Flux mechanism of compound flux on ash and slag of coal with high ash melting temperature☆
2019-08-07ChengliWuBeibeiWangJiuqiangZhengHanxuLi
Chengli Wu*,Beibei Wang,Jiuqiang Zheng,Hanxu Li
School of Chemical Engineering,Anhui University of Science and Technology,Huainan 232001,China
Keywords:Coal ash and slag Compound flux X-ray photoelectron spectroscopy Transformation of mineral structure Structure of kaolinite and mullite
ABSTRACT The melting temperature of Z coal ash was reduced by adding calcium-magnesium compound flux(WCaO/WMgO=1).In the process of simulated coal gasification,the coal ash and slag were prepared.The transformation of minerals in coal ash and slag upon the change of temperature was studied by using X-ray diffraction(XRD).With the increase of temperatures,forsterite in the ash disappears,while the diffraction peak strength of magnesium spinel increases,and the content of the calcium feldspar increases,then the content of the amorphous phase in the ash increases obviously.The species and evolution process of oxygen,silicon,aluminum,calcium,magnesium at different temperatures were analyzed by X-ray photoelectron spectroscopy(XPS).The decrease of the ash melting point mainly affects the structural changes of silicon,aluminum and oxygen.The coordination of aluminum and oxygen in the aluminum element structure, e.g., tetracoordinated aluminum oxide, was changed. Tetrahedral [AlO4] and hexacoordinated aluminoxy octahedral[AlO6]change with the temperature changing.The addition of Ca2+and Mg2+destroys silica chain,making bridge oxide silicon change into non-bridge oxysilicon;and bridge oxygen bond was broken and non-bridge oxygen bond was produced in the oxygen element structure.The addition of calcium and magnesium compound flux reacts with aluminum oxide tetrahedron,aluminum oxide octahedron and silicon tetrahedron to promote the breakage of the bridge oxygen bond.Ca2+and Mg2+are easily combined with silicon oxide and aluminum oxide tetrahedron and aluminum.Oxygen octahedrons combine with non-oxygen bonds to generate low-melting temperature feldspars and magnesite minerals,thereby reducing the coal ash melting temperatures.The structure of kaolinite and mullite was simulated by quantum chemistry calculation,and kaolinite molecule has a stable structure.
1.Introduction
At present,coal is still the most important primary energy source in China[1].Coal ash fusion temperature affects the efficient clean coal utilization.Coal slurry and pulverized coal gasification processes use liquid slag discharge technology, e.g., coal ash fusion temperature requires below1400°C,but near half of the coal mine resources that have been explored are of high ash melting point coals,and the use of these coals is a major problem[2].Minerals in coal are an important part of coal ash,and different mineral compositions at high temperature process produce different chemical compositions of coal ash,resulting in different coal ash melting characteristics[3].In order to ensure the normal application of high ash melting point coal in gasifiers,researchers used the method of coal blending or adding fluxes to reduce the coal ash melting temperature[4,5].Therefore,suitable fluxes were selected to reduce the amount of fluxes added.It is of great significance to make efficient use of coal resources and ensure the safe and stable operation of gasifier.
During gasification,the interaction between the flux and the minerals in coal and the formation of the eutectic melt make it impossible to accurately predict the behavior of the coal ash at high temperatures using conventional analytical methods.Therefore,XPS was used to analyze the chemical structure changes of O,Si,Al,Ca,and Mg elements during the fluxing process in this study, and the quantum chemical method was used to analyze the structural models and bonding laws of common minerals in coal ash slag.Based on the molecular point of view,the role of cations in flux in destroying the structure of coal ash and slag was explored,thereby reducing the ash melting point.
Xia et al.[6]used XPS to analyze Inner Mongolia coal surface and found that the carbon elements are mainly C=C,C--C,C--H,C--O(alcohol, phenol, ether), C=O (carbonyl and hydrazine), or O--C--O,COO--functional group structure.Xia[7]and others allowed the coalto weather naturally,and then studied the surface of coal with XPS.They found that the contents of C--C and C--H became less and the contents of C--O,C=O,and O=C--O increased.Xiang et al.[8]used XPS to study the existence and evolution of carbon and oxygen in the combustion process of coal.As a result,it was found that the graphitized carbon in the combustion process of carbon increases gradually,and the singlebond and double-bond carbon gradually decrease,and the oxygen increases with temperature increase.With the increase of temperature,the inorganic oxygen content gradually increases and organic oxygen decreases.Some researchers[9,10]used XPS to study the transformation of S, N, and other elements on the coal surface. However, there are few studies on the main elements silicon,oxygen,aluminum,calcium,and magnesium during coal gasification process.

Table 1 Proximate analysis and ultimate analysis of Z coal
Wang et al.[11]used quantum chemistry calculations to study the breakage of bonds in coal gasification.Yin et al.[12] correlations between the chemical bonds in the aluminosilicates and the metallic cations revealed that cations have a greater influence on the bridge oxygen bonds,and the bridge oxygen bonds have a certain relationship with their electronegativity. Transition metal ions (Fe2+) >alkaline earth metal ions (Ca2+, Mg2+) >alkali metal ions (Na+, K+). The study of aluminosilicate structure is helpful to the study of the structure of coal ash melt.Li et al.[13]used quantum chemistry to study the effect of kaolinite on the melting properties of coal ash.The study found that O(26)and O(22)are electrophilic,and they easily react with Na+and K+,thus promoting aluminum-oxygen bond cleavage. O2-alkali or alkaline-earth metal oxides easily react with Si(6)and Si(8),causing breakage of the bridge oxygen bond (Si--O--Si), and kaolinite is converted to sodium or potassium.High melting point minerals.Chen et al. [14] calculated the atomic structure of mullite and found that metallic ions can connect with the SiO2bond in mullite to form CaO·Al2O3·2SiO2.Kubich[15]calculated the characteristics of the SiO2bond using quantum chemistry and found that the bond length,bond angle, and bond energy have a certain correlation with temperature and pressure.
In this paper,the method of XPS combined with quantum chemical calculations was used to study the transformation of mineral structure during the fluxing process based on a view point of the change of molecular bonds.The findings are expected to explain the influencing mechanisms of adding fluxes on ash melting point.
2.Experimental
2.1.Characteristics of coal samples
A raw coal,namely Z coal from Huainan mining area,Anhui Province of China,and calcium oxide and magnesium oxide as fluxes were used in this research.Proximate analysis,ultimate analysis and ash composition analyses of the samples were conducted according to Chinese standards (GB/T212-2008, GB/T476-2008, and GB/T214-2007). The analysis results are shown in Tables 1 and 2.

Table 2 Ash composition analysis of Z coal(wt%)
Volatile content of the sample is more than 20%,and it belongs to typical bituminous coal.Moisture content is low,whereas ash content is high.The sum of silicon and aluminum(SiO2+Al2O3)of coal ash is more than 90%,and the ratio of silicon to aluminum is more than 1.8,while the content of alkaline oxide is only 5.64%.According to the previous experimental study,the coal ash fusion temperature of the same sample tested in this study can be reduced excellently when the content of calcium and magnesium compound added is 6% (coal basis, and WCaO/WMgO=1).
As shown in Table 3,the ash flow temperature of Z coal is higher than 1500°C,therefore,the ash fusion temperature(AFT)of Z coal is high and not suitable for liquid slag discharge in entrained flow gasifier.It is necessary to add flux to reduce the ash melting point.When 6%(WCaO/WMgO= 1) flux is added, the flow temperature decreased to 1290°C.

Table 3 Ash fusion temperatures of coal samples
2.2.Preparation of ash and slag samples
Calcium-magnesium complex flux (WCaO/WMgO= 1) was first mixed with Z coal and then transferred into a muffle furnace for ashing according to the Chinese standard(GB/T1574-2001).The resulting coal ash was put into a high temperature tubular furnace to simulate the ash melting process.The slag is heated to 1500°C and the atmosphere is CO:N2=6:4[16,17],and the slag samples made at different temperatures(1000,1200,1290,1400,1500°C),then ash and slag samples are rapidly quenched in cold water and ground into powder.
2.3.Analytical method
Ash chemical compositions were analyzed with an X-ray fluorescence spectrometer(XRF-1800,Shimadzu,Japan).The relative standard deviation(RSD)for the test results is about 0.6%.
X-ray diffraction(XRD,XD-3,Beijing Puxi General Company)was used to analyze the crystalline compounds in the ash/slag.Diffraction conditions:Cu target,tube voltage 36 kV,tube current 40 mA,scanning speed of 2(°)·min-1, scanning range: 10°-60°, scanning step: 0.02 degree.
X-ray photoelectron spectrometer(XPS,X Thermo ESCALAB 250XI,thermo Fisher Scientific,USA)was used to detect the species of O,Si,Al,Mg and Ca.The instrument parameters are:180ohemisphere energy analyzer, energy range: 0-5000 eV, Ka (HV = 1486.6 eV Al monochrome), power: 150 W. C1s at 284.8 eV was used for peak shift calibration.
Quantum chemical calculation method,the DFT method of ab initio quantization module Dmol3 is used to calculate and optimize the structure of kaolinite by plane wave pseudopotential formula,and its HOMO and LUMO orbit are obtained.

Fig. 1. XRD patterns of Z coal with adding 6% flux(CaO: MgO = 5:5) at different temperatures.A—SiO2;B—MgO;C—Al6Si2O13;D—Mg2SiO4;E—MgAl2O4;F—Ca(OH)2;K—Mg2Al4Si5O18;G—CaAl2Si2O8;H—Fe2O3.
3.Results and Discussion
3.1.Mineral composition and evolution in coal ash and slag
XRD was used to measure the mineral composition of Z coal with calcium-magnesium complex flux (WCaO/WMgO= 1) at different temperatures.
Fig.1 shows the transformation of minerals in coal ash and slag upon the change of temperatures.At 1000°C,the ash mainly contains quartz,hematite,periclase,mullite,forsterite and other minerals.With the increase of temperature,forsterite in the ash disappears,while the diffraction peak of magnesium spinel increases,the content of the calcium feldspar increases,and the content of the amorphous phase in the ash increases obviously. The minerals participate in the formation of forsterite and anorthite,respectively,and forsterite and anorthite form a eutectic with low melting point.At 1500°C,the majority of the slag becomes amorphous phase.
The formula for the main reaction mechanism is listed as follows:

Calcium oxide and magnesium oxide react with mullite at high temperatures to form anorthite and forsterite which possess relative low melting points,resulting in a decrease in coal ash melting temperature.
3.2.The existence form and change law of different elements
The coal ash and slag samples with addition of 6% flux (WCaO/WMgO= 1) were made at different temperatures. The slag samples were pressed and tablet samples were made. X-ray excites photoelectrons from the sample, passing electronic energy analyzer, back into the electronic probe, and electron binding energy of different elements and their strengths are obtained. Using XPS4.1 and origin8.5 for data processing and analysis, the five elements of O1s,Al2p, Si2p, Ca2p, and Mg1s were fitted by sub-peaks. The following results were obtained, Fig. 2 shows Z coal added 6% (WCaO/WMgO=1) XPS analysis of coal ash slag at 1000 °C. Table 4 shows the peak position, FWHM, and area of the peaks after fitting the five elements.

Fig.2.XPS peaks of Z coal adding 6%(WCaO/WMgO=1)of 1000°C slag sample.

Table 4 XPS analysis results of Z coal adding 6%(WCaO/WMgO=1)of 1000°C slag sample
Fig.2 and Table 4 show the results of the fitting of five elements O1s,Al2p,Si2p,Ca2p,Mg1s.The oxygen in the cinder is mainly inorganic oxygen.The main existence forms are composed of non-bridge oxygen Si-O-M (where M is a metal cation), Si--O--Si and free oxygen of M--O--M bridging oxygen in the oxygen structure.The corresponding binding energies are non-bridge oxygen (530 ± 0.5) eV, (531.1 ±0.3) eV, alumina bridging oxygen and silicon oxide are (531.8 ±0.3)eV and(532.65±0.3)eV.The combined energy of(530±0.5)eV is also likely to be oxygen in Mg--O or Ca--O.The relative content of different functional groups can be obtained by integrating the area of each small peak.
As a kind of amphoteric oxide,alumina acts as a bridge between the network generation and the outer body of the network.The binding energy shows that[AlO4]can be combined with tetrahedral was about(73.8 ± 0.4) eV at the eight surfaces of Al2p binding to [AlO6] was about(74.5±0.4)eV.The spectral peaks of Al2p are in between(74±1)eV,indicating that the aluminum in the ash slag is in the form of Al2O3,but there are two different ways of coordination.The silicon element in the coal ash mainly exists in silicate,and silicon element in silicate existed in the form of bridging oxygen silicon and non-bridging oxygen silicon,of which(102.4±0.3)eV is Si--O,but(103±0.4)eV is Si--O2.The two electron spin splitting peaks of Ca2p3/2 and Ca2p1/2 exists in Ca2p,and double peaks are characteristic phenomenon of Ca2p. The main structure is Ca--O. Binding energy of Mg1s is 1304.3 eV,and it is structure of Mg--O.
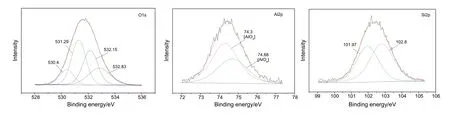
Fig.3.XPS analysis of Z coal adding 6%(WCaO/WMgO=1)of 1200°C slag sample.

Fig.4.XPS analysis of Z coal adding 6%(WCaO/WMgO=1)of 1290°C slag sample.

Fig.5.XPS analysis of Z coal adding 6%(WCaO/WMgO=1)of 1400°C slag sample.

Fig.6.XPS analysis of Z coal adding 6%(WCaO/WMgO=1)of 1500°C slag sample.
Figs.3-6 respectively show XPS peaks of Z coal adding 6%(WCaO/WMgO=1)analysis of 1200°C and 1290°C,1400°C and 1500°C.Due to the presence of calcium and magnesium in coal slag existing in single form of Ca--O and Mg--O,peak fitting of the main three elements O1s,Al2p,Si2p was conducted.XPS analysis results are as follows,O1s exists mainly in the form of non-bridging oxygen, oxygen and oxygen free bridge(Al2O3and SiO2in oxygen);Al2p is the main form of alumina,and there are two different coordination modes such as tetrahedron and Octahedron of Aluminum oxygen;XPS analysis of Si2p mainly exists in silicate form in coal ash slag, and two kinds of structure are Si--O and Si--O2.Therefore,after adding the calcium and magnesium compound flux into Z coal, the structure of Ca-O and Mg-O is not changed,which mainly affects the existence of O,Si and Al.
Calcium and magnesium compound flux was added into Z coal,in which Ca2+and Mg2+enter the mullite lattice,and mullite structure was destroyed,i.e.Si--O covalent bond breaking,and Ca2+and Mg2+combining with the non-bridge oxygen generate SiO2chain,and Ca2+and Mg2+make[AlO4]-,[AlO6]-and[SiO4]-combined to generate low melting point minerals such as calcium feldspar, olivine, spinel and magnesium cordierite,resulting in decreasing of coal ash melting temperature.

Fig.7.Optimized structure of kaolinite.
3.3.The simulation of minerals
Quantum chemical calculations were used to model different minerals, optimize the structure, obtain the HOMO and LUMO orbitals,and analyze the bond characteristics from different bond lengths,bond angles,and bond energies.The effects of calcium and magnesium complex fluxes on the transformation of mineral structure were studied and mechanisms of mineral action were explored.
3.3.1.Optimal calculation of kaolinite model
The common minerals (kaolinite, mullite) in coal ash slag were modeled and their structure was optimized.Through the analysis of the mineral compositions of two coal samples with high ash fusion temperature,it was found that the main crystal mineral in coal is kaolinite,and the presence of kaolinite is the main reason for the extremely high coal ash melting temperature.First of all,the DFT3 quantization module Dmol3 is used to calculate and optimize the kaolinite structure using the plane wave enthalpy formula.The specific calculations were performed.During the coal gasification process,the main minerals such as kaolinite and mullite,are converted into kaolinite and dehydrated at high temperatures to form mullite. Mullite reacts with CaO and MgO to form low-melting calcareous minerals at high temperatures.Magnesia minerals,thereby reduce the coal ash melting temperature.
Fig.7 shows the optimized structure of kaolinite.The result shows the number of Millken layouts of kaolinite. Kaolinite belongs to the biclinic system and consists mainly of aluminosilicate structure,which contains three polyhedrons [AlO6]-, [AlO4]-, [SiO4]-structures in which polyhedrons are connected by oxygen element.As can be seen from the data in Table 5, the number of layouts of O(1) and O(6) is the smallest,so metal cations in coal ash slag are easily combined with O(1)and O(6)in kaolinite to form other structures.
Fig.8(a)and(b)are the HOMO and LUMO diagrams of kaolinite.The HOMO orbital structure of kaolinite is compact and mainly consists of Al--O and Si--O--Al.The O composition of the method is that while the LUMO orbital structure of kaolinite is dispersed and mainly contains Si,O,and H atoms.Blue represents the minimum value in the electrostatic potential,mainly in the vicinity of oxygen element.The electrostatic potential represented by yellow is mainly in the vicinity of Si and Al.Since the electrostatic potential is high,it is easy to react with electrophilic reagents. The kaolinite molecular HOMO energy is-0.2495 eV,the LUMO energy is-0.0564 eV,and the energy difference between them is 0.1931 eV,so the kaolinite molecule has a stable structure.
3.3.2.Optimal calculation of mullite model
Mullite was dehydrated from kaolinite at high temperature,and the molecular structure of mullite was simulated by quantum chemistrycalculation.As shown in Fig.9,Al has three different sites,and oxygen element has four different sites.Silicon has only one kind of site.

Table 5 Mulliken atomic populations
Table 6 lists the molecular orbital bond lengths of mullite.The longer the bond length is,the smaller the bond energy is and the easier the bond is broken.From the results in the Table 6,Al(6)-O(8)and Al areshown in the aluminum oxide bond.The two longest bonds such as Al(6)-O(8)and Al(5)-O(7) are easily damaged.In the intermolecular chemical reaction,the first orbital edge orbits,and the edge electrons play a key role in chemistry. Therefore, when mullite is combined with electron acceptors(positively charged ions or ionic groups),electrons enter the lattice through the surface of the crystal,while O(2),O(4),O(8)(O(1),O(5),O(7)are in the lattice plane.O(1)electrons on the O(2),O(4),O(5),O(7),and O(8)atoms will transfer to other structures.When calcium and magnesium complex fluxes are added into,since the metallic cations Ca2+and Mg2+are both electron acceptors,there are the highly active oxygen atoms in the mullite lattice(O(2),O(4),O(8).The combination of Al(6)-O(8)and Al(5)-O(7)causes the transformation of the mullite structure and affects its physical and chemical properties.

Fig.8.Structure of kaolinite HOMO and LUMO.(White-H,Orange-Si,Red-O,Pink-Al,Yellow,blue-electrostatic potential)

Fig.9.Optimized structure of mullite.
4.Conclusions
(1) XPS was used to analyze five elements Al,Si,O,Ca and Mg in Z coal with the addition of 6%flux(WCaO/WMgO=1)during the ash melting process. It was found that aluminum in coal ash slag exists mainly in the form of Al2O3and is divided into two structures of[AlO4]tetrahedral and[AlO6]octahedron.Oxygen is mainly in the form of bridge oxygen,non-bridge oxygen,and free oxygen.Silicon is predominantly in the form of silicates including: silicon linked to bridge oxygen and silicon linked to non-bridge oxygen.Calcium and magnesium are predominantly in the form of Ca-O and Mg-O.
(2) Calcium and magnesium compound flux react with aluminum oxide tetrahedron and silicon tetrahedron to promote the fracture of bridge oxygen bonds. Ca2+and Mg2+combine with bridge oxygen to generate compounds with low ash melting point, which can effectively reduce the coal ash melting temperature.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Assessment of the TFM in predicting the onset of turbulent fluidization☆
- CFD study on double-to single-loop flow pattern transition and its influence on macro mixing efficiency in fully baffled tank stirred by a Rushton turbine☆
- Simulation of drop breakage in liquid-liquid system by coupling of CFD and PBM:Comparison of breakage kernels and effects of agitator configurations☆
- Heat transfer characteristics of molten plastics in a vertical falling film reactor☆
- Stabilizing silica nanoparticles in high saline water by using polyvinylpyrrolidone for reduction of asphaltene precipitation damage under dynamic condition
- Numerical simulation and experimental study on dissolving characteristics of layered salt rocks
