Process intensification and energy saving of reactive distillation for production of ester compounds☆
2019-08-19ChunliLiCongDuanJingFangHongshiLi
Chunli Li*,Cong Duan,Jing Fang,Hongshi Li
School of Chemical Engineering and Technology,Hebei University of Technology,Tianjin 300130,China
National-Local Joint Engineering Laboratory for Energy Conservation of Chemical Process Integration and Resources Utilization,Tianjin 300130,China
Keywords:Reactive distillation Process intensification Process coupling Thermal coupling
A B S T R A C T Reactive distillation(RD)process is an innovative hybrid process combining reaction with distillation,which has recently come into sharp focus as a successful case of process intensification.Considered as the most representative case of process intensification,it has been applied for many productions,especially for production of ester compounds.However,such problems existing in the RD system for ester productions are still hard to solve,as the removal of the water which comes from the esterification, and the separation of the azeotropes of ester-alcohol(-water).Many methods have been studying on the process to solve the problems resulting in further intensification and energy saving.In this paper,azeotropic-reactive distillation or entrainer enhanced reactive distillation(ERD)process,reactive extractive distillation(RED)process,the method of co-production in RD process,pressure-swing reactive distillation(PSRD)process,reactive distillation-pervaporation coupled process(RD-PV),are introduced to solve the problems above,so the product(s)can be separated efficiently and the chemical equilibrium can be shifted.Dividing-wall column(DWC)structure and novel methods of loading catalyst are also introduced as the measures to intensify the process and save energy.©2018 The Chemical Industry and Engineering Society of China,and Chemical Industry Press Co.,Ltd.All rights reserved.
1.Introduction
Esters are widely found in nature and are widely used by human beings.For example,commonly used in spices,esters of low carbon atoms are kinds of colorless liquid with fruit flavor,existing in flowers or fruits.Apples contain isoamyl valerate,bananas contain isoamyl acetate,and jasmine contains methyl benzoate. Esters of low carbon atoms are used as solvents,and many esters formed from branched alcohols are excellent lubricants.Esters can also be used in the industries of perfume,essence,cosmetics,soap and medicine.Nipagin esters can be applied to food,cosmetics,medicine and other industries as preservatives;dioctyl phthalate is a good plasticizer;and tributyl citrate is a kind of non-toxic,odorless,and excellent weather-resistant plasticizer[1].
Esters are generally prepared by esterification from alcohols and acids or by transesterification from esters and alcohols/acids/esters(different esters). The esterification reaction is reversible, where the esterification reaction and the hydrolysis reaction occur simultaneously.If we want to get the high yield of ester and shorten the reaction time,we should make the reaction take place under certain conditions,such as using catalyst,heating,make a certain reactant overdose and reduce the amount of water in the products [2]. In the ester solution, a few free alcohols and acids exist. Transesterification is based on the reversibility of the esterification reaction.Both the esterification and transesterification are limited by chemical equilibrium.The ester can also be produced by the reaction of the acyl halide or the acid anhydride with the alcohol, or by the reaction of the carboxylate with the halohydrocarbon.
RD,an innovative hybrid process combining chemical reaction with distillation,has the function of reaction and distillation in a single unit where both the reaction and the distillation process are intensified by each other.In the RD process,distillation allows the reaction products to be removed in time resulting in high reaction conversion; meanwhile,reaction can circumvent phase equilibrium limitations,such as distillation boundaries and azeotropes;besides,the heat released from the reaction can be used in the distillation process. RD has various advantages in performing reaction and separation simultaneously rather than sequentially including increasing conversion of the reactant in case of reversible reactions, improving selectivity of the desired product,avoiding azeotropic conditions,and preventing the formation of undesired products and hot spots [3]. Therefore, many reactions limited by chemical equilibrium like esterification, etherification,transesterification,hydrolysis,and acetylation have been carried out in RD processes successfully[4].
However,problems that exist in the RD system for ester production are still hard to solve. The removal of the water which comes from the esterification,and the separation of the azeotropes of ester-alcohol(-water)are two main problems.
With energy-saving potential, process intensification combining two or more conventional unit operations within one unit has been paid more attention in the chemical process engineering.Considered as the most typical case of process intensification,RD can help reduce operating costs,capital costs and environmental impacts.However,it can be intensified much further by process coupling,thermal coupling,and equipment improvement in order to solve the problems in conventional RD process resulting in much more energy saving.
Although process intensification brings many benefits like energy saving and equipment cost reduction,it also gives the challenge of coordination problem in process synthesis and design. It is an important principle to seek the synergistic effect among all the involving conventional unit operations.It is demonstrated by Huang et al.that simply combining two or more conventional unit operations together does not necessarily exploit the full potential of all the benefits of process intensification.After deliberately considering the synergistic effect in process synthesis and design,process intensification can be performed in the most effective manner.Furthermore,seeking synergistic effect appears frequently to yield improved process dynamics and provide additional redundancy to process operation because the underlying conflicts could be attenuated among all the conventional unit operations involved[5].
In the case of esterification,it is expected that the product(s)can be removed timely and efficiently,so the molar ratio of the reactants can be set close to stoichiometric ratio.However,the water and ester formed by the reaction can't be moved completely and efficiently in the RD process resulting in a potential of complete conversion[6].
To solve the problems above and intensify the RD process, many methods including process coupling,thermal coupling and equipment improvement have been proposed in forms of ERD,RED,PSRD,RD-PV coupled process,co-production,RDWCs and novel methods of loading catalyst.ERD process is proposed to make the water and the azeotropic agent form a new azeotrope which will be distilled from the top of the column. RED process will introduce a kind of extractive solvent to make the azeotropes disappear.And the method of co-production in the RD process is adding another reaction to react with water or to generate the corresponding entrainer(either azeotropic agent or extraction solvent)to remove the water.DWC structure is used to perform multicomponent separation in a single vessel which makes the process more energy efficient than the normal distillation sequence.Other methods like pressure-swing distillation, pervaporation process, and novel methods of loading catalyst can also be used to intensify the RD process.Since some articles[7-9]have summarized the RD process from fundamental researches to industrial applications,process intensification and energy saving in the RD process for production of ester compounds by esterification or transesterification over the past decade are more focused in this article.
2.Azeotropic-RD(ERD)
Azeotropic-reactive distillation(or entrainer enhanced reactive distillation,ERD),the integration of azeotropic distillation and reactive distillation which combines the characteristics of the two processes,can yield technical advantages from both heterogeneous azeotropic distillation and reactive distillation.Azeotropic distillation is a special case of multicomponent distillation used for separation of binary mixtures which are either difficult or impossible to separate through ordinary fractionation.It is commonly used to separate close-boiling mixtures with far fewer trays than in conventional distillation and with less circulation, resulting in reduction of equipment and energy costs [10].The method is widely used for esterification and transesterification in RD columns to increase the relative volatility of water by forming a minimum heterogeneous azeotrope with water,and this minimum heterogeneous azeotrope is separated from the top of the column and decanted to almost-pure water. Fig. 1 shows a flowsheet of the ERD process.
Named entrainer based reactive distillation, the selection of entrainers plays an important role to break the azeotropes and remove the water.The entrainer is selected in such a way that it should form a minimum heterogeneous azeotrope with one of the original azeotropic components(mostly with water)distilled from the top of the column,which facilitates the separation of the product from water, removes water to shift chemical equilibrium and further maintains the temperature of reaction section below the thermal stability limit of the catalyst.So the choice of entrainer depends on the reaction temperature,azeotrope temperature,and mutual solubility of heterogeneous azeotrope components,which should also fulfill the criteria mentioned in the article[11].Table 1 shows a set of possible entrainers for ERD to separate water.
The role of entrainer is important in terms of removing water from the reaction section to improve the conversion and obtaining desired selectivity of the products.Meanwhile,the hold-up of entrainer in the column and its volatility determine the temperature of the reaction section.With the presence of a proper entrainer in the column,the temperature of reaction section can be kept well below the permissible limit.In fact,the temperature of the entire column is lower than that without an entrainer,which can avoid acid(such as acetic acid)losses through the distillate,avoid corrosion due to some acid,and overcome the problem of acid-water separation[6,13].The following Table 2 reviews the studies and applications of the ERD in last ten years.
Mandagaran et al.studied the model of phase and chemical equilibrium on the quaternary system of AA,n-butanol,water and NBA.The results showed that chemical equilibrium constant had a strong influence on the azeotropic point[14].
Kang et al.proposed a thermodynamically infeasible region for the heterogeneous ERD process,determined by the constraint of reaction and phase equilibria.In addition,operating constraints were imposed on the feasibility evaluation in terms of the upper and lower internal reflux boundaries.Using these two kinds of feasibility constraints,they examined how the EA-and IPA-RD systems,which could not produce pure products because of four minimum-boiling azeotropes,led to a full conversion and pure product recovery with the introduction of an external entrainer inducing liquid-liquid splits.The pure acetate production in a single ERD column was possible because the composition trajectory approached the desired region in composition space without being blocked by both the critical composition region and the infeasible region confined by the internal reflux boundaries[15].

Table 1Possible entrainers for ERD to separate water[12]
As shown in Tables 2-4,the ERD process has many advantages and economic benefits,like shifting chemical equilibrium toward the removal of water, desired selectivity and high purity of the products.And,for such reactants as high boiling alcohol,high boiling di/trihydroxy alcohol,high boiling acid and high boiling dibasic acid,the temperature of the reactive zone may exceed the catalyst thermal stability limit.Further,corrosion can be severe in the presence of acetic acid vapors at temperatures above 100°C.In ERD process,the entrainer forms an azeotrope with water which facilitates the separation and further maintains the reactive zone temperature below a permissible limit[6].So it is a good choice for production of ester compounds.
3.Reactive-Extractive Distillation(RED)
Reactive-extractive distillation(RED),combining the advantages of reactive distillation with extractive distillation,can be an efficient alternative hybrid process to the conventional process.Just like azeotropic distillation process,extractive distillation is another case of multicomponent distillation used to separate close-boiling mixtures.However,the entrainer in extractive distillation is obtained in bottom products and needs to be further recovered through a solvent recovery column rather than being vaporized to the overhead and be refluxed from a decanter in azeotropic distillation.
In the RED process,the azeotrope formed by the product and the reactant can be destroyed by the entrainer,and the relative volatility of the components can be greatly enhanced by the added entrainer,resulting in the further increased conversion compared with the RD process without the entrainer [4]. A schematic diagram of the RED process is shown in Fig.2.
The selection of an appropriate solvent is the key factor for feasibility and economy in the RED process. Jing Fang et al. proposed a new method of screening mixed solvents of extractive distillation,which was according to the hydrogen bond formation and modified UNFAC model.Considering the synthetical evaluation to the solvents,the optimal mixed solvents to separate EA-ethanol were obtained.In the role of optimal mixed solvents,the relative volatility of EA-ethanol reached maximum which was higher than that by using any single solvent[30].
Ionic liquids(ILs)are chemicals composed of organic cations and organic or inorganic anions with non-volatile,low melting point,wide liquid range,high stability,recyclable,and other unique properties.They are considered to be green solvents used to replace the conventional volatile entrainers.ILs are easily separated from volatile chemicals in simple distillation due to its negligible vapor pressure,resulting in reduction of energy consumption compared with the conventional entrainer. Ionic liquids are thermally stable up to 200 °C, and exhibit Bronsted,Lewis,and Franklin acidity as well as superacidity,so ionic liquids can also be substituted for ion-exchange resin catalysts[31].
Jie et al.developed RED processes to conduct transesterification of methyl acetate(MA)with isobutanol catalyzed by ILs 1-sulfobutyl-3-methylimidazolium hydrogensulfate ([HSO3bmim][HSO4]) and oxylene as entrainer, through which high purity of the product and high conversion of the reactant can be achieved. The entrainer oxylene is used to separate the azeotropes of methanol-MA and isobutanol-IBA[4].The flowsheet of the process is showed in Fig.3.In the same year,Jie et al.also used ILs[HSO3bmin][HSO4]as catalyst and 1-butyl-3-methylimidazolium bis[(trifluoromethyl) sulfonyl]imide[BMIM][Tf2N] as entrainer to produce EA and methanol by transesterification of MA with ethanol via RED.The simulation results showed that the purity of ethanol was 0.9922 and the purity of EA was 0.9905 (molar fraction), and the conversion of MA was 0.9922 under the optimal operating and structural conditions[32].
Tian et al.studied the RED process to conduct esterification of AA with methanol using[Hmim]HSO4as catalyst and entrainer[33].
Jiménez et al.studied the production of NBA and methanol via RED from n-butanol and MA using o-xylene as the entrainer and Amberlyst-15 as the catalyst to exploit a methanol+MA mixture.O-xylene was identified as a suitable extractive agent and the reaction solvent[34].They also studied the dynamic behavior for the azeotropic separation,including the RED unit,and the solvent recovery system.High-purity methanol and NBA were obtained as products, and approaching 100%conversion of n-butanol was achieved.However,they proposed that the process had no profitability through a preliminary economic analysis [35]. And then, they determined the best solvent for the azeotropic mixture.A preliminary selection was completed in accordance with heuristics and physical properties.Selectivity at infinite dilution for 40 systems was measured using headspace gas chromatography.Above all,they found that n-alkanes,alkylbenzenes,and,in particular,o-xylene were the best alternatives[36].
If it is feasible to use one of the components that already existing in the system as the entrainer,the entrainer will be the priority because the solvent recovery column can be removed resulting in capital reduction and energy saving.In the EA system,Li et al.investigated how the amount of AA affected the composition of the azeotrope of EA-ethanol-water. They found that AA didn't form any azeotrope with the other three components and that the azeotrope of three components would turn to binary azeotrope of EA-water with the increasing amount of the AA in a certain range.So,AA could be the extractive entrainer of the EA reactive system. The purity of EA could reach 97 mol% after single-column distillation with a decanter[37].

Table 2Studies and applications on ERD

Table 2(continued)

Table 3TAC comparison of RD columns without and with an entrainer[18]

Table 4Comparison of the two-column process and the ERD process[23]
Based on the results of the research mentioned above,Li et al.investigated a RED process for the EA synthesis with the acidic ion-exchange resin as catalyst and AA as entrainer.After the simulation and lab experiment, they put it into industry operation with an annual output of 20,000 t EA,and operated very well[38].
Qi et al.investigated a semibatch RD process for IPA synthesis by esterification with AA as the entrainer.AA was the heaviest component of the IPA system and that it didn't form any azeotrope.So they used AA as the entrainer to extract isopropanol and direct it back to the reaction section and to allow IPA and water to pass upward into the distillate[39].
Zheng et al.proposed a packed-bed RED column to recover polyvinyl alcohol by-product MA through esterification from methanol and AA with AA as the entrainer over Amberlyst-35.According to the simulation results,the MA purity in the distillate and the methanol conversion could reach higher than 99%and 94%,respectively[40].

Fig.2.Schematic diagram of the RED process.
In the RED process, the entrainer needs to be further recovered though a solvent recovery column.ILs are easily separated from volatile chemicals in simple distillation.So,ILs are potential solvents for the RED process which are used to replace both the conventional entrainer and ion-exchange resin catalysts,resulting in reduction of energy consumption and capital cost.Using the components that already existing in the system as the entrainer will save a solvent recovery column which can also bring about energy saving and capital reduction.
4.Pressure-swing RD(PSRD)
Pressure-swing distillation is also a method to separate azeotropic mixtures sensitive to pressure by running the RD process under two different pressures to change the composition of azeotropic components. So pressure-swing distillation is another process to couple with RD very well for the azeotropic mixtures. PSRD provides the benefits of both reactive and pressure-swing distillation together to intensify the process.
However,the pressure change can affect the reaction in a PSRD process.Wang et al.studied the influences of pressure on the operation of RD columns involving kinetically controlled exothermic reactions.They found that the operating pressure could present nonmonotonic influences on process dynamics and operation and that the operating pressure could serve as an important decision variable to trade-off process design and operation[41].
So PSRD can be used as a method to separate binary systems which are difficult to separate in a conventional way after considering its effects on reaction and distillation in process design and operation.Bonet et al.studied the characterization of an original PSRD system by taking the MA transesterification with ethanol to produce methanol and EA as an illustrative example[42].
Modla proposed a novel double-column system for EA production by esterification in batch PSRD.The system was investigated by a feasibility study based on the analysis of reactive and non-reactive residue curve maps showed in Figs. 5 and 6. Two different process options were found to be feasible(Fig.4).In the option A process,the reactive column operated at the lower pressure (0.101 MPa), and the nonreactive column that removed EA from the system operated at the higher pressure(1 MPa).The option B allowed the reactive column to operate at the higher pressure(1 MPa),and the non-reactive column removed water at the lower pressure(0.101 MPa).The first option was studied by rigorous simulation based on less simplifying assumptions using a professional dynamic simulator.The influence of the most important operation parameters was also studied[43].

Fig.3.Process for the transesterification of MA with isobutanol[4].
Heat-integration is always conducted to the PSRD process. Suo et al. designed a novel partial heat-integrated PSRD process for transesterification of MA with isopropanol,which could save 33.41%energy consumption and 27.16%total annual cost(TAC)in comparison with conventional RD process(Fig.7).The basic and improved control structures for the process were studied by Aspen Dynamic[44].
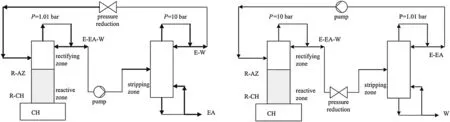
Fig.4.Sketch of the feasible column configuration(process option A&process option B)[43].(1 bar=0.1 MPa).

Fig.5.Quaternary residue curve map in 3D.(a)1.01 bar and(b)10 bar[43].(1 bar=0.1 MPa).
The PSRD process is always compared to the RED process.Luyben presented a quantitative steady-state and dynamic comparison of a PSRD process with a RED process to produce tert-amyl methyl ether with water as the entrainer.The RED process was found to be much more economical(40%lower capital investment and 60%lower energy cost).The plantwide dynamic controllability performances of the two systems were essentially equivalent[45].
Bonet-Ruiz et al.proposed a simplified cost function taking into account the main parameters to which were proportional the capital and operational costs.He provided an optimal operational window defined by a percentage over the minimum cost value by taking a PSRD process to produce EA and methanol by the transesterification as the example[46].
Pressure swing distillation is also an efficient separation method for separating pressure-sensitive azeotropic mixtures.But in the PSRD process, effects of pressure on reaction and the economical efficiency should be considered in process design and operation. And heatintegration is always considered to reduce the operating cost.

Fig.6.Reactive-residue curve map at different pressures(a)1.01 bar and(b)10 bar[43].(1 bar=0.1 MPa).

Fig.7.The cost comparison between the conventional RD,the PSRD and the partial heatintegrated PSRD processes[44].
5.RD-Pervaporation Coupled Process(RD-PV)
Reactive distillation-pervaporation coupled process(RD-PV)as a process that couples RD process and pervaporation process is also an effective way to separate the azeotropes during the ester production process. Pervaporation is mainly used in the separation of azeotrope system,dehydration of organic solutions and removal of trace organic compounds in water. Here for the ester system in the RD process,water removal and azeotrope separation problems can be solved by the pervaporation process.So,many applications of RD-PV have been introduced[47].
Lv et al.proposed a novel RD-PV coupling the PVA/ceramic composite membrane with the RD reboiler for EA production. The pervaporation membrane was located in the bottom stream in order to selectively remove the water from the reboiler and recycle the AA into the feed. The EA purity and ethanol conversion were improved from 82.4wt%to 85.6 wt%and from 81.3%to 84.8%,respectively owing to the removal of water and AA recycle from the reboiler via the pervaporation process[48].
Holtbruegge et al. studied the synthesis of dimethyl carbonate(DMC) and propylene glycol in a membrane-assisted RD process where RD was used to overcome the chemical equilibrium and vapor permeation was used to overcome the azeotrope[49].
Lee et al.proposed the evolutional design and control of EA process via RD-PV hybrid configuration for producing high purity-EA with further decreased energy consumption.A side stream with high EA purity was drawn from the rectifying section of the RD column, and pervaporation units were applied to remove water for producing high-purity EA product.The optimized design achieved 13%energy savings compared to the two-column process[50].
Harvianto et al.proposed a hybrid RD process with high selectivity pervaporation for NBA production via transesterification as shown in Fig. 8. The polyamide-6 membrane was used for the separation of a methanol and MA azeotrope,owing to its high selectivity for methanol while also allowing sufficient permeate flux.The high-purity MA recovered in the retentate stream leads to high conversion in the RD column,which enhances the energy savings(up to 71%)and TAC(up to 60%)of this process relative to conventional systems[51].They also proposed a thermally coupled RD-PV for NBA production which improved the energy efficiency of the RD process by preventing remixing effect in the RD column and eliminating the azeotropic nature of the methanol and MA in the recycle stream,respectively,resulting in improved energy efficiency and economics.The total reboiler duty and total annual cost were reduced to 63%and 43%,respectively,compared to those of the conventional case[52].
The chemical equilibrium can be shifted toward the production of esters through continuous removal of water from the reactive zone by pervaporation. However, there are some problems such as high cost and short using period of the membrane in the process of industrial application,which limit the process of large-scale industrialization.

Fig.8.RD-PV coupled process for NBA production.
6.Co-production in RD Process
The co-production in RD process here is a method to intensify the RD process by introducing a new reaction to consume the original products,or to generate a new entrainer to remove the water from the system via a RED or ERD process,or just to affect each other in some synergistic thermodynamic features. The reaction introduced can intensify the other reaction,or intensify the distillation process or both.
Applications of the co-production in RD can be the best description of this process.Dimian et al.proposed a novel approach based on dual esterification of fatty acid with light and heavy alcohols,namely methanol and 2-ethylhexanol to solve the key problem of the effective removal of water in view of protecting the solid catalyst and avoiding costly recovery of the alcohol excess.These two complementary reactants had an equivalent reactive function but synergistic thermodynamic features. The setup behaved rather as reactive absorption combined with reactive azeotropic distillation with heavy alcohol as co-reactant and water-separation agent.Another element of originality was the control of the inventory of alcohols by fixing the reflux of heavy alcohol and the light alcohol column inflow. This strategy allowed achieving both stoichiometric reactant feed rate and large flexibility in ester production resulting in a compact,efficient and easy-to-control multi-product reactive setup[53].
Tian et al.proposed a novel procedure for co-production of EA and NBA by RD as shown in Fig.9,whose feasibility was theoretically analyzed on the basis of the physical properties of EA/NBA system. The new procedure not only significantly reduced energy consumption,but also eliminated the AA purification column,as compared with the individual processes.The outputs of EA and NBA could be adjusted flexibly by changing the feed molar ratio of ethanol to n-butanol in a certain range.At last,TAC of the co-production was 3795912 U.S.dollars per year less than the individuals 5190921 U.S. dollars per year, when 10000 t EA and 26363 t NBA were produced [54]. And they also did the optimization of co-production of EA and NBA by RD[55].

Fig.9.Flow diagram of EA and NBA:I EA RD column and II NBA RD column.
Li et al.proposed a new process of co-production of methyl tert-butyl ether(MTBE)and tert-butyl alcohol(TBA)which could solve the difficult problem of separation of azeotrope of TBA and water,and eliminate the further separation of methanol and water.Finally the purities of 99.5%MTBE and 99.9%TBA were obtained,and the conversion of isobutylene of mixed C4is up to 99.9%[56].
As shown in Fig.10,Tavana et al.proposed a novel RD process for EA production intensified by the hydration of ethylene oxide(EO),which was an auxiliary reaction for removing the EA/water azeotrope.An optimal process design was achieved and the pure EA and ethylene glycol(EG)with low energy requirements were predicted[57].
Liu et al.also studied the use of EO hydration to intensify the RD to produce EA.The result obtained presented that the mass fraction of EA was 99.3%,the mass fraction of EG was 96%at the bottom of the column and the energy consumption of production process was 366 kW[58].
Wang studied the use of ethylene hydration to intensify the RD process to produce EA by consuming water,generating ethanol and releasing heat,resulting in high purity of the products,low consumption of the reactants with Aspen Plus simulation[59].
Tong et al.studied a RD process of MA hydrolysis intensified by reaction of methanol dehydration.The simulations of RD were performed using a three-phase and non-equilibrium model implemented by gPROMS.Two novel processes for recovery of MA in PVA production were developed.The simulation results showed that the high purity of dimethyl ether could be achieved with a complete conversion of MA,and a large amount of energy demand and equipment costs could be reduced[60].
The RD process is intensified by adding another reaction to react with water or to generate the corresponding entrainer (either azeotropic agent or extraction solvent)to remove the water.The way of co-production in the RD process is a very innovative method which could not only intensify both the reaction and distillation process but also increase the production efficiency.
7.Reactive Dividing-wall Columns(RDWCs)
Reactive dividing-wall columns(RDWCs)are highly integrated systems that can simultaneously perform chemical reactions and multicomponent separations within the same vessel. The DWC performs multicomponent separations that normally require multiple distillation columns,in a single piece of equipment,which is achieved through the unique feature, a partial vertical partition that exists inside of the column.Although popular in chemical processes due to their ability to present significant capital and operating cost savings,RDWCs have not been commercially adopted[61].The development from a conventional distillation column(DC)to a RDWC is showed in Fig.11.The design and operational variables involved with running a RDWC are shown in Fig.12.
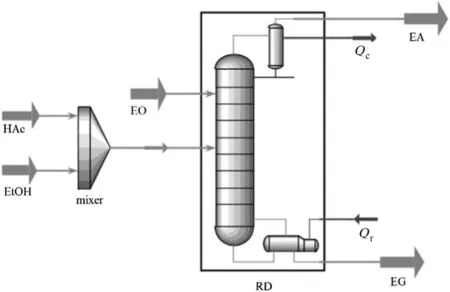
Fig.10.Configuration of the RD process to co-production of pure EA and EG[57].

Fig.11.The development from DC to RDC[62].

Fig.12.Design and operational variables involved with running a RDWC[61].
Cho et al.studied how to use the RDWC to recover lactic acid from a fermentation process which generated excess water and impurities with high boiling points.The presence of high boiling points and nonvolatility of lactic acid made the separation of lactic acid very difficult.Esterification of lactic acid with methanol followed by hydrolysis of the separated MA was proposed as a technique to overcome these difficulties.And a RDWC structure helps to reduce the capital and energy costs[63].
Muller and Kenig did the numerical investigation of the RDWC with a simulation tool which applied the rigorous rate-based modeling approach[64].
Barroso-Munoz et al.did a series of researches on RDWCs.At first,they studied the thermally coupled distillation systems for production of EA, using three distinct thermally coupled distillation systems to carry out the purported reaction-separation process: the thermally coupled system arranged with the side stripper (Fig. 13a), arranged with the side rectifier (Fig. 13b) and Petlyuk column (Fig. 13c). The Petlyuk distillation column turned out to be the most conveniently proposed system which could reduce the energy demanded by utilizing the traditional process, remove additionally the water produced in the chemical reaction avoiding the known azeotrope condition for this mixture,and allow a higher EA compound production[65].Hernandez and Barroso-Munoz later conducted a parametric study and obtained dynamic closed-loop responses of the Petlyuk column by Aspen Plus™and Aspen Dynamics™.The steady state results showed that most of the water produced in the esterification reaction could be removed by the use of side columns or a side stream.This favored the production of EA and only the EA-water heterogeneous azeotrope was formed in the distillate product.The dynamic results showed that a PI controller could be used to control the molar composition of the EA in the organic liquid phase[66].Barroso-Munoz et al.also did a research on implementation and operation of a RDWC by a dividing wall and a side tank in order to manipulate the internal flows associated with energy consumption.The results showed that it was possible to obtain an azeotrope of EA-water as top product [67]. They also (Hernandez et al.)found that the reactive Petlyuk column could achieve set point changes in two control loops of temperature.Also,for load rejection,the control loops could eliminate the effect of the disturbances in the feed composition.These results and previous knowledge reported about thermally coupled distillation columns and RD were considered to design and implement a RDWC[68].They(Delgado-Delgado et al.)then presented the experimental results for the production of EA in a RDWC.The experimental results were in agreement with those obtained using steady state simulations with Aspen plus, which were possible to validate most of the previous simulation results[69].Then a series of experiments for the production of EA in a RD pilot column were presented.The experimental validation was shown to be essential to provide realistic hydrodynamic parameters,to understand the sensitive parameters such as heat losses and to adapt values for the catalyst holdup as a function of the system[70].They also studied the esterification of oleic acid and methanol using sulfuric acid as a homogeneous catalyst in a RDWC.The conversion of the free fatty acid was found to depend strongly on the molar ratio of methanol/oleic acid.The reaction time directly influenced the conversion of the free fatty acid,and this conversion would decrease when temperature increased[71].

Fig.13.a.Thermally coupled system arranged with the side stripper[69].b.Thermally coupled system arranged with the side rectifier[69].c.Petlyuk column[69].
Safe et al.studied the model on the reduction and optimization of a batch RDWC inspired by response surface methodology and differential evolution with the advantages of overcoming the known azeotrope conditions,high purity for EA and decreasing the batch time compared to simple reactive batch distillation.The corresponding dynamic simulation was carried out by simultaneously solving the model-associated system of differential and algebraic equations[72].
Wang et al.designed the mixed acid esterification of mixed acid(AA and propionic acid)with methanol via incorporation of DWCs.Optimal design results showed that the RDWC system saved steam consumption by 45.2%and reduced TAC by 34.5%compared with conventional RD sequence[73].
Santaella et al.made a comparison of different RD schemes for EA production using sustainability indicators(i.e.,conventional,RD,PSRD,DWC with reactive reboiler and a novel configuration using a RDWC).The results shown in Fig.14 indicated that the novel RDWC configuration proposed in this work turned out to be the most energy efficient and cost effective (46% energy and 26% cost savings compared with the conventional process)and also it was characterized by the best sustainability indicators[74].
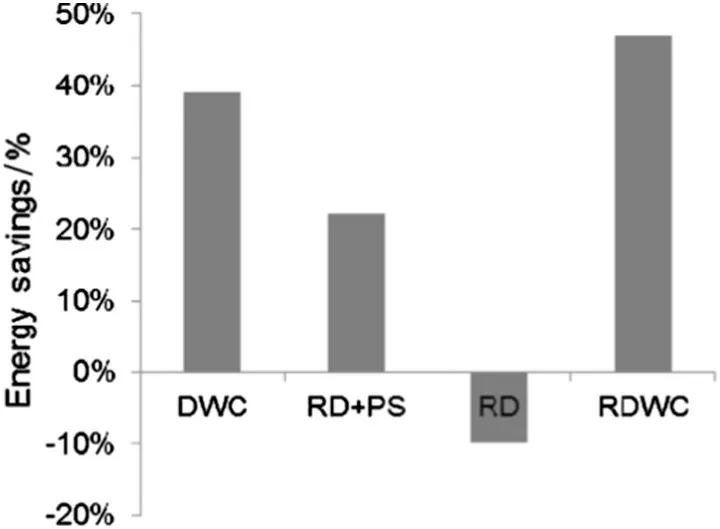
Fig.14.Comparison of different RD schemes on energy savings.
Dai et al.performed the design and control of a RDWC for the synthesis of NPP.The RDWC process was shown in Fig.15.Sensitivity analysis of whether the vapor split ratio could maintain the purity of the product was studied.The results showed that the RDWC could save 12.4%of energy consumption and 16.4%of the minimum TAC compared with the two-column design. Two control structures for the three-column model were considered[75].
Suo et al.did an investigation about energy saving for synthesis of IBA in the RDWC via transesterification between MA and isobutanol with Amberlyst-15 as catalyst whose process was shown in Fig. 16.

Fig.15.a.Integration scheme for the RDWC process.b.Equivalent scheme for the RDWC process.
The experimental kinetic parameters were obtained by the application of the Arrhenius equation. The energy consumption and the TAC of the RDWC process could be reduced by 26.40%and 18.25%,respectively,in comparison with the conventional RD process[76].
Shi et al. studied the intensifying RDWC processes via a vapor recompression heat pump(VRHP).They explored the feasibility and effectiveness of this technology in two representative RDWC processes with different operation characteristics through in-depth evaluations of the steady-state performance of the VRHP reinforced RDWC (i.e.,the proposed RDWC-VRHP).Simulation results demonstrated that the RDWC-VRHP could substantially reduce utility consumption of RDWC.Especially,much more economic benefit could be secured by VRHP for the RDWC with small column temperature difference and high latent heat in the overhead vapor[77].
Zheng et al. synthesized diethyl carbonate (DEC) by transesterification from DMC and ethanol with the use of homogeneous catalyst sodium ethoxide. The process was shown in Fig. 17. The results showed that the RDWC could save 18.7%of the energy consumption and 13.9%of the TAC compared with the conventional RD process.A control structure was also proposed[78].

Fig.16.a.Integration scheme for the RDWC process.b.Equivalent scheme for the RDWC process.
Rodriguez et al.presented the control of an extractive and a reactive DWC.They established the decentralized structure as well as a model predictive control and compared both approaches[79].
In China,many researchers are interested in the RDWCs especially for Sun et al.They(Sun et al.)proposed a novel process of RDWC for the hydrolysis of the MA simulated by Aspen Plus in 2008. They got the result of energy reduction by 19.6%compared with the conventional RD process[80].Later,they studied the control strategy of the RDWC for hydrolysis of MA based on steady-state simulation. They studied through steady-state sensitive analysis firstly, and then the control strategy was simulated by Aspen Dynamics.The results showed the PI control scheme with three temperature loops could achieve reasonable performance[81].Then they(Yang,Sun et al.)proposed a catalyzed rectifying partition wall column for the synthesis of EA simulated with Aspen Plus in 2009.They studied the effects of liquid split ratio.The energy consumption was reduced by 25.7%[82].In 2010,they proposed a novel RDWC process to convert MA to NBA simulated with Aspen Plus.The energy consumption was reduced by 17.34%[83].Then they proposed a design and optimization procedures of a RDWC sequence in 2011.MA transesterification system was investigated as an example.The procedures could be applied to retrofit a conventional twocolumn-RD sequence to a RDWC sequence and guarantee the optimal values of the design variables[84].And they(Chen et al.)performed the control of the RDWC process to produce NBA in 2016.An improved control strategy(CS3)was proposed,which showed a great advantage in reducing overshoot of the purity of the products[85].
Zong et al.performed the experiments to study the process parameters in a RDWC for producing EA and gave the optimum process parameters[86].
Tian et al.proposed a RDWC process to produce NBA,resulting in the reduction by 30%of the energy consumption[87].
Man et al.proposed a RDWC process to produce n-propyl acetate simulated with Aspen Plus.Energy consumption of the novel process could be reduced by above 15%in comparison with the conventional catalytic distillation process.The cost of equipment is decreased,too[88].
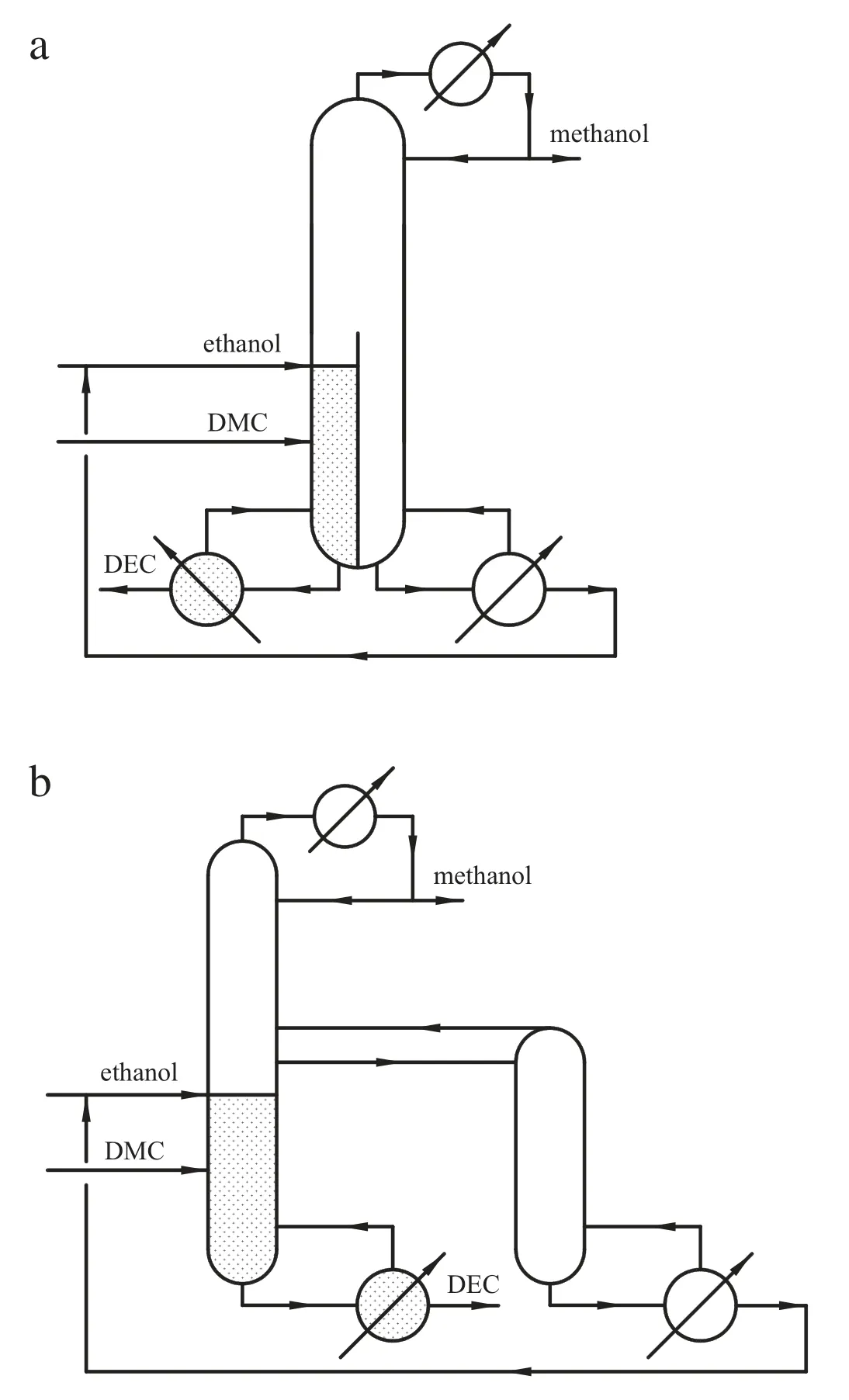
Fig.17.a.Integration scheme for the RDWC process.b.Equivalent scheme for the RDWC process.
Hao et al.proposed a novel RDWC with a single reaction section for transesterification of NBA and ethanol. The TAC could be decreased compared to the conventional RD process,and it could achieve complete separation of the mixtures[89].
Li proposed a RDWC process with two external recycles to deal with the problem existed in RD with a top-bottom external recycle,where the separation of quaternary reacting mixtures may be limited because the unconverted lightest or heaviest reactants within them will unavoidably pass through the side-draw stage.The novel process reduced the amount of the unconverted lightest and heaviest reactants passing through the side-draw stage by limiting them in the feeding side of the dividing-wall and both ends of the column.Esterification of lactic acid and methanol was investigated to evaluate the strategy resulting in a TAC reduction by 27.92%[90].
Bao et al.used the RDWC to produce isopropyl alcohol through the transesterification of isopropyl acetate and methanol with sodium methoxide as catalyst. The results showed that the RDWC process could get more than 99.8%purity of isopropyl,98.54%yield of isopropyl alcohol,and 20.4%energy saving[91].
For the production of fine chemicals,enzymes have become an attractive alternative to traditional chemical catalysts due to their increased regio- and enantioselectivities [92]. Egger and Fieg did experiments and model validation on an enzymatic catalyzed RDWC(eRDWC).This apparatus enabled the simultaneous production and separation of up to 4 pure product streams.Comprehensive experiments with the reference system of the transesterification of hexanol and NBA in a DN 65 pilot scale column (Fig. 18) showed the feasibility of steady operations for this process [93]. They also presented the first comprehensive experimental study of the dynamic behavior of RDWCs.The reference system of enzymatic catalyzed NBA transesterification with hexanol had been employed to investigate the start-up and open loop behavior under different operating conditions. Two different start-up strategies were tested and compared.The experiments demonstrated the reliable and secure start-up and stable operation of a RDWC.Additionally,a developed rigorous dynamic RDWC model was presented that considered the dynamic influence on the vapor distribution in the dividing wall section. A detailed model validation was carried out using the obtained experimental data.The comparison of simulation results and experimental values showed good agreement over a wide range of operating conditions[94].
RDWCs can reduce the number of heat exchangers,DCs and reactors resulting in reduction of capital cost.Meanwhile,RDWCs can reduce the energy consumption of condensers and reboilers because of the reduction of the remixing of middle-boiling components.RDWCs have the potential to add value in chemical processes involving reactions and multicomponent separations compared to the traditional process such as reactors in series with distillation columns. Although popular in chemical processes due to their ability to present significant capital and operating cost savings, RDWCs have not been commercially adopted[61].

Fig.18.Scheme of the RDWC pilot plant with packing sections(S),temperature sensors(T),collector/distributors(CD).
8.Novel Methods of Loading Catalyst
The RD process requires the reaction and distillation performing in one unit.Thus,the equipment integration of the units is very important.The improvement of the equipment can be considered in terms of the distillation process and the reaction process.
Von Harbou et al.studied the D+R tray as shown in Fig.19,which was a novel type of laboratory equipment used by the heterogeneously catalyzed RD.For both test systems,the most important process parameters such as feed rate and the amount of catalyst were systematically varied.High conversion of the reactants and high purity of the products were achieved[95].
Sun et al.developed a novel structured catalyst parking,which had a number of advantages including low pressure drop,high flooding gas velocity,stable liquid holdup,large catalyst loading fraction,and easy parking and removal of catalyst[96].

Fig.19.Schematic drawing of a D+R tray:I distillation section,II reactive section,(1),(2),(6) downcomers, (3) sieves, (4) upper catalyst port, (5) lower catalyst port, (7) gas chimney,(8)venting pipe,(9),(10)sampling port,(11)temperature measurement.The gray area indicates the catalyst bed[95].
Huang et al.developed a new type of multi-layer wire mesh packing PACTU-800 with a big operating flexibility and holdup,which is better to be used under high vapor and liquid load,and was featured by a large flux,small change of pressure drop,long residence time and high efficiency.Its hydrodynamics and mass transfer performances were tested by the designed pilot-scale equipment[97].
Li et al.did an experimental research for the reaction magnitude in the solid reactive spray tray(SRST,shown in Fig.20)by using the strong acidic ion-exchange resin as catalyst and taking the reaction of AA with ethanol as an example.The effects of catalyst height,ethanol/AA molar ratio and flow rate on reaction magnitude were studied.An empirical equation of reaction magnitude was regressed from the experimental data.A model was established to describe the gas and liquid phase compositions of EA of each tray in the SRST based on the empirical equation and mass fraction of EA.The model was verified by experiments in industrial equipment[98].

Fig.20.Structure of SRST.
9.Multiple Coupling
The author's group (Li et al.) proposed and studied a process of azeotropic-reactive dividing wall column(A-RDWC)shown in Fig.21 to produce EA via esterification with Amberlyst-15 as the catalyst and NBA as the entrainer.An experimental set was built up and the results of the simulation agreed well with the experiment.The influences of the amount of entrainer,liquid split ratio,reflux ratio and the concentration of AA were simulated with Aspen Plus.Compared with the traditional RD process,the A-RDWC process could avoid the backmixing of the intermediate component and separate the heavy component and intermediate component clearly [99]. Xie et al. then proposed a novel method of loading catalyst as shown in Fig.22.And they studied the reliability of the model of the simulation which could be verified through the experiments in a RDWC as shown in Fig.23.They found that the height (H) between the catalyst beds was an important parameter that affected the pressure drop.The structural parameter H had an optimal value to achieve the optimal contact between the gas and liquid phases in the reaction section of the column.The results showed that the RDWC could save 34.7%of total operating costs and 18.5%of TAC compared with the traditional RD column[100].
Li et al. proposed a process for co-production of EA and NBA in a RDWC with NBA as not only the product,but also the entrainer to remove the water generated by the two esterification reactions.The experimental results were in good agreement with the simulation results. Two kinds of RDWC structures (RDWC-FC and RDWC-RS)were proposed as shown in Figs.24 and 25,and the operating parameters of the two types of RDWCs were optimized by Aspen Plus respectively. The results showed that the reboiler duty of the two types could be reduced by 20.4%and 17.0%respectively,and the TAC could be saved by 23.6%and 17.0%respectively compared with the traditional RD process[101].
There are also other process intensification measurements in the RD process for the production of esters like adsorption-RD[102,103]and microwave-assisted RD[104].

Fig.21.A flowsheet of A-RDWC.
Like all the methods mentioned in this article,solving the problems which exist in the conventional process is the purpose of process intensification in the RD process,resulting in energy saving and cost reduction.Such problems as the separation of the azeotropes and the removal of water to get a high conversion of the reactants or selectivity and purity of the products can be solved by process intensification through process coupling,thermal coupling and equipment improvement.

Fig.22.Schematic diagram of catalyst loading inside the reaction section.

Fig.23.Schematic diagram of the RDWC.

Fig.24.A flowsheet of RDWC-FC.

Fig.25.A flowsheet of RDWC-RS.
Process intensification is a popular trend in the chemical industry.In the RD process for ester production,the coupling of new energy-saving technology,improvement of catalyst loading method and equipment integration are the main ideas to improve and intensify the process,which will be more effective after seeking synergistic effect among all the involving conventional processes.Deliberate consideration of synergistic effect in process synthesis rather than the simple combination can help reduce the energy costs and capital costs.Analyzing and evaluating all the coupling for intensification from conceptual feasibility to application feasibility are necessary.In this way,more potential of energy saving and capital-cost reduction can be developed from concept to industrial application.
Nomenclature
AA acetic acid
A-RDWC azeotropic-reactive dividing-wall column
ARD/ERD azeotropic reactive distillation/entrainer enhanced reactive
distillation
DC distillation column
DEC diethyl carbonate
DMC dimethyl carbonate
DWCs dividing-wall columns
EDC ethylene dichloride
EG ethylene glycol
EO ethylene oxide
eRDWC enzymatic catalyzed reactive dividing-wall column
IBA isobutyl acetate
ILs ionic liquids
IPA isopropyl acetate
MA methyl acetate
MTBE methyl tert-butyl ether
NBA n-butyl acetate
NPP n-propyl propionate
PI proportional integral
PSRD pressure-swing reactive distillation
PVA polyvinyl alcohol
RD-PV Reactive distillation-pervaporation coupled process
RD reactive distillation
RDWCs reactive dividing-wall columns
RED reactive-extractive distillation
TAC total annual cost
TBA tert-butyl alcohol
TCC total capital cost
SRST solid reactive spray tray
VRHP vapor recompression heat pump
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Recent advances in acid-resistant zeolite T membranes for dehydration of organics☆
- Process intensification in vapor-liquid mass transfer:The state-of-the-art☆
- Extractive distillation:Advances in conceptual design,solvent selection,and separation strategies☆
- Beyond graphene oxides:Emerging 2D molecular sieve membranes for efficient separation☆
- A review of internally heat integrated distillation column☆
- Recent progress and future prospects of oil-absorbing materials☆
