in separation engineering due to its facile and large-scale production via layer-bylayer assembly method[30,31].
As a derivative of graphene, GO is a typical inorganic 2D nanomaterial and has been widely used in the field of membrane separation,such as water treatment,gas separation,solvent dehydration,etc.[32-34] In addition to the general characteristics of inorganic 2D nanomaterials, GO contains a large number of hydroxyl and epoxy groups on the basal plane and carboxyl groups at the edges [35].These oxygen-containing functional groups are of great benefit to assemble the individual GO nanosheets into the laminated GO membrane by interlayer hydrogen bonding interactions.The adjacent interlayer distance of the stacked GO sheets could be effectively adjusted by different methods.The laminated channels provide passageway to water and block the species larger than the channel size.Apart from the size exclusion mechanism,the oxygen-containing functional groups offer electrostatic interaction with hydrated ions, which enhance the selective permeation of the ions through the membrane.The functional groups also provide a key active site for modification during the process of GO membrane fabrication[36-39].
In this review,we aim to overview the experimental technologies of improving the performance,stability and anti-biofouling ability of the laminated GO membranes.Section 2 focuses on improving the performance of the laminated GO membranes by tuning the size of the lamellar channels.Section 3 reviews the methods of enhancing the stability of the laminated GO membranes in the application of water treatment.Section 4 summarizes the works on improving the anti-fouling and anti-biofouling abilities of the GO membranes to bacterium,microorganism and fouling. Finally, we make a summary and outlook of the progress in developing the laminated GO membranes for water treatment.
2.Structure Tunability and Surface Modification to Enhance Permeability and Selectivity
Permeability and selectivity are two key parameters to evaluate the performance of the laminated GO membranes in the application of desalination. The size exclusion mechanism dominates the water flux and ion rejection of the membrane.The spacing between adjacent GO layers and the surface properties of the GO nanosheets are two dominant factors determining the permeability and selectivity of the GO membranes.Therefore,a vast number of investigations on tuning the GO interlayer spacings and the surface properties of the GO nanosheets have been carried out to improve the separation performance of the laminated GO membranes. Table 1 provides the performance of the GO-based membranes modified by different methods.
2.1.Composite structure by intercalation
The methods of intercalating substance into the GO interlayer space have been widely used to tune the spacing of adjacent GO layers[40].A few hydrophilic nanoparticles(NPs)such as TiO2,SiO2and Si3N4were intercalated into the stacked GO laminates.Intercalating the NPs with different sizes could achieve the stacked GO laminates with different interlayer spacings,as shown in Fig.1.Charged NPs were also used to benefit the rejection of many charged solutes.Thus electrostatic repulsion and physical size sieving could dominate the selectivity of the laminated GO membrane to charged solutes and large molecules.The expanded interlayers by NPs improve the water permeability through the membrane. Especially, the membrane intercalated with TiO2NPs shows higher water flux(137.7 L·m-2·h-1·MPa-1)than the pristine membrane(76.2 L·m-2·h-1·MPa-1),and succeeds in gaining high organicdye rejection abilities, such as >85% for basic fuchsin (BF), >92% for methylene blue(MB),>99%for methyl orange(MO),and 99.85%for evans blue(EB).

Table 1Separation performance of the GO-based membranes modified by different methods
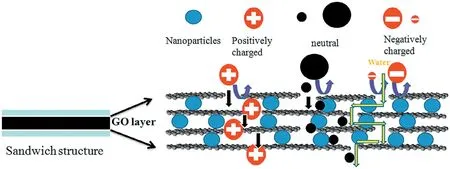
Fig.1.Mechanism of the NPs intercalated GO laminates rejecting large neutral molecules and/or charged solutes[40].
Zhang et al.[41]introduced size-and density-controllable Fe3O4NPs as a kind of intercalant into the interlayers of rGO nanosheets by in-situ synthesis,generating uniformly expanded nanochannels and ordered laminar structures.The improved GO membrane exhibited ultrahigh water permeability of 2960.3 L·m-2·h-1·MPa-1,which is two orders of magnitudes higher than the pristine rGO membrane. Meanwhile,the membrane also presented excellent rejection of >95%for the organic dyes(i.e.MB,MO and RhB),and 99.81%of Cu2+,98.59%of Cd2+.Currently,the rejection to the heavy metal ions reached the maximum value.
Metal organic frameworks(MOFs)are a class of functional porous materials consisted of coordination association between metal ions and organic linkers.It was reported that MOFs were intercalated into the GO interlayer spacing via in situ fabrication between metal ions and carboxyl groups on GO plane[42],as shown in Fig.2.The porous MOFs endowed the MOF@GO laminates with low resistance to water permeation.Further research also indicated that the amino modified MOFs could enhance the surface potential of the MOF@GO laminates,facilitating the removal of charged solutes.The membrane exhibits efficient rejection of copper(II)up to ~90%,and maintains a relatively high flux of ~310 L·m-2·h-1·MPa-1[43].
As we know, reduced GO (rGO) nanosheets with low oxygencontaining functional groups exhibit thinner thickness than GO nanosheets. Accordingly, intercalating the thinner rGO sheets into the stacked GO membrane could lower its interlayer spacing[48].Previous report showed that orderly lamellar nanostructure of GO sheets doped with partially reduced GO(prGO)sheets lowered the interlayer spacing and stabilized the composite GO membrane. Fig. 3 presents the comparison of the structure and stability of the GO laminates and GO/prGO laminates.It was found that the prGO nanosheets have strong π-π attraction interaction with the GO nanosheets,thus weakens the swelling of the laminated GO membrane.
Oxide carbon nanotubes(OCNTs)were also used to enlarge the GO interlayer spacing via layer-by-layer self-assembly techniques[44,45,49,50](Fig.4).The intercalated OCNTs provide stable supporting points and help to construct the interconnected water channels through the GO membrane.The OCNTs in the alternate sandwich structure also provide additional routes for rapid water transport[51],and enhance the selectivity of the GO membrane.Furthermore,the oxygen-containing functional groups of the OCNTs produce extra Donnan effect on ions and dyes,which enhances the membrane selectivity.As the content of OCNT increased, pure water flux increased from 12 to 113 L·m-2·h-1·MPa-1,and the Na2SO4rejection rate increased from 58.8% to 80.0% and the NaCl rejection rate increased from 12.1% to 35.3%.As a result,suitable intercalation of OCNTs could simultaneously increase the permeability and selectivity of the composite GO membranes,which can be achieved by controlling proper concentration of OCNTs in the GO suspension during the membrane fabrication.

Fig.2.Schematic illustration of the structure and separation mechanism of the MOF@GO membrane[42].

Fig.3.Comparisons of the structure and stability of the common GO and the proposed GO/prGO membranes[48].
Recently,the GO interlayer spacings were successfully adjusted and stabilized by intercalating cations into the GO interlayers[52].The laminated GO membrane was immersed in the saline solution,and then the cations were adsorbed into the GO interlayers.The successful intercalation of the cations was confirmed by molecular dynamics(MD)simulation and density functional theory(DFT)calculation.On one hand,it was found by MD simulation that the hydrated cations could bind the GO sheets through a stable hydrogen-bond network. On the other hand, DFT calculations indicated that the region where the oxidized groups and the aromatic rings coexist provided the most stable site for adsorbing the cations.Therefore,the concentration of the saline solution determines the interlayer spacings of the cation-intercalated GO film.The GO interlayer spacings were fixed by the strong noncovalent interaction including the cation-π interaction between the hydrated cations and the basal plane of the GO nanosheets,as well as the interaction between the hydrated cations and the oxygen-containing groups on the GO sheet.

Fig.4.Illustration of simultaneous improvements in permeability and selectivity by intercalating OCNT into RGO membrane[44].
2.2.Graft by functional groups
Sulfuric acid was adopted to graft sulfonate group with high hydrophilicity on the surface of GO sheets[53,54].The sulfonated GO nanosheets are endowed with enhanced hydrophilicity.The sulfonic groups on the GO surface have strong hydrogen bonding interaction with water,thus producing a layer of water molecules on the membrane surface. It was claimed that reasonable sulfonation could improve the water permeability of the laminated GO membranes, and the water permeability increases with the increasing the sulfonation degree.Nevertheless,excessive sulfonation has negative impact on the improvement of water permeation through the laminated GO membranes.
To improve the surface property of the GO nanosheets,Kochameshki et al. [46] grafted hydrophilic/charged polymer chains on the GO planes. It was reported that 2-bromopropionyl bromide (BPB)was first anchored to the GO surface for linking the chain transfer agent (i.e. O-ethyl xanthate). The linked O-ethyl xanthate reacted with Azobisisobutyronitrile (AIBN) to produce Poly(diallyldimethylammonium chloride) (PDADMAC) via the control living radical polymerization(i.e.the reversible addition fragmentation chain transfer polymerization(RAFT)technique).Fig.5 shows a schematic illustration of the procedure of grafting PDADMAC on the GO surface (GO-PDADMAC) by the RAFT technique. It was found both the porosity and hydrophilicity of the GO film were improved. The GO membrane showed high rejection capabilities for various heavy metal ions,such as Cu2+for 86.68%and Cd2+for 88.68%.
Similarly,Zhu et al.[47]also grafted the poly(sulfobetaine methacrylate) (PSBMA) chains with zwitterionic property on the GO surface by the reverse atom transfer radical polymerization (RATRP) technique. The hydrophilic groups of the grafted zwitterionic PSBMA chains endow the laminated GO membrane with simultaneously enhanced permeability and selectivity. It was showed that the charge distribution of the zwitterionic polymers dominates the membrane selectivity to the ions of specific valence. The permeability increased from 64.4 to 119.8 L·m-2·h-1·MPa-1. Besides, the membranes also showed high rejection to Reactive Black 5 (99.2%) and Reactive Red 49 (97.2%), but relatively low rejection to bivalent salts (10% for Na2SO4).
3.Improvement in Laminated GO Membrane Stability
There exist a large amount of oxygen-containing functional groups on the surface of the GO nanosheets.These functional groups endow the GO sheets with good hydrophilicity.If the layer-by-layer stacked GO membrane is used to purify the aqueous system, the membrane will inevitably swell and the GO sheets will successively detach from the membrane surface.Consequently,long-term stability limits the applications of the laminated GO membrane in water treatment.To solve this problem,the methods of cross-linking and physical confinement have been proven to be effective in the improvement of the stability of the laminated GO membranes.Meanwhile,the interlayer spacing is related with the physical confinement reagents and the size of the crosslinkers.For example,the introduced crosslinkers would decrease the permeation of water and ions across the membrane[55-57].
3.1.Stability enhancement by cross-linking methods
The amine-containing molecules can react with the oxygencontaining functional groups (especially the epoxy groups and carboxyl)of the GO sheets via the acylation affinity addition reaction.The amine groups(--NH2)interact with the epoxy group of GO by nucleophilic reaction,in which the epoxy ring opens and forms the C--N bond with the crosslinker and an additional C--OH bond;meanwhile,the amine groups (--NH2) could also interact with the carboxyl groups (O=C--OH) of GO to form an amide bond (O=C--NH), as shown in Fig. 6. Furthermore, the crosslinker could also anchor the laminated GO film to the support membrane. Diamine and amide have been widely used to crosslink the laminar GO nanosheets supported by organic microporous membrane. For example,ethanediamine (EDA) was used not only to crosslink the laminated GO layers but also to fix the crosslinked GO film on the BPPO membrane[58].After immersing the pristine and crosslinked GO/BPPO membranes in DI water for one month,the crosslinked membrane showed robust performance for desalination,but the salt rejection of the tested pristine GO membrane decreased by half.

Fig.5.The RAFT procedure of grafting PDADMAC on the GO surface(GO-PDAD MAC)[46].
As we know,urea contains two amine groups,thus can be also used to crosslink the laminated GO layers via the C--N bonds formed by the reaction between the amine groups of urea and the oxygen-containing functional groups of GO.Zhang et al.[59]employed urea to crosslink the GO film supported by the cellulose acetate (CA) membrane. It was found that nucleophilic addition reaction occurred between the carboxyl or epoxide groups of the GO film and the acyl groups of the CA membrane surface.The crosslinked GO film maintained good structural stability despite it was rinsed in water for several times.Besides,some other diamine-type molecules,such as propylene diamine(PDA)[56],o-phenylenediamine (ODA) [56], hexamethylene diamine (HMDA)[56],butane diamine(BDA)[57],p-phenylenediamine(PPD)[57],mphenylenediamine(MPD)[60],1,4-cyclohyxanediamine(CDA)[61]and p-phenylenediamine (pPDA) [61], were also used as the crosslinkers to improve the stability of the GO composite membranes[56,57,60-62].
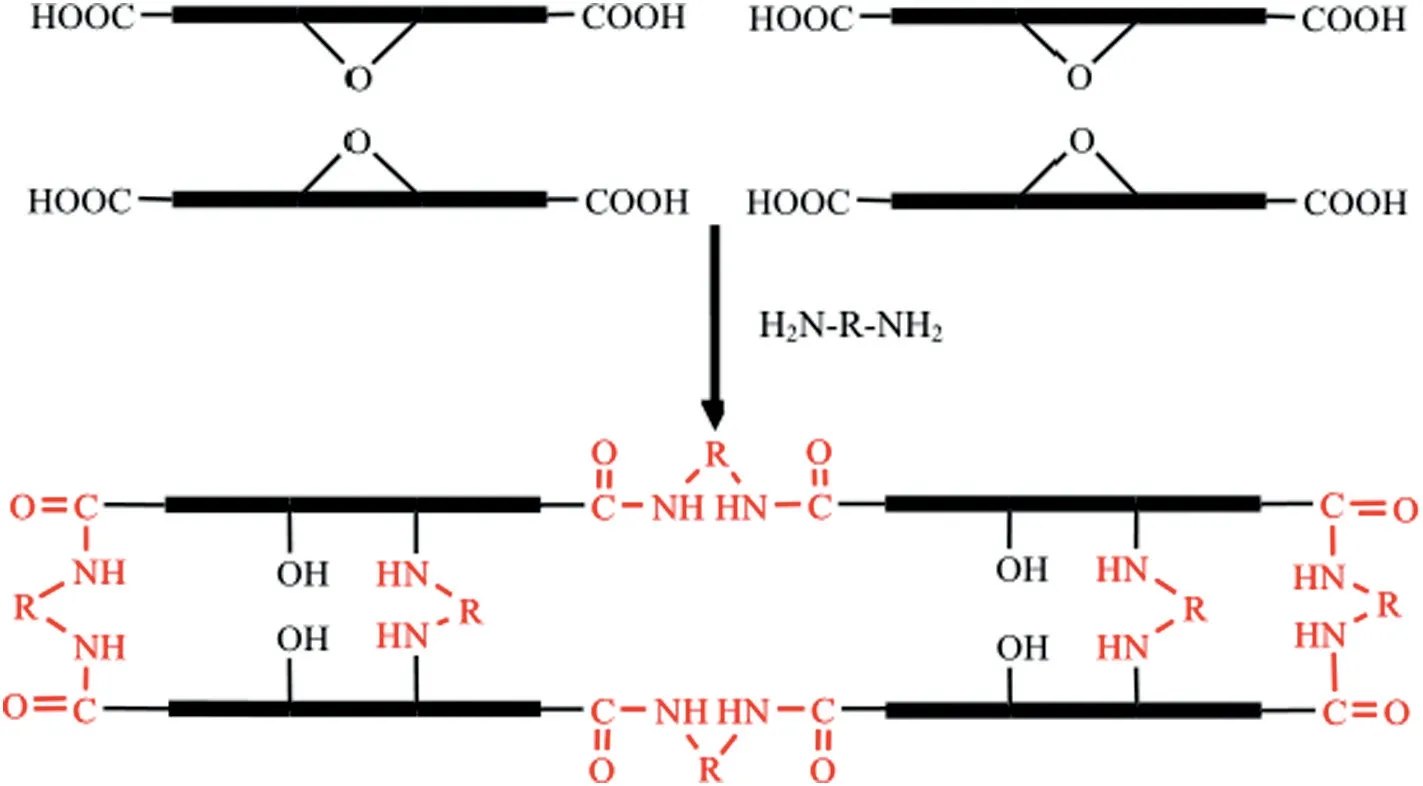
Fig.6.Reaction mechanism of the catalytic amidation cross-linking between GO nanosheets[56].
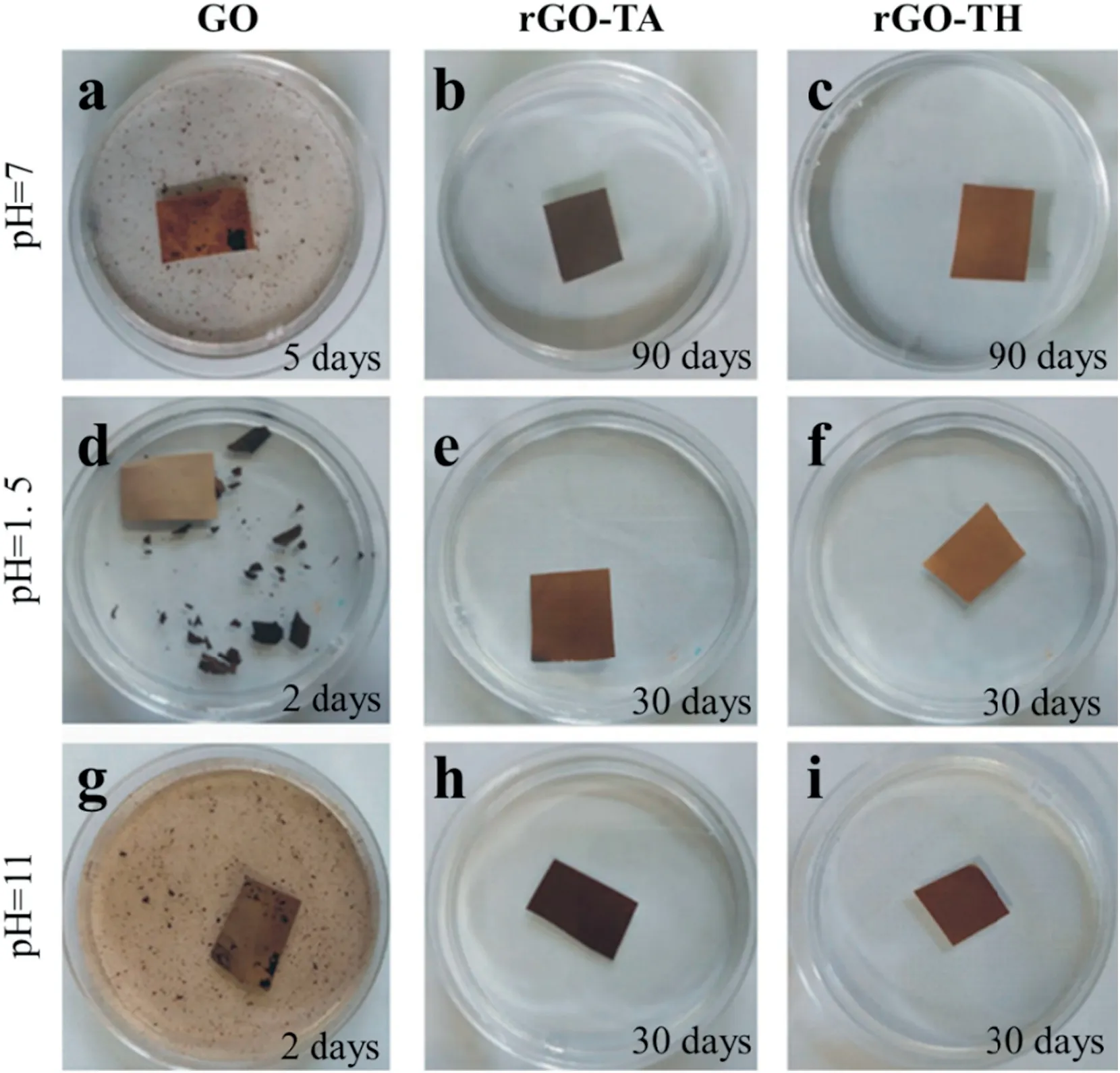
Fig.7.Stability tests of the pristine and crosslinked GO membranes:(a-c)water(pH=7),(d-f)acid solution(pH=1.5),and(g-i)alkaline solution(pH=11)[63].
Amino acid is a species of molecules with several hydroxyl,carboxyl and amino groups,thus reaction could occur between amino acid and reduced GO(rGO)nanosheets.Tannic acid(TA)and theanine amino acid(TH)have been successfully adopted to crosslink the laminated GO layers via the reaction between the oxygen-containing functional groups of rGO and the carboxyl groups of TA and TH[63,64].The rGO film was stabilized by the hydrogen bonding interaction between the unreacted functional groups of rGO and the crosslinkers. The crosslinked rGO film showed intact structure after soaking in water for 90 days.In addition,the GO membranes were strongly robust after immersing in the acid(pH=1.5)and alkaline(pH=11)solutions for 30 days,as shown in Fig.7.
Sarkar et al.[65]employed glutaraldehyde to crosslink the GO film supported by the methyl cellulose membrane.The two aldehyde groups of glutaraldehyde react with the hydroxyl groups of the GO nanosheets and the supporting membrane, respectively. It was found that crosslinking could greatly enhance the stability and mechanical strength of the GO membrane.TGA showed that as the crosslinking degree was enhanced,the degradation temperature of the as-fabricated GO membrane was elevated from 310 to 400°C for 10% weight loss.The tensile strength and Young's modulus of the membrane are also increased by enhancing the crosslinking degree.
Besides, poly(vinyl alcohol) (PVA) was used as the crosslinker to connect the laminated GO layers via the acetylation reaction[67].The PVA-crosslinked GO membrane exhibited. Isophorone diisocyanate(IPDI) was also used as a crosslinker due to its two isocyanates(N--C=O),which could react with the epoxy group of the GO nanosheets [68]. The IPDI-crosslinked GO membrane maintains stable water permeability and salt rejection after shaking in water for 48 h.Similarly, toluene 2,4-diisocyanate (TDI) with two isocyanates was found to stably crosslink the laminated GO layers[69].
Recently,Xia et al.[66]fabricated the GO-doped TiO2gel with crosslinking structure. The membrane showed integrated and crack-free layer with smooth surface and without concave pores. Fig. 8 shows the procedure of fabricating the crosslinked GO-doped TiO2gel. The GO nanosheets were first crosslinked by the Ti--O--C structure with the hydrolyzed titanium alkoxide precursor in an alkaline-catalyzed process.Subsequently,the triblock copolymer of L64(PEG-PPG-PEG)was introduced to produce a stable crown ether-type GO-doped TiO2sol. The as-fabricated sol was transformed to a gel with crosslinked structure. The dynamic mechanical analysis showed that the doped GO nanosheets obviously improved the storage modulus and loss modulus of the membrane.Meanwhile,the GO-TiO2membrane still exhibited the water permeability of 283.2 L·m-2·h-1·MPa-1.
3.2.Stability enhancement by physical-constraint methods
The structure of the laminated GO layers has been strongly stabilized with physical confinement methods[55].Most investigations focused on the in-situ polymerization of polymeric monomers throughout the GO laminates to achieve the physical-constraint,referred to as confined interfacial polymerization.It was also reported that the interlayer spacing could be controlled by spatial confinement method.
To achieve the confined interfacial polymerization inside the GO film,the polymer monomers were firstly well dispersed in the film by forcing their precursor solution to permeate through the laminated GO layers. Subsequently, the trapped monomers reacted with each other by the interfacial polymerization of free radical to produce a continuous polymer network through the GO film.Accordingly,the interconnected polymer could restrict the laminated GO layers from swelling or disintegrating in the process of water treatment[70-73].For example,MPD and TMC monomers were successfully polymerized to form interconnected PA polymer through the laminated GO film[70],as shown in Fig.9.It was found that the permeability and selectivity of the as-fabricated GO-PA composite membrane remained stable after immersing in the aqueous NaClO solution for 24 h.Meanwhile, the membrane still exhibited fast water transport of 30 L·m-2·h-1·MPa-1and high NaCl rejection of 99.7%.Calcium alginate and polyvinyl alcohol(PVA)were also employed to synthesize a highly crosslinked double-network hydrogels through the laminated GO layers,where the physical constraint and the intermolecular hydrogen bonding interaction could constraint the structure of the GO film[74].The GO/double-network composite hydrogel exhibited low swelling ratio even in the aqueous environment.As a result,the confined interfacial polymerization technique could stabilize the intrinsic structure of the laminated GO film.
Abraham et al.[55]embedded the stacked GO film in the epoxy to physically restrict it from swelling in water.The physical confined GO membranes by the rigid epoxy plate could not swell in water over time,as shown in Fig.10.Comparing with the crosslinked GO membranes, this membrane showed comparable water permeation rate but much higher salt rejection under favorable interlayer spacings.

Fig.8.An illustration of the formation mechanisms of the GO-doped TiO2 gel[66].
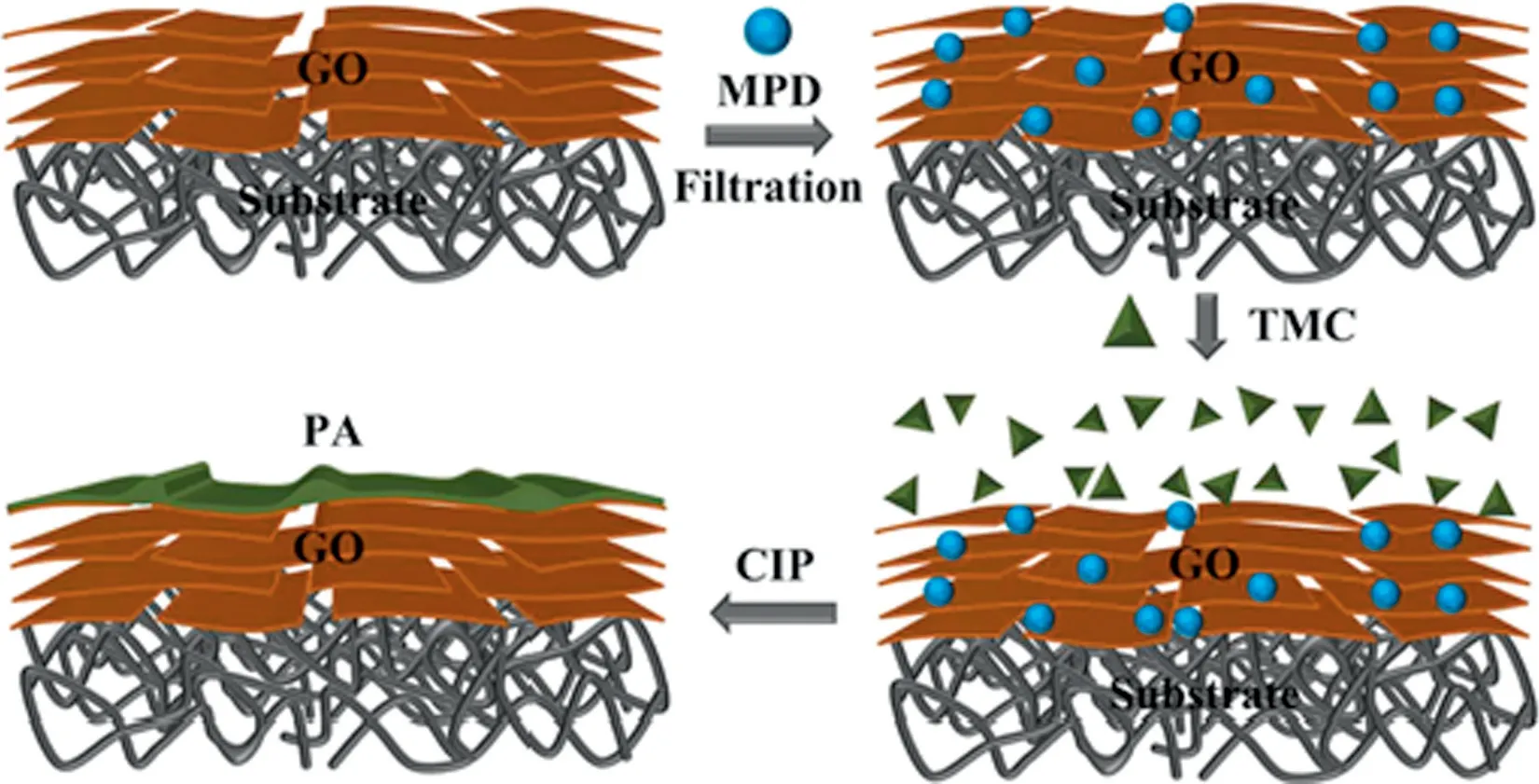
Fig.9.Schematic illustration of the preparation of the PA-GO membrane formation via confined interfacial polymerization[70].
4.Anti-biofouling Enhancement
Membrane fouling and biofouling significantly limit the long-term and extensive applications in separation technology for seawater desalination and wastewater treatment[1].Anti-fouling and anti-biofouling are always great challenges posed to the industrial applications of the membranes.The fouling and biofouling are mainly caused by the accumulation of organic,inorganic and biological substances on the membrane surface. It is widely accepted that biological pollution is still a serious challenge to the membrane for water treatment[75].Irreversible deposition and growth of microbial cells lead to the accumulated microorganisms and produce biofilm on the membrane surface or even inside the membrane matrix [76,77]. As a result, the adhesion and subsequent growth of the microorganisms are the dominating processes of membrane biofouling.In order to recover the permeability of the biocontaminated membrane,it is of significant importance to remove the biofilm from its surface and/or interior.However,due to the strong adhesion of the biofilm to membrane,it is hard to effectively achieve the removal of the accumulated microorganisms from the membrane.GO-based membrane has better antimicrobial than regular polymeric membrane due to its physical and chemical effects on the microorganisms[78].Moreover,surface modification and nanoparticle doping were still employed to further improve the anti-biofouling ability of the GO membranes.

Fig. 10. Physically confined GO membranes with tunable interlayer spacing [55].(a)photograph of a physical confined GO membrane glued into a rectangular slot of a plastic disk with 5 cm diameter;(b)optical micrograph of the membrane cross-section,which shows 100-μm-thick GO laminates (black) embedded in epoxy (light yellow);(c)SEM image of the red-marked region in(b).
4.1.Doping with Ag nanoparticles
Considering the low effect of the anti-biofouling enhancement on human health,silver nanoparticle(AgNP)has been widely used to improve the anti-biofouling ability of the laminated GO membranes.Fig.11 shows a possible synergistic antibacterial mechanism of the Ag nanosystem.The AgNPs were firstly oxidized in order to release Ag+.Meanwhile,the AgNPs could catalyze the reactions of the dissolved oxygen in the solution to produce excess free radical,such as superoxide anion,hydroxyl free radical and hydrogen peroxide.As we know,ROS is a natural byproduct of the bacterial metabolism, but excess ROS could produce oxidative stress and have negative effect on the bacterial cell functions.As a result,the produced excess free radicals played the critical role of reactive oxygen species(ROS)[79]in inhibiting the formation and growth of bacteria.Highly reactive Ag+and ROS can also destroy the functions of bacterial cells[80].For example,Ag+and ROS attack the cytomembrane of bacteria and disturb its transmembrane transport,attack respiratory enzyme and suppress the production of adenosine triphosphate(ATP),attack protein and damage its physiological functions,attack mRNA and perturb its transcription,attack DNA and impede its replication,etc.[81].As a result,Ag NPs could be introduced into the GO interlayers to enhance the antimicrobial ability of the GO membranes.
Pounraj et al.[82]doped AgNPs into the mixed matrix of chitosan(Ch) and GO nanosheets to fabricate a hybrid nanocomposite film (GOns-Ch-AgNPs) with excellent antimicrobial properties. The GOns-Ch-AgNPs nanocomposite membrane could inhibit the formation of bacterial biofilm, such as the Escherichia coli (E. coli) and Bacillus subtilis films. It was found that the excellent anti-biofouling ability mainly resulted from the interactions between GO and bacteria(such as anti-adhesion,shape puncture through the cytomembrane,and oxidation of cellular components)as well as the electrostatic interaction between the bacterial cytomembrane and the nanoparticles or chitosan.As shown in Fig.12,compared with the control image clearly,biofilm could not be observed on the surface of the four nanocomposite films.

Fig.11.Schematic of silver nanoparticle interaction with bacterial cells[75].
Furthermore,GO functionalized with Ag(Ag-GO)was adopted as the surface coating to enhance the anti-biofouling ability of the laminated GO layers[83].The Ag-GO coating layer was stably supported on the GO membrane surface. The anchored AgNPs could lower the surface adhesion to bacteria and inactivate the adhered bacteria.Besides, the Ag-GO coating endowed the GO membrane with more smooth and negative zeta potential surface than the pristine GO-stack membrane.

Fig.12.FESEM images of E.coli bacteria attached on(A)control surface,(B)GOns-Ch film,(C)GOns-AgNP film,(D)Ch-AgNP film and(E)GOns-Ch-AgNP film[82].
Shuai et al.[81]also intercalated AgNPs into the interlayers of the laminated GO layered, and achieved a layer-by-layer structure with GO nanosheets and AgNPs supporting to each other,as shown in Fig.13.As the bacteria approaches to the surface of the GO-Ag membrane,it will suffer from cellular structure destruction and function damage.It was found that the antibacterial property mainly resulted from the physical damage of the shape puncture of the sharp GO edges into the bacterial cytomembrane and the chemical attack by Ag+and ROS.
Faria et al.[84]synthesized a bacteriostatic layer of AgNPs on the surface of TFC membrane by in-situ nucleation and growth of AgNPs on the top GO layer.The dynamic biofouling tests showed that the in-situ produced GO-Ag layer exhibited good anti-biofouling ability.During the tests,a few dead bacteria were observed on the far side of the biofilm from the GO membrane surface. It indicates that the improved membrane could kill the bacteria in indirect contact with the membrane surface.This is due to the fact that the released Ag+ions could diffuse to the surrounding area and thus endows the modified GO membrane with non-contact antimicrobial ability.
4.2.Doping with oxide nanoparticles
Kim et al.[85]found that GO-MoS2nanocomposite possessed the bacteriostatic characteristic to E. coli. The improved GO membrane showed that bacterial cells were inactivated and lost their viabilities,as shown in Fig.14.The refractive index(RI)distribution of each voxel in the bacterial cell was detected by holotomographic(HT)microscopy.It indicated that some bacterial cells near GO-MoS2were apoptotic after 90 min.Besides,it was also found that GO-MoS2could produce the ROS(i.e. superoxide anion, O2•-), which has the oxidative stress on the bacterial intracellular components such as proteins, phospholipids,and nucleic acids. The oxidation of the intracellular components leads to the damage of the bacterial cytomembrane. Accordingly,the bacteria with severely damaged cytomembranes then lose activity.Fig. 14 shows a collection of bacterial cells were killed after 3 h for incubation.
ZnO has excellent properties of hydrophilicity,antibacterial,anticorrosion,thermal and mechanical stability,thus it is a good additive material to improve the anti-fouling and anti-biofouling ability of membrane[86].However,the nano-sized ZnO particles(ZnONPs)are apt to aggregate together;thus they cannot be well dispersed in the polymer matrix.Recently, Chung et al. [87] prepared ZnO-GO nanohybrid via the sol-gel method to confine the ZnONPs aggregation,which improved the dispersion of ZnONPs in the membrane.Similarly,ZnONPs could generate ROS,which disrupts the bacterial cell integrity.Thus the formation of the bacterial cells could be suppressed by the presence of ZnONPs.
4.3.Surface modification by chitosan
GO composite nanosheets were also prepared by surface modification of with chitosan[88-91].Condensation reaction between the carboxyl group of GO and the amine group of chitosan occurs to form the amide covalent bond, which stabilizes the hydrophilicity of the GO membrane surface.Fig.15 depicts the schematic process of synthesizing the GO nanosheets modified by chitosan.The grafted chitosan enhances the hydrophilicity and smoothness of the membrane surface.This is the key to improve the anti-biofouling property of the GO membrane.Furthermore,the positively charged--NH2of the chitosan chains could have electrostatic interaction with the anionic groups of the microbial cell membrane. The electrostatic interaction weakens the cytomembrane permeability and even the destruct the cytomembrane,resulting in apoptosis of the microbial cells.
5.Conclusions

Fig.14.The structure and the antibacterial ability of GO-MoS2 nanocomposite[85].
Graphene oxide is a novel material with many advantages that enable the laminated GO membrane with excellent properties for water purification and desalination. A fundamental understanding of the structure-property relationships is critical to effectively improve the performances, stability and anti-fouling of the laminated GO membranes.The single-atom-thick layer structure and the hydrophilic property endow the laminated GO membrane with low flow resistance.The molecular size exclusion effect and Donnan effect dominate the ions or molecular selectivity of the GO membrane.Intercalation of ions or nanoparticles with various sizes could achieve tunable nanochannels within the laminated GO membranes,which provides a versatile approach for controlling the water permeability and the molecular size exclusion effect.Surface modifications of the GO nanosheets have been employed to well adjust the Donnan effect on the membrane selectivity.The rich oxygen-containing groups benefit the modification for adjusting the GO interlayer spacing and membrane stability.Accordingly,crosslinking technology becomes the most popular method to stabilize the laminated GO layers.Specifically,the mechanical and chemical robust and the bacteriostatic properties guarantee the long-term operation of the membrane in water treatment. Nevertheless, a few investigations were also carried out to further enhance the anti-biofouling ability of the laminated GO membranes.At present,introducing silver nanoparticles is the most widely used method to inhibit the formation of bacterial biofilm on the GO membranes.Some other inorganic materials such as MoS2and ZnO also exhibit antibacterial property and contribute to enhancing the anti-biofouling ability of the GO membranes.From the perspective of industrial application,the facile and large-scale production enables the laminated GO membrane to become the next-generation membrane for efficiently solving the global water crisis.Although tremendous progresses have been made to push the GO-based membrane technology forward,further efforts are still needed to promote its performances and robust operation capability.

Fig.15.The synthesis process of the composite GO/chitosan nanoplates[88].
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Recent advances in acid-resistant zeolite T membranes for dehydration of organics☆
- Process intensification in vapor-liquid mass transfer:The state-of-the-art☆
- Extractive distillation:Advances in conceptual design,solvent selection,and separation strategies☆
- Beyond graphene oxides:Emerging 2D molecular sieve membranes for efficient separation☆
- A review of internally heat integrated distillation column☆
- Recent progress and future prospects of oil-absorbing materials☆
