Recent advances in acid-resistant zeolite T membranes for dehydration of organics☆
2019-08-19YiweiLuoWaseemRazaJianhuaYangLiangqingLiYingLu
Yiwei Luo,Waseem Raza,Jianhua Yang,*,Liangqing Li,Ying Lu
1 State Key Laboratory of Fine Chemicals,School of Chemical Engineering,Dalian University of Technology,Dalian 116024,China
2 Collaborative Innovation Center of Fine Chemical and Materials Technology,School of Chemistry and Chemical Engineering,Huangshan University,Huangshan 245041,China
Keywords:Dehydration of organics Epitaxial growth Zeolite T membrane Membrane pervaporation
A B S T R A C T Zeolite membranes offer outstanding potentials in separation of many molecular mixtures due to their molecular sieving selectivity and the high thermal and mechanical stability that allow them to operate at harsh conditions.Development of durable and high separation performance membranes with lower fabrication and operation cost are highly demanded for industrial applications. Zeolite T membrane possesses good acid-resistance with excellent hydrophilic properties as compared to NaA zeolite membrane and can be extended to industrial organic dehydrations under an acidic environment.In the present review the research advances in development of zeolite T membranes for the dehydration of organic mixtures in acidic conditions are summarized.Especially the low temperature synthesis,and epitaxial growth of the zeolite membrane with high performance are well addressed,besides emphasis is particularly placed on ensemble synthesis of hollow fiber zeolite T membrane module and its future prospects for industrial separations.©2019 The Chemical Industry and Engineering Society of China,and Chemical Industry Press Co.,Ltd.All rights reserved.
1.Introduction
The dehydration of organic mixtures is essential in many industrial processes including petrochemical,pharmaceutical and biotechnology[1,2]. Distillation as one of the traditional separation technique has been widely used in industrial process; however, it displayed high cost,low efficiency,and secondary pollution,especially for azeotropes and close-boiling mixtures.In current industrialization,energy saving,environmental-friendly, increasing the share of renewable energy,cleaner and energy-efficient separation technologies are important for sustainable development. For this, membrane-based separation demonstrates great power to lower energy consumption and carbon footprint by providing alternatives to many conventional techniques such as distillation,drying and evaporation[2-4].
In many industrial processes such as esterification reaction,acetic acid production and other organic chemicals,dehydration in acidic media is highly demanded because of their usage in commodity chemicals[5,6].For this type of acidic medium dehydrations,membrane based separation process is found very suitable due to ecofriendly nature. Till now, many membrane materials including polymeric,composites,zeolites,and hybrids have been applied for the dehydration of industrial solvents.However,the dehydration of organics under acid condition is often restricted due to low durability under acid condition. Novel membrane materials and synthesis methods must be developed to overcome the problem of stability in acidic mediums.
Zeolite membranes are regarded as promising modules in industrial separations as they exhibit efficient separation abilities due to their uniform structure.The good chemical and thermal stability,adsorption and molecular sieving properties make these materials much suitable for dehydration of the organic solvents with better separation abilities over polymer membranes. Generally, the Si/Al ratio(denoted as Si/Al)of the framework significantly affect the zeolite physichemical properties. As the Si/Al ratio of the framework decreases the hydrophilicity of zeolites increases while the acid stability decreases.Zeolite A membranes with framework Si/Al of 1 and crystalline pore window size of 0.41 nm are good for dehydration of organics and have been commercialized for the dehydration of solvents and biofuels in small-to medium-scale plants[7-9].However,zeolite LTA membranes[10]are found unstable under acidic conditions or with higher content of water because of Al leaching from the zeolite LTA framework. T type zeolite membranes with framework Si/Al of 3-4 possess acid resistance while sustaining relatively high hydrophilicity, therefore the zeolite T membrane can be extended to organic dehydration under an acid environment with pH as low as 3 [11,12]. The MOR membranes with tunable Si/Al larger than 5 and ZSM-5 membrane of Si/Al of 10~∞possess higher acid stability than the lower Si/Al zeolite membrane A and T.Indeed,MOR[13] and ZSM-5 [14] membranes are reported for dehydration of 90wt%acetic acid/10wt%water mixture(pH about 1)where zeolite T membrane cannot compete for this purpose.However,the zeolite T membrane outweigh the MOR and ZSM-5 membrane for dehydration of organics under weak acid condition because of the better separation performance and lower production cost.
In the present review efforts will be solely focused on the research advances and fundamental aspects of the acid resistant zeolite T membranes for the dehydration of organic mixtures. The MOR and ZSM-5 membranes for dehydration of organics in acid media will not be covered. Especially the low temperature synthesis, epitaxial growth and the ensemble synthesis of hollow fiber T zeolite membrane module are focused. We hope this study will provide deep insights for the fabrication of acid resistant T type zeolite membrane for industrial applications.
2. Research Advances in Synthesis of Acid Resistance Zeolite T Membranes for Dehydration of Organics under Acidic Solution
Zeolite T with Si/Al ratio of 3-4 is an intergrowth-type zeolite of erionite and offretite and its effective pore size for permeation is the pore size of erionite (0.36 nm × 0.51 nm) because of the stacking faults of erionite sheets in the framework[15,16].Zeolite T membrane can be extended to dehydration of organics under acidic environment. Great efforts ranging from optimizing the seed size, synthesis temperature and seeding method to support configuration were devoted to development of zeolite T membranes with improved performance for the purpose of practical industrial applications.
2.1.Zeolite T membranes by low temperature synthesis
In the early stage, synthesis of the zeolite T membrane was investigated at relative low temperature of 373 K by secondary hydrothermal growth.Ken-Ichi Okamoto et al.[11]prepared zeolite T membrane with thickness of 20 μm at 373 K for 24-30 h by using different silica sources for the dehydration of acetic acid.This synthesis method opens new doors for the acid resistant membranes.Further efforts were focused on investigation of the seed size,heating way either microwave heating or conventional heating,the seeding method and careful modification of synthesis composition on the formation and separation performance of zeolite T membranes [12,17-22]. Yang et al. [12] synthesized randomly oriented zeolite T membranes with thickness of 5-6 μm using MAHS method(microwave-assisted hydrothermal synthesis),as shown in Fig. 1 in their extended work [17], they reported a & b- oriented zeolite T membrane with thickness of 7 μm from the a&b-oriented T zeolite seed layer by conventional heating for 8 h at 373 K and combined microwave heating for 1 h at 413 K.Both of these zeolite membranes showed excellent stability and separation performance in acidic media.
In our previous work [18],zeolite T membrane with thickness of 7 μm were crystallized at 373 K for 30 h via two varyingtemperature hot-dip coating seeding method using large seeds of 2 μm as filler for modifying the pore size of support and small seeds of 0.4 μm for inducing crystal growth. This new seeding procedure was found flexible and very effective for seed size,coating temperature and seed suspension optimization resulting in smooth and defect free zeolite T membrane. All previously reported zeolite T membranes,synthesized at low temperature of 373 K,showed a flux of 1.23-2.52 kg·m-2·h-1with separation factor of 1301 to >10000 for 90 wt% EtOH/10wt% H2O (a flux of about 1.5 kg·m-2·h-1with separation factor almost 2000) and a higher flux of 1.52-2.52 kg·m-2·h-1with higher separation factor >10000 for 90 wt%IPA/10wt%H2O.
Regarding the acid resistance,the T zeolite membranes were found to possess good hydrothermal stability and acid stability for 10 wt%ethanol/water mixtures to pH ~3 at 338 K up to 140 h[12].Chen et al.[23]also investigated the acid resistance of zeolite T membrane prepared through micro sized seeding strategy.The membrane was applied for dehydration of binary to ternary mixture such as ethanol/water mixtures and ethanol/water/acetic acid mixtures.The excellent separation performance (flux of 2.50 kg·m-2·h-1, separation factor of 13000(water/isopropanol) and proved the good stability of these T type zeolite membranes.
In membrane synthesis process,various factors are involved to control the production economics of the membrane such as synthesis time,calcination temperature and material involved through the process.In addition to separation performance membrane synthesis time is also very important and many researchers added their novel ideas to shorten the synthesis time. The addition of fluoride ions into the membrane synthesis solution and increase in crystallization temperature were used to shorten the synthesis time and improve the flux by Chen et al. [23-25]. The T zeolite membrane prepared at 423 K for 35 h was composed of two layers with about 15 μm intermediate layer and 20 μm C-oriented top layer as shown in Fig.2.This T membrane showed similar separation performance as obtained at low temperature 373 K as listed in Table 1.They reported that the addition of fluoride ions into synthesis gels for the zeolite T membrane could shorten the crystallization time from 35 to 4-6 h at 423 K[24,25].The obtained membrane showed an improved flux on the stainless steel tube of 6.7 kg·m-2·h-1with separation factor larger than 10000 for 90 wt%IPA/H2O mixture at 348 K.
The zeolite T membranes as discussed above showed good separation performance and acid resistance either in binary and ternary mixtures containing acidic media.However,the fluoride media or a long synthesis time or complex synthesis steps are required that are hindering the commercialization of this membrane. Thus, it is essential to further improve the preparation process and separation performance for economic industrialization of these membranes.

Fig.1.Microwave-assisted hydrothermal synthesis of zeolite T membrane[12].
2.2.Zeolite T membrane by epitaxial growth at high temperature
Zeolites T is an intergrowth of offretite (60 wt%-97 wt%) and erionite, in their structure, each erionite cage involves a large pore(12-ring) in c-direction completely blocked by 6-ring channels with opening channel for molecular permeation small to 0.25 nm,which resulted in a“dead-end”effect to the large pore of offretite[30].This effect forces the molecule diffusion through 8-ring channel in the a- or bdirection (perpendicular to c-direction) with pore window size of 0.31 nm,leading to the shape-selectivity.Thus,small amount of erionite intergrown in offretite is expected to key control even the offretite can absorb large molecules into zeolite.Therefore,the preferred orientation for the water molecular(0.276 nm)permeation is a-or b-axis of zeolite T,the a&b-oriented zeolite T membrane are supposed to possess high separation performance.

Fig.2.FE-SEM surface and cross-sectional photographs of various membranes prepared in fluoride media[25].
2.2.1.Epitaxial growth of zeolite T membrane
As mentioned above,Yang et al.[12]obtained a&b-oriented zeolite T membrane from the a&b-oriented T zeolite seed layer by combiningconventional heating of 8 h at 373 K and microwave heating of 1 h at 413 K.Recently,we reported[29]a simple but highly effective strategy through epitaxial growth of the seeds solely by regulating the kinetics of zeolite nucleation and crystal growth as shown in Fig.3.By this method,highly permeable and selective zeolite T membrane can be obtained in short time on various supports.The molar composition of the synthesis solution is 1 SiO2:0.05 Al2O3:0.26 Na2O: 0.09 K2O: 35 H2O, same as Zhou et al.[18].
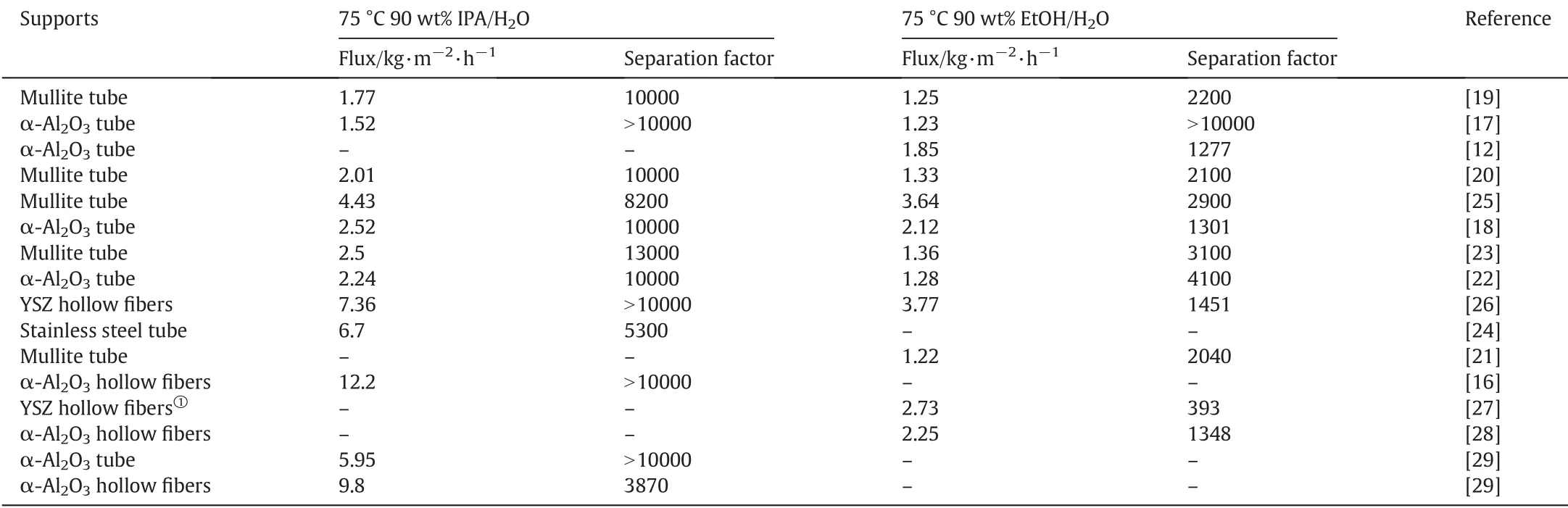
Table 1Pervaporation performance of the T zeolite membranes reported in the literatures

Fig.3.Schematic of a zeolite T membrane by epitaxial growth mechanism[29].
As shown in Fig. 4, a continuous, smooth and pinhole-free a &b-oriented seed layer is significant for a & b oriented membrane growth within the seed layer on both Al2O3support and hollow fibber support. The SEM image of the membrane revealed that a continuous and dense layer composed of well intergrown rod-like crystals was formed on the support surface,which is quite distinct from the rice-like seeds.
Moreover,the membrane possessed a thickness of 3-4 μm,which was similar to the seed layer.It indicates that the seeds grew along a-and b-direction in-plane.The time tracking observations revealed that the membrane was formed by an epitaxial growth of seeds with closing of the voids between the seeds. From Fig. 5, after 1 h of growth,some small particles emerged on the seed crystals.With increasing in crystallization time to 2 h, the seeds became well intergrown, with one end of some rice-like seeds elongating to have an appearance of a rod-like“tail”.At 3 h,the membrane surface was compactly covered by well intergrown rod-like crystals.When the time increased to 4 h, the rod-like crystals elongated further with increased intergrowth.The key element of this growth process is that the membrane thickness remained the same during an initial growth period of 3 h with minor changes in thickness observed at the end of 4 h of growth.Therefore,oriented zeolite T membranes were obtained through high crystallization temperature and controlling of seed growth.
The zeolite T membrane prepared on Al2O3support exhibited high performance with water flux 5.95 kg·m-2·h-1and water selectivity over isopropanol(IPA)larger than 10,000 for the dehydration of 90 wt% IPA aqueous at 348 K, while on hollow fibers showed high flux of 9.80 kg·m-2·h-1and separation factor 3790.Therefore,dense a&boriented zeolite T membranes were formed via epitaxial growth.The high performance and largely shortened membrane growth time 4 h are favorable for urging wide industrial implementation of membrane separation because of the reduced cost.
2.2.2.Crystallization temperature and seeds microstructure effects on epitaxial growth mechanism
The controlling of membrane microstructures including preferential grain orientation[31],the membrane morphology[32],the membrane thickness[33],and grain boundary defects[34]was a key to the formation of a high performance zeolite membrane.Recently,it was noted that among the microstructures,the orientation of zeolite membranes were found to have significant effects on the separation performance,many efforts[17,31-35]have been made to study the orientation of zeolite membranes. We [29] recently synthesized an a & b-oriented zeolite T membranes on the Al2O3hollow fibers and macroporous alumina supports through epitaxial growth mechanism, further,it is interesting to investigate the crystallization temperature and seeds microstructure effects on the epitaxial growth process,i.e.the orientation of zeolite T membrane.
According to the tracking observations, the membrane growth process was further investigated at various temperatures of 373 K and 393 K and using seeds with different morphology and were found almost same as the one at 423 K. At the early stage <10 h for 373 K and <6 h for 393 K,the seeds grew along a-and b-direction,resulting in the size increase of the seed grains and the closing of seed voids,õbesides,few new nuclei appeared on the seed layer(SEM images are referred to reference[36]).However,the formed nuclei were dissolved into the solution to provide nutrient for the crystal growth according to Ostwald Ripening[37].Then the seeds mainly grew in the c-direction,leading to the formation of rod-like crystals. It should be noted that low temperature led to long time to obtain dense zeolite T membrane layer with good pervaporation performance as 373 K for 23 h,393 K for 12.5 h,respectively.The membrane formation process for different synthesis temperature revealed the same growth mechanism,namely the zeolite T membranes were formed by an epitaxial growth of seeds with closing of the voids at the synthesis temperature 373-423 K.
We further investigated the seeds size 0.4-3 μm effects on the formation of zeolite T membranes[36].The zeolite T membrane morphologies prepared by different size seeds were quite distinct, from wheat-like,stick-like to rod-like with zeolite T seed size decreasing.The influences of the seed size on the oriented zeolite T membrane morphology revealed that the epitaxial growth for the gel solution were the same despite the different seed layer microstructures.
3.Thermal and Acid Stability of the Zeolite T Membranes
3.1. Thermal and acid stability of the zeolite T membranes in various environments
Zeolite T membranes prepared by epitaxial growth showed good hydrothermal and acid resistance in ethyl acetate/water, isopropanol/water/acetic acid and tetrahydrofuran/water/HCl mixtures as shown in Figs.6-9.In Fig.6,the total flux through the zeolite T membranes stabilized at about 12 kg·m-2·h-1,and the water permeate concentration remained at 100 wt% during 270 h vapor permeation test in 95 wt%Ethyl acetate/water(pH ~6)mixtures at 373 K,indicating a quite stable hydrothermal resistance at pH ~ 6. While for pH ~3, 90 wt%isopropanol/water/acetic acid mixtures (in Fig. 7), the total flux decreased from 8.8 kg·m-2·h-1to a steady-state of 4.2 kg·m-2·h-1,and the water permeate concentration kept above 99.5 wt%, acetic acid was not detected in permeate.Considering the industrialization of zeolite T membrane in acidic environment,the water dehydration stability for 96.5wt%/3.5 wt%tetrahydrofuran/water(added HCl, pH<1)mixture was also evaluated at 333 K for 219 h.The total flux of zeolite T membranes stabilized at about 0.7 kg·m-2·h-1,and the water permeate concentration remained above 99.3 wt%through the test.All those results demonstrated that the synthesized a&b-oriented zeolite T membranes had good stabilities in acidic solution.

Fig.4.SEM images of the M1 seed layer(a)&(b)and the zeolite T membrane(c)&(d)on a macroporous Al2O3 tube,and the M2 seed layer(e)&(f)and the zeolite T membrane(g)&(h)on a hollow fiber(HF)support[29].
It was reported that zeolite A membranes were unstable either in acid condition or under water concentration larger than 15 wt%for separation of water/organics mixture.The hydrothermal stability of zeolite T membrane was investigated using 80 wt%IPA/20 wt%at 348 K for 250 h.The water flux decreased the initial value of 11.7 kg•m-2•h-1to a steady-state value of 7.0 kg·m-2·h-1,and the water permeate concentration remained at 100 wt%through the whole test experiment.The as-synthesized zeolite T membranes were stable for 80 wt%IPA/20 wt%at 348 K during the investigated time.
3.2. Zeolite T and other zeolite membranes for dehydration of organics:a comparison
The available materials for the dehydration of organics have proven to be effective in various operations.However,each material has its own limitations in various testing conditions under which they can be applied.NaA zeolite membranes are one of the most commonly used membrane materials for the dehydration of organics at commercial levels(Mitsui engineering).However,these membranes are stable just limited to feed lower water contents smaller than 20 wt%and in pH range of 6-8[38].Thus the zeolite NaA membranes are not feasible in industrial processes involving acidic media.CHA type zeolite membranes low Si/Al of 2.5 are also very promising in dehydration of organics in acidic mediums including acetic acid and others.This material is also reported for feed containing water 50 wt%so far[38].CHA membranes are also available at commercial levels manufactured by both Mitsubishi chemical corporation and Hitachi Zosen Japan[38].The production cost of the CHA zeolite membranes is relatively higher than the T zeolite membrane owing its longer synthesis time or complex synthesis strategy.Nevertheless,CHA zeolite membranes are highly promising for practical application for the purpose of dehydration of organics especially for alcohols[39].

Fig.5.SEM images of top view and cross section of prepared zeolite T membrane at 423 K for different crystallization times:(a1),(a)and(b)for 1 h,(c1),(c)and(d)for 2 h,(e1),(e)and(f)for 3 h[29].

Fig.6.Vapor permeation performance of zeolite T membrane as a function of operation time for a 95 wt%Ethyl acetate/water mixture at 373 K(pH ~6)[36].

Fig.7.Vapor permeation performance of zeolite T membrane M1 as a function of operation time for a 90 wt%isopropanol/water(added acetic,pH ~3)mixture at 373 K.

Fig.8.Pervaporation performance of zeolite T membrane as a function of operation time for a 96.7 wt%/3.5 wt%tetrahydrofuran/water(added HCl,pH <1 at 333 K)mixture at 333 K[36].
Generally, T zeolite membrane with Si/Al ratio 3-4 is highly hydrophilic and can withstand at quite harsh acid conditions including 50 wt.%. acetic acid aqueous solution.These properties make zeolite T membrane much feasible for the dehydration of acidic mixtures as well as adsorption and drying of acid gasses.For the MOR and ZSM-5 membranes with the lowest Si/Al of 5 and 10 respectively they were reported for dehydration of 90 wt%acetic acid/10 wt%water mixture(pH about 1), where zeolite T membrane cannot compete for this purpose. Although the acid stability strength of T zeolite is weaker than the MOR and ZSM-5 membranes,the synthesis cost of the zeolite T membrane is lower than MOR and ZSM-5 membrane due to the lower synthesis temperature and much shorten membrane growth time.Therefore,for the dehydration of organics in the weaker acid condition(PH >3)the zeolite T membrane is prior to the MOR and ZSM-5 membranes because of the better economic performance.
Zeolite T membranes were reported to have been industrialized and manufactured commercially by Mitsui Engineering&Shipbuilding Co.,Ltd.in the patent literature for PV/VP applications[40].However,no study has been reported so far for real plant based zeolite T application so far.Due to excellent hydrophilic properties of zeolite T membranes, these have great potential to get in industrial sector for the separation of commodity chemicals. Many researchers including Japanese companies are exploring the ways to commercialize these types of membranes in order to get a full benefit from academia.
The synthesis of zeolite T membranes through epitaxial growth opens new avenues for industrialization application of these zeolite membranes because of the shortened synthesis time, improved flux and good hydrothermal and acid stability. Further modification to control more optimum Si/Al ratio in this method can lead to production of economical membranes with better selective for the dehydration of industrial solvents even in harsh acidic conditions.
The potential of zeolite membrane for already discussed applications make it preferable over hydrophilic zeolite membranes such as A,X and Y.However,researchers are further exploring the economical method for the production of zeolite T membranes in acidic media by maintaining excellent separation performance. In this concern, the novel synthesis of zeolite T membranes through epitaxial growth can facilitate the shortest route for the synthesis of industrial zeolite T membranes.

Fig.9.Dependency of pervaporation performance of zeolite T membrane as a function of operation time for 80 wt%IPA/20 wt%H2O at 348 K[36].
4.Ensemble Synthesis of Hollow Fiber Zeolite T Membrane Module
So far,T-type tubular zeolite membranes have demonstrated excellent and stable separation performance in acidic media.However,due to many factors including membrane cost and long synthesis time except for the epitaxial growth,the zeolite T membranes are still not yet industrialized effectively.For this,porous ceramic hollow fibers(HFs)are introduced with very thin wall thickness as promising candidate for zeolite membranes(e.g.LTA,T-type,and MFI)[26,41,42].The hollow fiber membranes are now getting much attention due to their high permeation flux and higher packing densities.The packing density of the hollow fiber membrane can be further increased up to 1000 m2·m-3(three times greater than tubular membrane) to achieve high flux[41].In HF membranes,the asymmetric hollow fiber membrane development is getting much attention in pervaporation application due to various favorable factors including (1) high surface area to volume ratio,and(2)self-supporting type structure(no need of any support and can withstand by itself).A few of the studies have been reported for the development of T-type zeolite membranes supported on hollow fibers for pervaporation applications.Gu et al.[26]prepared high performance T-type zeolite membranes supported on yttria-stabilized zirconia (YSZ) through secondary growth method. The HF supported Ttype zeolite membranes reported in this study showed permeation flux of 7.36 kg·m-2·h-1(10000 separation factor)at 75°C for 90 wt%isopropanol dehydration. They suggested rubbing method to prepare best HF supported T-type zeolite membrane, however for commercial preparation of these membranes,a vacuum assisted coating was found favorable. Wang et al.[26] reported synthesis of T zeolite membrane on Al2O3hollow fiber with ultrasonic pretreatment prior to secondary growth, the resulting T-type zeolite membrane synthesized in 24 h with 0.5 h ultrasonic pretreatment has a separation factor of >10000 and a flux of 12.2-14.3 kg·m-2·h-1for 90.0 wt%isopropanol aqueous solution at 75°C.The α-Al2O3HF support largely contributes to this ever high flux.
Recently, Gu. et al. [27] investigated different configurations of HF supported T-type zeolite membranes for the dehydration of ethanol/water mixture and suggested that geometric configuration of the membrane modules strongly affect the separation efficiency.They further reported fabrication of HF supported T-type zeolite membrane using ensemble synthesis route[28]as shown in Fig.10.Briefly,ceramic base was used to assemble a bundle of Al2O3hollow fibers by enamel sealing and different synthesis parameters including seed size, seed solution concentration, coating and crystallization time. In this study, high quality HF-T type zeolite membrane showed excellent pervaporation performance with flux:2.25 kg·m-2·h-1and SF:1348 for the dehydration of ethanol.The stability of these membranes was further investigated using acidic solution of ethanol (pH = 3) for about 450 h and a very stable performance was reported for the investigated time period.The reported ensemble strategy to fabricate HF supports zeolite membrane solves the problem of sealing by providing increased fabrication efficiency.This strategy can be further applied to other type of HF supported zeolite membranes for industrial separation applications.
Due to high package density,and excellent separation performance HF based zeolite membranes are cost effective and can be industrialized effectively for various organic dehydrations.The introduction of hollow fibers in fabrication of various zeolite membranes will open new doors for stable and high performance commercial membranes ultimately leading membrane technology to a peak level.
5.Future Prospects of Acid Resistant Zeolite T Membranes
The sustainable future of hydrophilic membrane modules for pervaporation greatly relies on broadening the existing range of membrane applications as well as investigating new materials and fabrication procedures in membrane technology.Dehydration of organics in acidic mediums has received much attention over the last few years and is now a well-defined and challenging area of research in separation technology through pervaporation.The major challenges for organic dehydration include:(1)high cost of membrane materials;(2)poor stability of membrane materials.Thus for these problems new stable and selective membrane materials are highly demanded for the better separation economics.

Fig.10.(a)The ensemble synthesis strategy for HF-T-type zeolite membrane module(b)hollow fiber supports and membrane modules[28].
The epitaxial growth developed in our group for the fabrication of zeolite T membranes has shortened the synthesis time effectively by maintaining their excellent separation performance.Due to excellent properties of zeolite T, the application of these membranes can be further extended to many industrial separations especially for the dehydration of alcohols in mild acidic media.For this researchers are exploring the ways to introduce these membranes commercially such as optimization of capital cost by various synthesis methods(epitaxial growth) and also to avoid reduction in flux by maintaining its initial high flux.
Further,optimization such as Si/Al ratio for zeolite T membrane can give higher flux membranes for industrial operations.Another strategy,to introduce zeolite T membranes at commercial levels is hollow fiber membrane technology,the investigation of these modules for zeolite T can lead to amazing industrial separation economics.Beside this,zeolite T membrane fabrication on low cost tubular supports is also great point of interest for further optimization of capital cost.
6.Conclusions
In this review,research advancements of zeolite T membranes for the dehydration of organic mixtures in acidic conditions have been addressed.Also,low temperature synthesis and epitaxial growth of T zeolite membranes along with ensemble synthesis of hollow fiber zeolite membrane modules have been highlighted.Further,we extended our discussion on stability of these membranes in acidic conditions. It is worth mentioning,that zeolite membrane with high acid resistance is a new generation of membrane technology,in terms of industrial practical application,as NaA zeolite membranes were not stable under acid environment due to their inherent poor acid resistance.Thus,for large scale applications, efforts are needed to improve zeolite framework chemistry and innovations in membrane modules such as hollow fiber module.The hollow fiber module T zeolite membranes can be a milestone for its wide range of practical applications.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Amorphous and humidity caking:A review☆
- Process intensification in vapor-liquid mass transfer:The state-of-the-art☆
- Extractive distillation:Advances in conceptual design,solvent selection,and separation strategies☆
- Beyond graphene oxides:Emerging 2D molecular sieve membranes for efficient separation☆
- A review of internally heat integrated distillation column☆
- Recent progress and future prospects of oil-absorbing materials☆
