Neoadjuvant endocrine therapy: A potential strategy for ER-positive breast cancer
2019-08-14LiTongYaoMoZhiWangMengShenWangXueTingYuJingYiGuoTieSunXinYanLiYingYingXu
Li-Tong Yao,Mo-Zhi Wang,Meng-Shen Wang,Xue-Ting Yu,Jing-Yi Guo,Tie Sun,Xin-Yan Li,Ying-Ying Xu
Abstract
Key words: Breast cancer; Neoadjuvant endocrine therapy; Neoadjuvant chemotherapy;Aromatase inhibitor; Palbociclib; Ki67; Genomic assay
INTRODUCTION
Neoadjuvant chemotherapy (NAC) has been defined as a standard treatment option for localized or locally advanced breast cancer.NAC is used to downgrade and downsize the tumor,which can decrease the extent of surgery and increase the likelihood of breast-conserving surgery (BCS).Moreover,it can improve the long-term prognosis for patients whose operative specimen showed a pathological complete response (pCR)[1,2].Clinical evidence has demonstrated that the status of hormone receptor (HR) and human epidermal growth factor receptor 2 (HER2) leads to a corresponding response and efficacy of NAC[3].However,patients with estrogen receptor (ER)-positive,HER2-negative (ER+/HER2-) breast cancer show limited results from NAC,with a lower pCR and poor objective response rate (ORR)[4].Thus,an alternate neoadjuvant approach is required for breast cancer of this subtype.There is now increasing data that neoadjuvant endocrine therapy (NAE) may be a more appropriate treatment strategy than NAC[5].
Traditionally,NAE has been reserved for locally advanced breast cancer in chemotherapy intolerant or feeble senior patients due to its indolent clinical response and difficulties in efficacy evaluation[6].However,since the development of thirdgeneration aromatase inhibitors (AIs),several studies were conducted to evaluate the effects of NAE on ER+ breast cancer[7,8].Neoadjuvant AI has comparable efficacy to NAC in terms of pCR,ORR,and BCS,suggesting the feasibility of this well-tolerated strategy,mainly for postmenopausal patients[9].The choice of chemotherapy or endocrine therapy (ET) as neoadjuvant treatment depends on disease characteristics and patient subtypes.We will discuss the most relevant clinical trials to illustrate whether NAE,either monotherapy or combination therapy,can serve as a potential option in patients with ER+/HER2- breast cancer.
Furthermore,optimal NAE settings can optimize treatment response and achieve the maximum therapeutic effect for ER+ breast cancer[10].Emerging evidence has revealed the efficacy of different endocrine agents in presurgical application,including AIs,tamoxifen,and fulvestrant.The identification of optimal agents can result in tailored treatment for both postmenopausal and premenopausal patients.Combining targeted agents with AI or fulvestrant yields promising effects for postmenopausal patients with advanced or metastatic breast cancer[11,12].The results are being translated to neoadjuvant settings and provide possibilities to fulfill the requirements of drug resistance mechanism exploration and new drug development[13].Available neoadjuvant endocrine settings are generally favorable for ER+ breast cancer patients,but not all patients benefit equally.The neoadjuvant period can provide a usable platform for tumors to receive biopsy on treatment,leading to the exploration of predictive tools that can screen suitable individuals and select treatment options in breast cancer[14,15].Current evidence suggested that compared with traditional tumor staging,biomarkers,especially Ki67,and genomic assays offered more accurate prediction information.This evidence provides novel insight into the development of biomarker-based strategies in NAE.
In this review,we will detail the most relevant evidence to verify the potential role of NAE.We identify optimal settings,including optimal duration,optimal endocrine agents,and optimal targeted agents,and then evaluate the prognostic role of biomarkers and genomic assays in NAE.NAE can act as a prospective strategy and scientific platform for optimizing treatment efficacy,screening suitable individuals,and investigating mechanisms of drug resistance in ER+/HER2- breast cancer.
NAE AS A POTENTIAL APPROACH IN THE NEOADJUVANT SETTING
NAE monotherapy
Several studies have already proved that NAE had a similar beneficial therapeutic effect to NAC for ER+ breast cancer (Table 1).A phase II clinical trial published by Semiglazovet al[16]suggested that there was no significant difference between the NAC arm (doxorubicin plus paclitaxel) and NAE arm (anastrozole or exemestane) in terms of clinical response (63.6%vs64.5%),ultrasound response (46%vs40%),or mammographic response (63%vs60%).In comparison with the NAC arm,the NAE arm showed an improved BCS rate (24%vs33%,P= 0.058).Similar results were provided in the GEICAM/2006-03 trial between the NAC arm (epirubicin,cyclophosphamide,and docetaxel) and NAE arm (exemestane) in both clinical response rate (CRR 66%vs48%,P= 0.075) and BCS rate (47%vs56%,P= 0.2369)[5].Meanwhile,a subgroup analysis suggested that NAC had a clear advantage over NAE in premenopausal women (CRR,75% and 44%,P= 0.027),whereas in postmenopausal women,this advantage disappeared.
Regarding the safety of neoadjuvant settings,grade 3/4 toxicity was more common in NAC therapy than in NAE therapy (47%vs9%,P< 0.001)[5].The recent,multicenter NEOCENT trial also proposed that the NAC group had more serious adverse effects(AEs),such as alopecia,vomiting,stomatitis,and anemia,than the NAE group,affirming the safety and tolerability of NAE[17].Furthermore,a meta-analysis including five randomized controlled trials with 538 patients,of whom 267 (49.6%)received NAE and 271 (50.4%) underwent NAC,can be considered the best evidence.This meta-analysis indicated that NAE was as efficacious as NAC in clinical response and increased the rates of BCS and wide local excision,with better tolerability[9].Given the efficacy and low toxicity correlated with NAE,this treatment option as a potential alternative treatment strategy to NAC,especially in postmenopausal patients,was encouraged.
NAE combination therapy
Based on this promising knowledge of NAE,neoadjuvant chemo-endocrine therapy(NCET),which combined NAE and NAC,was a potential treatment option for hormone-sensitive patients (Table 1).NCET has been explored in comparison with either NAE or NAC therapy.Nakayamaet al[18]compared the efficacy between the single-agent anastrozole group and the anastrozole plus UFT (tegafur/uracil) group in the Neo-ACET BC trial.There was a greater tendency of tumor degeneration in the NCET group than in the NAE group,although the study was halted due to altered liver function.A recent trial enrolled 63 primary invasive breast cancer patients with initial exemestane treatment followed by response-dependent addition of cyclophosphamide.The results suggested that the clinical response of nonresponders was improved to be comparable to that of responders by the addition of cytotoxic agents to the endocrine agents (CRR for nonresponders and responders at weeks 24 and 36; 54%vs85% and 71%vs71%)[19].NECT has a broad-range antitumor activity and favorable efficacy over NAE monotherapy.
In comparison with NAC and NECT,Mohammadianpanahet al[20]indicated that the addition of letrozole simultaneously with neoadjuvant FAC (5-fluorouracil,doxorubicin,and cyclophosphamide) therapy significantly increased clinical and pathologic response rates compared with chemotherapy alone.The CSCSG-036 trial confirmed that concurrent NAC and estrogen deprivation provided higher effectiveness in ER+ breast cancer (CRR,84.8%vs72.6%,P= 0.02),especially for those with high Ki67 expression (91.2%vs68.7%,P= 0.001)[21].Similar toxicities were observed in the NCET and NAC arms,further supporting the role of NCET as a potential neoadjuvant treatment option.Owing to its promising clinical response and acceptable toxicity,NCET has shown great development prospects for ER+/HER2-breast cancer patients.
OPTIMAL SETTING OF NAE
To understand the optimal settings for NAE,we will discuss clinical trials to identify the optimal duration,endocrine agents,and targeted agents (Tables 2 and 3).
Optimal duration of NAE
The duration of NAE treatment in most clinical trials was approximately 4-6 mo,based on experience; however,some investigators noted that this common duration might not be sufficient to achieve the best results in tumor shrinkage[22-24].Severalclinical trials were established to assess the optimal duration of neoadjuvant AIs that would permit tumor regression and BCS eligibility for initially unsuitable patients[23].A study comparing the tumor size of patients receiving exemestane treatment at 3 and 6 months revealed that extended exemestane therapy had a potential to significantly reduce tumor volume[25].A phase IV clinical trial verified this conclusion with a slightly larger number of participants and suggested that 7.5-mo neoadjuvant letrozole therapy was optimal to achieve beneficial shrinkage in tumor volume and facilitate BCS,in comparison with 4-month conventional treatment[22].Overall,longterm neoadjuvant treatment achieves further tumor reduction and increases the feasibility of the BCS rate,but the optimal treatment duration for NAE is still unknown and needs to be further investigated.
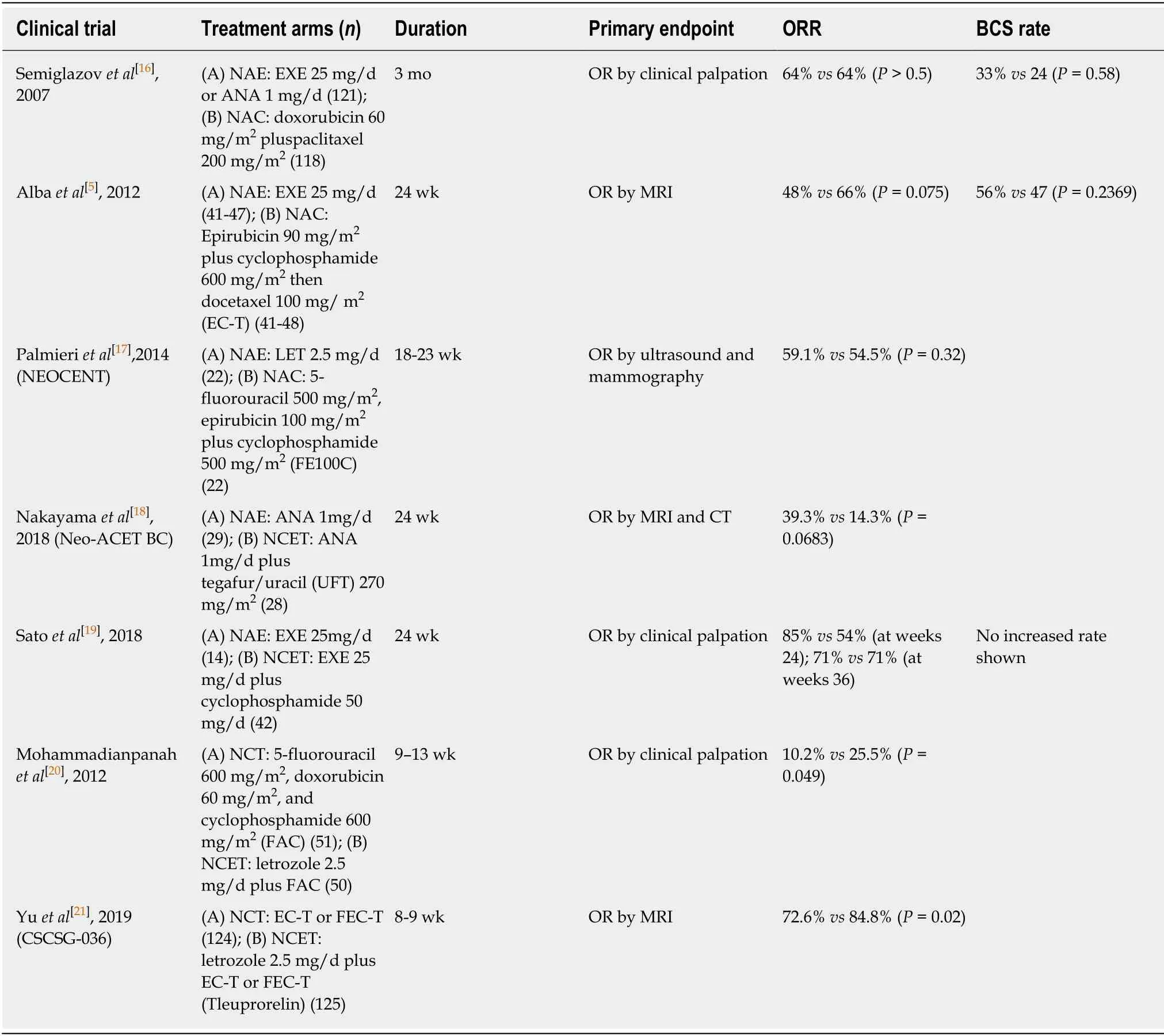
Table 1 Neoadjuvant endocrine therapy and neoadjuvant chemo-endocrine therapy as potential approaches in the neoadjuvant settings
Optimal endocrine agents for NAE
In the following paragraph,we will review clinical trials that were conducted to evaluate the efficacy of different endocrine agents and identify the optimal choice in both premenopausal and postmenopausal patients.
Neoadjuvant AIs vs tamoxifen:Clinical evidence favoring the three third-generation AIs,letrozole,anastrozole,and exemestane,rather than tamoxifen,in neoadjuvant treatment was established in several randomized clinical trials for ER+ breast cancer.For both the CRR and BCS,letrozole was superior to tamoxifen and had less toxicity,as shown in the P024 trial[7].There was no statistically significant difference betweenanastrozole and tamoxifen in ORR; however,anastrozole was more effective than tamoxifen in certain clinical subgroups of patients in the IMPACT and PROACT trials[26,27].Semiglazovet al[16]proved that the exemestane group exhibited higher CRR and BCS rates than the tamoxifen group,but there was no difference in the effectiveness,as shown by ultrasound and mammography.A meta-analysis of seven randomized trials further demonstrated the efficacy of neoadjuvant AIs.There was significantly higher clinical and radiological response rates (OR = 1.69 and 1.49,respectively,P< 0.001) and BCS rate (OR = 1.62,P< 0.001) in the AI arm than in the tamoxifen arm[10].As illustrated,neoadjuvant AIs treatment possessed better efficacy than tamoxifen.

Table 2 The optimal duration and optimal endocrine agents of neoadjuvant endocrine therapy
Neoadjuvant AIs vs fulvestrant:Fulvestrant is a selective ER degrader that is recommended by NCCN guidelines as first-line ET for HR+ metastatic breast cancer after progression on TAM or AI[28].Limited reports have appraised the appropriatetreatment dosing and clinical value of fulvestrant in NAE.The phase II NEWEST trial reported that 500 mg fulvestrant was significantly related to greater early reduction in the levels of ER (-25.0%vs-13.5%,P= 0.0002) and Ki67 (-78.8%vs-47.4%,P< 0.0001)than 250 mg.Meanwhile,individuals also had better responses at the recommended 500 mg dose (CRR at week 16,22.9%vs20.6%)[29].A high-dose regimen of fulvestrant would improve clinical response and biological activity for ER+ breast cancer in NAE.Together with the same dose-dependent advantages in adjuvant settings,we support the application of 500 mg fulvestrant in future clinical practice.

Table 3 The optimal targeted agents
To determine the efficacy of fulvestrant,a short-term neoadjuvant study first compared the biological activity of fulvestrant plus anastrozole treatmentvseither agent alone.Quenel-Tueuxet al[30]evaluated the utility of these two agents in 120 postmenopausal breast cancer patients who were not eligible for primary BCS,with different results.They demonstrated that the fulvestrant arm yielded equal effectiveness as the anastrozole arm in both objective response rate (53.8%vs58.9%)and BCS rate (50.0%vs58.9%)[30].The CARMINA 02 trial also showed that the efficacy and tolerability of fulvestrant were similar to those of anastrozole[31].These outcomes suggested the excellent therapeutic effects of fulvestrant and encouraged further exploration to verify whether it can serve as an ideal agent to replace well-recognized and valuable AIs in neoadjuvant therapy.
Choice of different aromatase inhibitors: To explore the choice of the beneficial AIs,the ACOSOG Z1031A trial involved 377 postmenopausal women with stage II/III ER+ (Allred score,6 to 8) breast cancer.These patients were randomized to treatment with presurgical exemestane,letrozole,or anastrozole for 16 wk.The three AIs had clinically and biologically equivalent effects,as the CRR was 60%,72%,and 68% and the geometric mean percentage change in Ki67 was 87.2%,82.1%,and 78%,respectively[32].Thus,the clinical and biological efficacy did not significantly differ among the three AIs in neoadjuvant settings.
Optimal endocrine agents for premenopausal patients:Current limited data encouraged the efficacy of AI plus ovarian function suppression (OFS) in neoadjuvant endocrine settings for premenopausal patients.Torrisiet al[33]confirmed the efficacy of NAE with letrozole plus gonadotropin-releasing hormone (GnRH) analogue in premenopausal breast cancer patients.Over half of the patients achieved clinical response,and none of patients progressed during treatment[33].The STAGE trial randomized 204 premenopausal patients into either the neoadjuvant anastrozole arm or tamoxifen arm,accompanied by goserelin,for 24 wk.They indicated the superiority of anastrozole over tamoxifen by assessing CRR (70.4vs50.5%,P= 0.004)and BCS (86%vs68%)[34].Furthermore,the suitable selection of OFS has been discussed in premenopausal individuals.The TREND trial was conducted to evaluate the efficacy of degarelix (a GnRH antagonist)vstriptorelin (a GnRH agonist) in patients receiving neoadjuvant letrozole.Individuals treated with degarelix responded more quickly in inducing optimal OFS than those receiving triptorelin[35].This finding supported the use of additional studies to assess whether degarelix optimizes treatment efficacy in neoadjuvant treatment and to screen for the optimal agents for OFS.In conclusion,AI plus OFS is a suitable selection in NAE for premenopausal patients,and it demands prospective validation in more clinical trials.
Optimal targeted agents of NAE
The application of targeted agents was supported to promote endocrine response and reveal drug resistance mechanisms.We will review the promising antiproliferative effects of targeted agents,including cyclin-dependent kinase (CDK) 4/6 inhibitors and phosphatidylinositol 3-kinase (PI3K)/AKT/mammalian target of rapamycin(mTOR) pathway inhibitors.
CDK 4/6 inhibitors:CDK 4/6 inhibitors are suppressors of the cell cycle from G1 to S phase.By inducing retinoblastoma protein (Rb) hypophosphorylation,they effectively inhibit mitosis and thus subsequently prevent cell proliferation and tumor progression (Figure 1)[36].CDK4/6 inhibitors in combination with endocrine therapies have yielded a good prognosis and clinical benefits for ER+ patients,and provoked thoughts about applying CDK4/6 inhibitors in NAE.The following several paragraphs will review clinical trials discussing the efficacy of CDK4/6 inhibitors,especially palbociclib,and addressing the mechanisms of drug resistance.
Newly published in 2018,PALLET,the largest phase II clinical study,has obtained worldwide focus for its concern with efficacy evaluation of a CDK4/6 inhibitor in NAE.In this trial,patients were randomized to letrozole monotherapy or letrozole plus palbociclib therapy for 14 weeks.The combination group showed remarkable superiority over letrozole monotherapy,with larger change in Ki67 (-4.1vs-2.2),higher rate of complete cell-cycle arrest (CCCA,90%vs59%),and greater cleaved poly(ADP-ribose) polymerase (c-PARP,-0.80vs-0.42),indicating that the combination of palbociclib and letrozole could induce the suppression of cell proliferation[37].In NeoPalAna single arm trial,patients received neoadjuvant anastrozole,adding palbociclib on cycle 1 day 1 (C1D1) and leaving study if Ki67 was > 10% on C1D15.CCCA rate,as the primary endpoint,was significantly higher in C1D15 than C1D1(87%vs26%),and rebound of Ki67 expression occurred following withdrawal of palbociclib[38].The significant improvement of CCCA rate and Ki67 suppression was observed in either luminal A or B subtype and with both PIK3CA mutant or wild type(WT) status,which is parallel to data from the PALOMA-3 trial[39].Considering molecular remission,researchers pointed out that the antiproliferation effect corresponding to biomarkers was important to identify patients who might benefit and provide an effective endpoint for clinical research.
The POP trial investigated the antiproliferative action of palbociclib in the Rb phosphorylation process.Antiproliferative response,defined as lnKi67 < 1 at day 5,was the primary endpoint,and it was improved in the palbociclib armvsthe placebo arm (58%vs12%).Greater Ki67 and phospho-Rb decrease were observed in the palbociclib group[40,41].Phospho-Rb was related to palbociclib activity,and changes in Rb phosphorylation might be an indicator for CDK4/6 inhibitors.In the MONALEESA-1 trial,patients randomly received either letrozole alone (A) or in combination with ribociclib at different dosages (B:400 mg/d,C:600 mg/d).The mean decreases in the Ki67-positive cell fraction from baseline were A,69%; B,96%;and C,92%; this finding indicated the possible antiproliferative effect of combined letrozole and ribociclib[42].Regarding abemaciclib,224 postmenopausal patients were randomly assigned to undergo neoadjuvant abemaciclib monotherapy,anastrozole monotherapy,or combination therapy for 2 weeks in the neoMONARCH trial.Abemaciclib,alone or in combination with anastrozole,reduced Ki67 in more patients than anastrozole alone (59%,66%,and 15%,respectively)[43].Investigators concluded that CDK 4/6 inhibitors might serve as antiproliferative agents with manageable toxicities and may be predictive of improved disease-free survival.However,this conclusion is still uncertain due to small sample sizes and limited data,and continuous application should be guaranteed to maintain this effect.

Figure 1 The crosstalk between estrogen receptor and growth factor receptor intracellular signaling pathways.
PI3K/AKT/mTOR pathway inhibitors:The PI3K/AKT/mTOR pathway was found to be a key survival mechanism responsible for endocrine resistance (Figure 1).Regarding PIK3CA inhibitors,the LORELEI trial randomized postmenopausal patients with ER+ HER2- operable breast cancer into two arms to investigate the effect of letrozole plus taselisibvsletrozole plus placebo for 16 wk[44].The primary results showed that the improvement of ORR was significantly related to the addition of taselisib in the general patient population as well as PIK3CA mutant participants compared with letrozole monotherapy.The results encouraged the antitumor value of taselisib to be investigated in further research.
In preclinical studies,MK-2206 has been proved to be an allosteric pan-AKT inhibitor[45].A clinical trial explored whether the addition of MK-2206 to anastrozole can lead to the pCR improvement of PIK3CA mutant ER+ breast cancer.This trial selected 22 PIK3CA mutant patients,of whom 16 received the experimental drug.The combination of anastrozole and MK-2206 had no further inhibitory effect on cell proliferation and did not promote apoptosis on C1D17 compared to anastrozole monotherapy[46].The possibility of improving the efficacy of anastrozole by adding MK-2206 is low,and we disagree with the necessity to continue studying MK-2206 in the target population.
Everolimus,an analog of rapamycin,might competitively bind to the target protein of rapamycin and block downstream signal transduction to suppress tumor cell proliferation[47].Everolimus utility in NAE was first discussed in a 2009 phase II randomized study carried out by Baselgaet al[48].Postmenopausal patients with ER+breast cancer were treated with neoadjuvant letrozole plus everolimus or letrozole monotherapy.Investigators assessed CRR (68.1%vs59.1%) and calculated the percentage of patients with Ki67 < 1% (57%vs30%)[48].The everolimus group was superior to the placebo group in both endpoints,indicating the better efficacy of everolimus in NAE.Clinical trials in this area were not adequate until Wuet al[49]compared neoadjuvant letrozole plus everolimus with neoadjuvant chemotherapy in ER+/HER2- nonmetastatic breast cancer,and a network meta-analysis by Wanget al[50]in 2016 concluded that letrozole in combination with everolimus was the most effective treatment in the neoadjuvant setting.Furthermore,we can expect outcomes in several ongoing neoadjuvant studies combining AI with everolimus,in an attempt to enhance the clinical response of NAE.
BIOMARKERS AND GENOMIC ASSAYS FOR PREDICTION OF NAE BENEFIT
The molecular and genetic expression profiles measured in tumor specimens upon neoadjuvant treatment can provide a remarkable opportunity to explore predictive tools.In the following paragraph,we will discuss the relevant research that elaborates the value of biomarkers and genomic assays and establish a potential biomarkerbased strategy in neoadjuvant settings.
Ki67 and PEPI biomarkers
Ki67,as a biomarker,can be commonly expressed in all stages of the cell cycle,except for G0,and it is of great significance in measuring tumor proliferation in breast specimens[51].Current evidence showed that the decrease of Ki67 during NAE with endocrine or targeted agents can reveal anti-proliferation effects,and Ki67 level was inversely correlated with prognosis[52,53].We encourage a broad development prospect for biomarker-based estimates of prognosis in the neoadjuvant therapy field.
Several clinical trials discussed the relationship between prognosis and Ki67 expression at baseline or after short-term treatment.DeCensiet al[54]assessed the levels of Ki67 at baseline and after 4 wk of presurgical tamoxifen treatment,and the multivariable hazard ratio for baseline and posttreatment Ki67 labeling index was 1.007 (95%CI:0.975-1.041)vs1.034 (95%CI:1.001-1.068)[54].A PerELISA study first indicated that patients with postmenopausal ER+ breast cancer who gained a reduction in Ki67 after 2-wk neoadjuvant letrozole treatment achieved a meaningful pCR rate without chemotherapy[55].However,a recent study showed different results at baseline.The POETIC trial compared peri-surgical AI treatment (pre- and postsurgery) with no treatment in 4000 ER+ breast cancers[56].The results announced at the SABCS 2017 conference suggested that Ki67 levels both at baseline and after short-term therapy were both predictive of efficacy.In conclusion,sufficient evidence has demonstrated that Ki67 levels measured after short-term neoadjuvant therapy were meaningfully related to survival.Whether Ki67 level at baseline can act as a predictive tool is controversial and needs further confirmation.
The preoperative endocrine prognostic index (PEPI) combines Ki67 level with ER status,pathological tumor size,and node status in the surgical specimen following NAE[57].The predictive role of PEPI for relapse-free survival (RFS) was discovered by Elliset al[32]in the P024 trial and validated in the independent IMPACT trial.Patients with a PEPI score equal to 0 (pT1 or pT2,pN0,Ki67 ≤ 2.7%,Allred score < 2) had an extremely low risk of relapse and can be exempt from adjuvant chemotherapy,while PEPI > 0 recognizes a higher relapse risk.Recently,the predictive value of PEPI was verified in the ACOSOG Z1031B trial[32].After a median follow-up period of 5.5 years,the incidence of recurrence in patients who completed the neoadjuvant AI period was significantly different.Kaplan-Meier analysis identified the relationship between RFS and PEPI,and the recurrence HR in patients with a PEPI score equal to 0vsPEPI > 0 patients was 0.27 (P= 0.014).Moreover,the relapse risk was only 3.6% without chemotherapy in patients with a PEPI score equal to 0[58].These results supported the use of the PEPI score to identify drug-sensitive or drug-resistant patients and guide tailored treatment decisions on avoiding chemotherapy.
Genomic assays
Recent evidence indicated the predictive role of genomic assays including Oncotype DX,EndoPredict,MammaPrint,BluePrint assays,and a four-gene predictive tool(Table 4).
Oncotype DX Recurrence Score assay: The Oncotype DX®Breast Recurrence Score(RS) assay is a 21-gene validated genomic tool developed by Genomic Health to assess recurrence risk for patients who received adjuvant ET with ER+/HER2- early stage breast cancer,regardless of lymph node status[59,60].The RS assay can predict the likelihood of benefit from adding chemotherapy to ET in the adjuvant settings.A lowrisk RS tended to have a greater clinical response to ET[61].Additionally,several studies have already incorporated the RS assay in the neoadjuvant setting and illustrated that the approach can be used to guide the decision of neoadjuvant systemic therapy.
We discuss the relevant trials considering the predictive role of RS assay in NAE.Uenoet al[62]indicated that the low-risk group (RS < 18) was more likely to benefit from presurgical exemestane treatment than the high-risk group (RS ≥ 31) in CRR(54%vs22%,P< 0.001) and BCS (91%vs47%,P= 0.003).A 2017 multicenter study reported that the successful BCS rates of the low-intermediate risk group (RS 11-25)after NAE were 75% and 72%,respectively,which were higher than that of high-risk NAC[63].NAE was found to be a scientific strategy for low-risk patients.Moreover,NAE was not inferior to NAE in midrange RS score,and this finding mirrored the results in similar adjuvant settings in the TAILORx trial[64].Most recently,the larger,multicenter TransNEOS trial validated the feasibility of RS assay in predicting clinical response and successful BCS with neoadjuvant letrozole in 295 ER+/HER2-postmenopausal patients.The low RS-score group was considered to have animproved BCS rate,implying a greater likelihood of response to NAE rather than NAC (CRR:54%vs22%,P< 0.001)[65].In conclusion,the Oncotype DX assay would be a significant predictive tool for providing useful information to screen patients who would benefit from neoadjuvant systematic therapy,with NAE used for low-risk group and NAC for high-risk group.
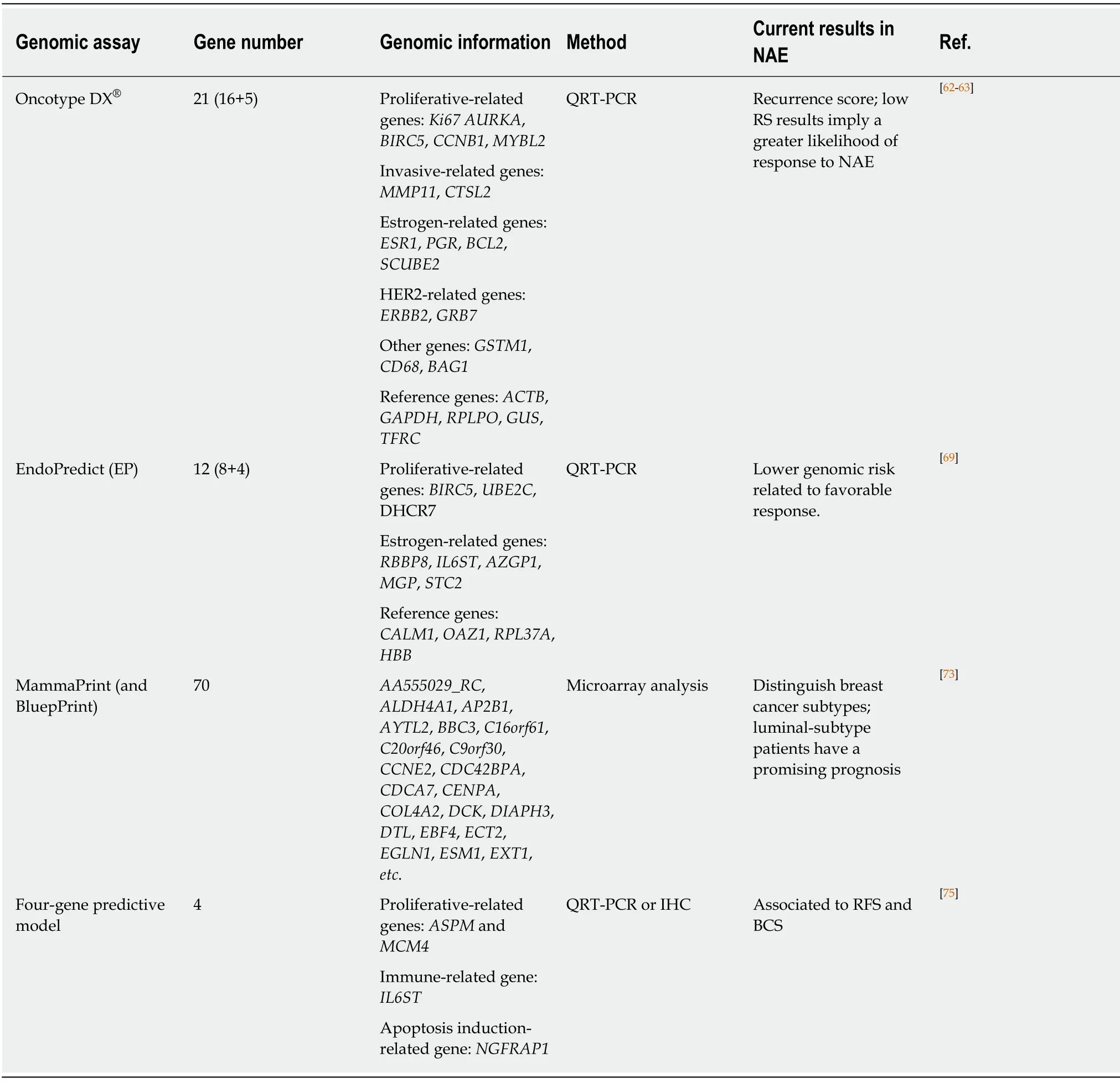
Table 4 Genomic assays to predict outcome in neoadjuvant endocrine therapy
EndoPredict assay:The EndoPredict®(EP) assay is a 12-gene signature test based on eight proliferation-related and differentiation-related cancer genes and four reference genes.EP score low-risk and high-risk categories were specified in previous studies,providing a score between 0 and 15 to assess recurrence risk[66].EPclin is a diagnostic arithmetic genomic assay derived from the EP score by integrating clinical factors including nodal status and residual tumor size,which are also involved in PEPI[67].EP and EPclin assays were shown to be prognostic for early and late distant recurrence[68].Chowet al[69]enrolled 20 eligible patients with neoadjuvant letrozole plus palbociclib treatment in four repeated cycles.The EP score was significantly reduced after NAE,and patients in the high PEPI category had high EPclin scores,indicating that EPclin might be a better predictive marker than PEPI[69].A retrospective analysis of ABCSG 34 reported at SABCS 2017 also examined the predictive role of EP score.The results after 6 months of neoadjuvant letrozole treatment showed that 27.3% of low-risk and 7.7% of high-risk patients achieved residual cancer burden[70].The EP assay may help guide neoadjuvant therapy.Lower genomic risk is related to a favorable response to NAE and worse response to NAC.Further studies should be conducted to verify whether EP and EPclin scores can act as alternative parameters for prognosis in NAE settings in a wide range of population.
MammaPrint and BluePrint assays: The MammaPrint®assay,a 70-gene genomic assay,was of great significance in the accurate guidance of treatment decisions for breast cancer patients in adjuvant settings[71].BluePrint®,a molecular profile that integrates the expression levels of 80 genes,can act as a complement with MammaPrint[72].According to MammaPrint and BluePrint assays,patients were classified into four molecular subgroups:Luminal A,Luminal B,HER2,and Basal type.The identification of chemosensitivities or endocrine sensitivities in patients with different subtypes can provide insight into the response and prognosis of neoadjuvant therapy.The NBRST trial was designed to include patients with histologically proven breast cancer for selecting optimal therapy[73].Approximately 68% of patients with BluePrint Luminal breast cancer who received AI therapy and 29% who received tamoxifen had a clinical response in NAE.Patients with MammaPrint Luminal A-subtype tumors had similar clinical efficacy to relatively high-risk Luminal B-subtype patients (CRR:68.6%vs66.7%)[74].We discovered that luminal-subtype patients determined by these genomic assays could be valuable candidates for NAE and had a promising prognosis.Limited research focused on MammaPrint and BluePrint prognostic assays for the effective stratification of breast cancer patients in neoadjuvant therapy; therefore,further prospective development is needed to guide clinicians’ decisions.
Four-gene predictive model:A clinical trial reported by Turnbullet al[75]provided a four-gene predictive model combining two pretreatment genes (immune-relatedIL6STand apoptosis-relatedNGFRAP1) and two on-treatment genes (proliferationrelatedASPMandMCM4) after 2 weeks of letrozole therapy to forecast clinical response with an accuracy of 96%[75].Another blinded independent setting of patients receiving anastrozole yielded similar results,with an accuracy of 91%.The gene signature can be significantly associated with RFS and BCS,and accurately measured and performed by PCR and immunohistochemistry,which can give confidence to guide treatment decisions and facilitate further applications[76].Although it is a viable result as a predictive biomarker based on one small,retrospective analysis,it needs to be confirmed in large,prospective clinical trials.
DISCUSSION AND FUTURE OUTLOOK
NAE is a favorable alternate approach treatment to NAC for patients with ER+ breast cancer.Given efficacy and good tolerance associated with NAE,especially as NECT,consideration and identification of the optimal settings are of great significance for precision treatment.We consider that extended NAE therapy has a greater potential to result in tumor regression and BCS eligibility,but the optimal treatment duration remains to be further validated.The optimal endocrine agents have been widely discussed in neoadjuvant settings.For postmenopausal patients,AIs demonstrated superiority over TAM,and clinical efficacy is biologically and clinically equivalent among letrozole,anastrozole,and exemestane.For premenopausal patients,AI plus OFS is a beneficial strategy in both adjuvant and neoadjuvant settings despite limited data,as mentioned.The effective role of fulvestrant in NAE has also been indicated above.Additional relevant studies are demanded for the helpful knowledge of endocrine agents to define the most appropriate medication and investigate combination approaches in both postmenopausal and premenopausal patients in NAE.
Multiple trials have demonstrated that combination approaches with targeted agents are effective in inducing cell cycle arrest and preventing tumor progression.The potential antiproliferative effect of CDK4/6 inhibitors,especially palbociclib,has been confirmed to be broadly applicable to ER+ breast cancer patients in NAE.However,insufficient data have been published for PI3K/AKT/mTOR pathway inhibitors.Attempts to promote endocrine response and address mechanisms of both“de novo” and acquired endocrine resistance by application of targeted agents through specific intracellular signaling pathways are encouraged.
Apart from that,NAE will also provide a well-recognized scenario for biomarker research related to cell proliferation.The establishment of the Ki67 biomarker,which can replace the conventional clinical endpoint of tumor shrinkage,offered a feasible approach to reveal the antiproliferative effect of different drugs[77].Moreover,Ki67 levels in postsurgical biopsies have been validated as an effective predictive tool for prognosis and facilitated the development of biomarker-based prognosis estimation.PEPI integrating four risk parameters associated with survival was further confirmed to predict RFS.The ongoing and highly anticipated ALTERNATE trial aimed to assess the validity of Ki67 level measurement following 4 wk treatment and a modification of the PEPI score prospectively responding to anastrozole,fulvestrant,or combination therapy[78].If a biomarker-based strategy is ultimately determined,it will help guide the choice of treatment options and achieve the goal of individualized and precise treatment.
Compared with the molecular profiles,gene analysis provided more accurate information in predicting response,as the results remained the same during the treatment or washout period.The predictive role of genomic assays in NAE is in its infancy.The risk stratification through genetic analysis provided a unique opportunity to guide neoadjuvant systemic therapies.Different genomic assays could evaluate recurrence risks of individuals based on specific related genes and statistical algorithms and provided various risk stratification.Oncotype Dx has been widely recognized as the most useful potential assay in NAE to screen endocrine or chemosensitive individuals and divide individuals according to RS scores into low-risk,moderate-risk,and high-risk groups.Inconsistent with Oncotype Dx,Endopredict and MammaPrint assays have utility to classify candidates into high-risk and low-risk groups.Similarly,the low-risk group has a potential to benefit from NAE and exempt from adjuvant chemotherapy.Thus for,there are still insufficient retrospective and prospective studies to confirm the predictive role of genomic assays.We believe that with the publication of more clinical research results,genomic assays will become a useful predictive tool for clinicians to judge prognosis and guide clinical treatment.
CONCLUSION
In conclusion,NAE can serve as a potential strategy for ER+ breast cancer.It allows the identification of suitable individuals with a good response and guides the decisions for clinical systemic treatment.We can meet our requirements of precise treatment through this platform.Although potential strategies have been proposed,the clinical practicability is lacking validity.Further explorations with large-range populations and long-term follow-up periods are demanded to verify the value of NAE.
杂志排行
World Journal of Clinical Cases的其它文章
- Bone alterations in inflammatory bowel diseases
- Extrahepatic hepcidin production: The intriguing outcomes of recent years
- Vestigial like family member 3 is a novel prognostic biomarker for gastric cancer
- HER2 heterogeneity is a poor prognosticator for HER2-positive gastric cancer
- Changes in corneal endothelial cell density in patients with primary open-angle glaucoma
- Myocardial bridge-related coronary heart disease: lndependent influencing factors and their predicting value
