Intravitreal treatment realities and experiences in diabetic macular edema
2019-08-15
Abstract
INTRODUCTION
Diabetes mellitus (DM) is a global health disease and has an increasing prevalence worldwide. The ophthalmic manifestation diabetic macular edema (DME) is one of the major causes of visual impairment[1]. DME can emerge within the first five years of type 1 diabetes. The DME prevalence increases from 0-3% to 28-29% in patients who have type 2 diabetes for 20y[2-3]. It is estimated that nearly 750,000 people are affected by this central vision-threatening disease in the United States. As well, DME will cause at least three lines of visual loss in 24% of eyes in 3y[4-5]. In DME, mostly vascular endothelial growth factor (VEGF) and other inflammatory factors like interleukin-6, intercellular adhesion molecule-1, and monocyte chemotactic protein-1, have been considered responsible from abnormal vascular permeability[6-7]. In the majority of patients, macular laser photocoagulation is inadequate in visual improvement[8]. RIDE and RISE, READ-2, RESTORE and RESOLVE were ranibizumab based randomized controlled clinical trials in which outstanding visual outcomes were achieved with ranibizumab when compared with macular laser or sham injection[9-12]. Recently, the three commonly used intravitreal VEGF inhibitors, bevacizumab (Avastin, Genentech), ranibizumab (Lucentis, Genentech), aflibercept (Eylea, Regeneron Pharmaceuticals), and the slow-release intravitreal steroid dexamethasone implant (Ozurdex, Allergan) have been shown to be safe and effective for DME treatment[13-16]. Aflibercept, ranibizumab, and dexamethasone implant are Food and Drug Administration (FDA) approved for DME treatment but bevacizumab which has not been approved for any ocular indication is widely used off-label[17].
Visual gain and the benefits of anti-VEGF therapies of DME are evaluatedclearly in pivotal studies but “Can we obtain those results in real life under real-life limitations?” The purpose of this study is to explore the real life experiences and outcomes in DME patients who were treated with bevacizumab, ranibizumab or aflibercept.
SUBJECTS AND METHODS
All patients from Ankara Numune Training and Research Hospital Retina Service who had received an initial intravitreal anti-VEGF treatment related to central-involved diabetic macular edema, and had follow-up duration over than 6mo after the injection between June 2012 and December 2016 were selected. This study was designed as a cross-sectional retrospective study and followed the tenets of the Declaration of Helsinki and carried out with ethical approval from the Institutional Ethics Committee. The real life data were captured from patient records which were investigated retrospectively.
Each patient had bilateral assessment for the median logarithm of the minimal angle of resolution (LogMAR) visual acuity (VA) converted from Snellen measurements, anterior segment and fundus examination and spectral domain optical coherence tomography (SD-OCT) Zeiss Cirrus HD-OCT 4000 (Carl Zeiss Meditec AG, Germany) 6×6 mm scans for macular morphology and thickness at each visit scheduled 1mo apart. SD-OCT image database was used to assess the retinal images with reports.
All patients were also evaluated with fundus fluorescein angiography. Patients with non-proliferative diabetic retinopathy who had central macular thickness (CMT) over than 300 μm with intraretinal and/or subretinal fluid in SD-OCT and best-corrected visual acuity between 1.2 LogMAR and 0.3 LogMAR were included in the study. A standard or fixed treatment protocol wasn’t followed, and all the treatment decisions were given according to patient requirements. An appointment system was used to perform all intravitreal injections within 1 to 5d after the last visit in an operating room. Then, patients were recalled at 1mo intervals. Retreatment with an intravitreal anti-VEGF was planned when the central macular thickness was >300 μm in OCT scans with remaining intraretinal and/or subretinal fluid in the follow-up visit. A dry macula was defined as CMT ≤300 μm without

Figure1ChangesofLogMARvisionacuitybetweenbaselineand24mo.
any residual fluid or with some small amount of cystoid cavities in the fovea resembling a chronic degenerative change but unlike a cystoid macular edema. Switching to another anti-VEGF, laser rescue therapy or intravitreal steroid implant were discussed in persistent DME cases who had less than 10% decrement in CMT after 3 or 4 monthly intravitreal anti-VEGF injections without a visual gain. Patients who received intravitreal steroids were not included in the study. A laser rescue therapy (as deferred laser therapy) with PASCAL Streamline Yellow (wavelength, 577 nm) was planned six months (24wk) after the initial intravitreal therapy[18].
Currently, we use individualized therapies of proactive regimens, but during the study period, our treatment regimen fitted more to the pro re nata (PRN) approach of the anti-VEGF treatment of DME. Subjects having an epiretinal membrane, vitreomacular traction, vitreous haemorrhage, proliferative diabetic retinopathy, fluorescein angiographic macular ischemia, any type of age-related macular degeneration, degenerative myopia, uveitis, previous trauma, and posterior segment surgery or intraocular surgery, past laser history or sequential intravitreal injections, less than 6mo follow-up were excluded from the study.
Visual acuity, CMT, the number of total visits and injections at baseline and months 3rd, 6th, 12th, 18th, and 24thwere the assessment parameters.
Statistical analyses were performed with the SPSSversion 17.0 (SPSS Inc., Chicago, IL). The Related Samples Wilcoxon Signed Rank Test was used to determine the statistical significance of the changes in the VA and SD-OCT. A value ofP<0.05 was considered statistically significant. Other results were given as means±SD.
RESULTS
Fifty-four eyes of 40 patients were included in the study. 22 of the total subjects (55%) were female and 18 (45%) were male. The mean ages were 60.59±8.9 and 62.3±11.17 years in female and male subjects, respectively. The mean duration of diabetes mellitus was 14.7±4.9y. The mean follow up time was 21.22±6.88mo. 14 eyes had local peripheral ischemic pattern in their four quadrant fluorescein angiographic images. None of them had macular ischemic pattern in their fluorescein angiography.

Figure2Meantotalinjectionsandvisitnumbersin3,6,12,18and24mo.
The changes of LogMAR vision during the follow-up period is given in Figure 1. The differences in visual acuity between baseline and 3rd, 6th, 12th, 18thand 24thmonths were not statistically significant (P>0.05, Wilcoxon Signed Ranks Test).
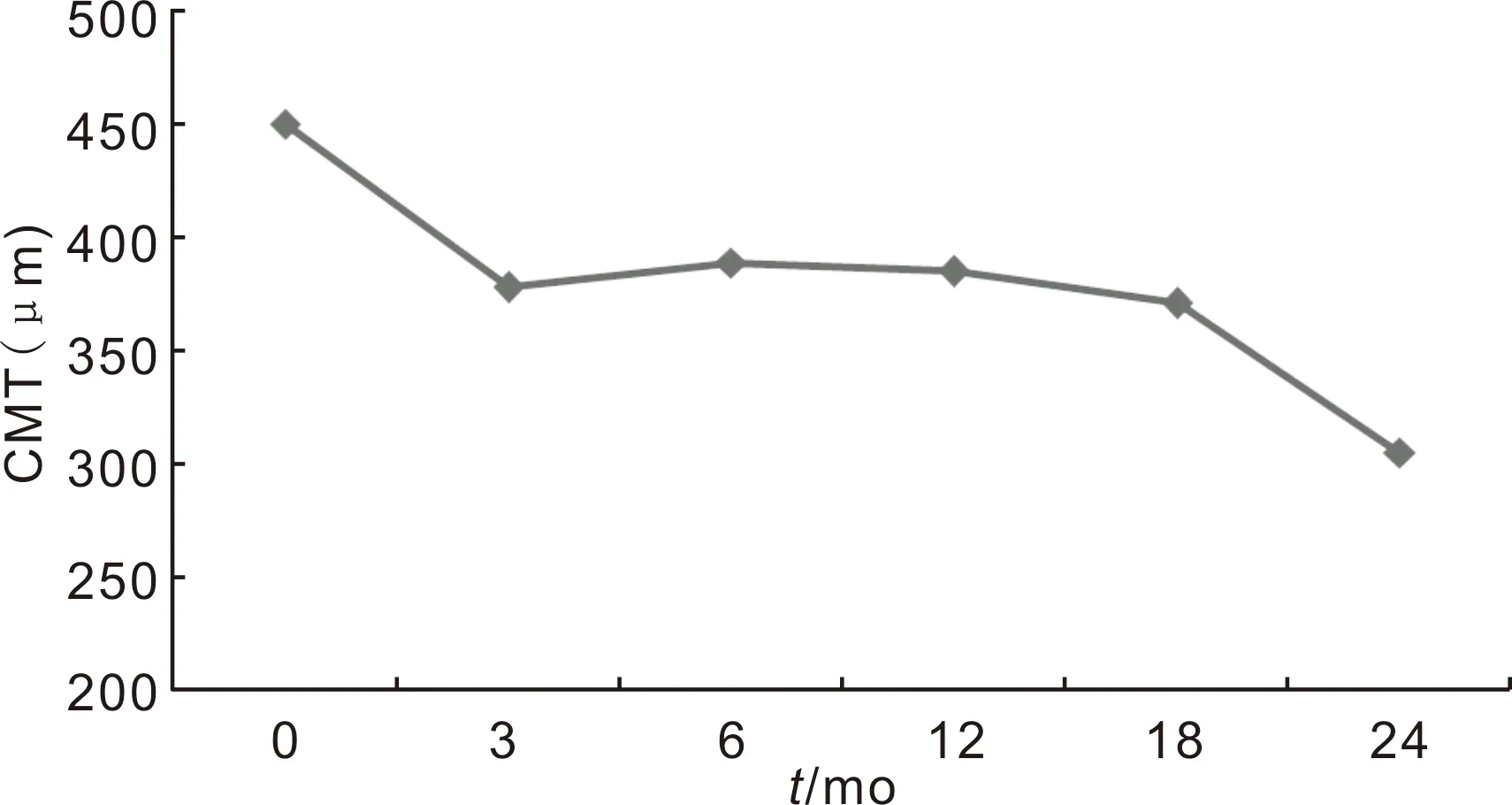
Figure3Changesofcentralmacularthicknessbetweenbaselineand24mo.
The mean number of intravitreal injections was 3.52±2.19 and 6.15±3.65 in the 12 and 24mo, respectively. The average number of patient visits were 9.0±2.39 in the 12mo, and 15.48±4.8 in the 24mo follow-up (Figure 2).
The mean baseline CMT was 450±153 μm and decreased to 385±141 μm at 12mo. The final CMT was 305±111 μm in the second year. The reduction in CMT at 3th, 6th, 12th, 18thand 24thmonths from baseline were statistically significant (P=0.001,P=0.014,P<0.001,P=0.025 andP<0.001, respectively for Wilcoxon Signed Ranks Test,P<0.05) (Figure 3).
Ranibizumab was the most frequently used intravitreal agent for DME. The peak of intravitreal treatment application term was between 6-12mo. Furthermore, the preference of bevacizumab injections decreased gradually while aflibercept preference increased throughout the second year. DME affected eyes receiving an intravitreal agent increased throughout the first year, and intravitreal treatment requirement continued at the same intensity in the second year (Figure 4).
Eleven eyes received laser rescue therapy (only focal treatment) between 6-12mo after the baseline assessment. 48 (89%),46 (85%) and 43 (80%) eyes completed the 12mo, 18mo and 24mo visits respectively. All the intravitreal treatment agents were tolerated well, and none of the patients had procedure-related serious adverse events.
DISCUSSION

Figure4Theintravitrealinjectionprofileofanti-VEGFagentsusedforcentral-involvedDMEamong0-3mo,3-6mo,6-12mo,12-18moand18-24mo.
This study investigates the real life outcomes of intravitreal anti-VEGF treatment in DME patients under real-life limitations and decisions. Visual gain is one of the primary goals of intravitreal anti-VEGF treatment in DME, and the visual gain was maximum in the third and twelfth month of this study. Throughout the second year, the visual gain achievement was relatively maintained with intravitreal treatment and VA was fixed slightly above the baseline value.
The first real life data was from ADMOR study that assessed ranibizumab efficacy in South Asian DME patients[19]. Also, Hraratetal[20]reported a mean gain of 10.7 letters at the 12mo of the treatment in a real life study with ranibizumab. The best corrected visual acuity (BCVA) changed from 48.3 letters (20/100=0.7 LogMAR) to 59 letters (20/63=0.5 LogMAR) in this study. Vyasetal[21]found that the VA increased from 0.80 LogMAR to 0.68 LogMAR at 6wk, 0.63 LogMAR at the 3mo and 0.6 LogMAR at the 6mo with bevacizumab injections. Mushtaqetal[22]found a mean visual gain of 0.12 LogMAR (6 letters) with bevacizumab injections at 12mo.
PRN treatment protocol is described as retreatment on proof of exudative disease activity on monthly visits. The intraretinal and/or subretinal fluid is a marker of disease activity and treatment requirement[23]. In a treat and extend (TAE) protocol, monthly injections are used until the exudation is resolved. Then, the time to retreatment is lengthened 1-2wk as long as a sign of recurrent exudation is absent. In patients with recurrence of exudation, treatment interval is reduced. In TAE protocol the main objective is to obtain and maintain a dry macula without recurrences as in PRN treatment. It is believed to reduce the treatment burden by fewer visits, diagnostics and therapeutics[24].
In the ranibizumab therapy arm of the RESTORE study; the mean number of injections in the 1styear was 7 injections. In the RELIGHT study, the patients received 8.5 injections over 18mo[11,25]. The mean number of injections in real life studies ranged between 3.3 to 5 over the 12mo follow-up period[22,26]. Our results are similar to the study by Mushtaqetal[22]with a mean of 3.52±2.19 injections. Sugimotoetal[27]reported a mean of 8.8 TAE injections in 24mo period and we completed the second year with a mean of 6.16±3.65 injections. They discussed that subjects with the TAE regimen required fewer office visits (range 7-9 in 2y) and had better vision stability. We believe that our low mean injection values originated from more PRN approach.
Granströmetal[26]resulted mean of 14 visits (10-19) and Hraratetal[20]resulted mean of 8.8 visits (4-13) per a patient over the 12mo. We have approximate results with Hrarat, and we completed the second year with a mean of 15.48±4.84 visits, approximately 1.5mo apart between each visit. The patient visits for these long-term follow-up patients were stable and regular.
The mean baseline CMT reduction in OCT after intravitreal treatment was 10, 11 and 12% in the 3, 6 and 12mo respectively. It was completed with 30% at the end of the 2y. Mushtaqetal[22]found 33.6 μm OCT decrement in group 1 (baseline CMT thickness <400 μm) and 146 μm in group 2 (baseline CMT thickness >400 μm) in the first year. The CMT proportional reduction in group 1 was similar to our results for the first year. Also, mean CMT reduction from baseline to 12mo was 127±158 μm (approximately 30%) in RELIGHT study[25]. These results suggest that the decrease in CMT after intravitreal anti-VEGF treatment is higher in patients with higher baseline CMT if the baseline CMT is higher.
The major limitation of this study was the absence of a standard treatment model. Three different anti-VEGF forms were used to assess the effects of intravitreal therapy on whole functional and anatomical outcomes. The number of used anti-VEGFs (Figure 4) and clinical severity of diabetic macular edema in intravitreal treatment subgroups were not proportional to each other, so we couldn’t compare the efficacy of intravitreal agents. However, it is evident that a unique treatment protocol of PRN, TAE or monthly regimen cannot be used in all subjects under real life clinical circumstances.
In DRCR net Protocol T, over 40% of study eyes required a deferred laser and more than 30% of ranibizumab-treated eyes (every 4wk in 24mo) needed a macular laser in phase 3 RISE and RIDE trials[9,17]. Currently, we think laser treatment is still an essential step in diabetic retinopathy and macular edema treatment and should be used in necessary conditions. Also, some of the ongoing studies focused on if the laser treatment can be used in combination with anti-VEGFs[28]to reduce intraocular VEGF-load, treatment burden and risks of intravitreal therapy while stabilizing DME. It has been shown that PRP reduces plasma VEGF levels in 4mo after treatment[29].Also, it is known that the use of sub-threshold macular laser therapies causes minimal collateral damage, and when used in selectively targeted areas like peripheral ischemia they may provide better results both in diabetic retinopathy and DME treatment.
In general assessment, we believe that the number of visits was adequate in our study. If we accept VA and CMT as the criteria of success in the intravitreal treatment of DME, the first and second-year results are satisfactory. We maintained and protected the baseline VA. Also, we think it is a satisfactory result that 80% of the eyes completed the second year follow-up. This is likely to increase if sufficient awareness is raised about the severity of diabetic macular edema in patients. Mainly, in the first half of the study period, ranibizumab was the most available and frequently preferred anti-VEGF worldwide, as well it was the first approved anti-VEGF for DME by FDA. We think that was the most influential reason in the treatment decision and high ranibizumab percentages in our clinical applications. Also, clinicians moved away from off-label medicines while new and approved options became available (Figure 4).
We believe that age, presence of systemic comorbidities, poor economic and social attainment to treatment, poor education, and awareness of the disease, uncontrolled diabetes, PRN treatment without loading dose applications, the presence of an appointment system for intravitreal injections are the most significant limitations for intravitreal treatment effectivity. As a result of more PRN approach in this study, the visit numbers are adequate, but according to pivotal studies’ results, our injection numbers reflect undertreatment. So, a PRN protocol with loading doses or TAE of a proactive regimen might be an advantageous option to increase treatment effectivity or decrease treatment burden which was indicated in RETAIN study[30].
In conclusion,PRN approach in DME treatment may keep the VA stable and decrease CMT, but probably similar or better treatment effectivity could be obtained with a lower visit burden in proactive regimens.
