CH4/N2 separation on methane molecules grade diameter channel molecular sieves with a CHA-type structure
2019-08-07HuaShangYupingLiJiaqiLiuXuanTangJiangfengYangJinpingLi
Hua Shang,Yuping Li,Jiaqi Liu,Xuan Tang,Jiangfeng Yang,3,*,Jinping Li,3
1 Research Institute of Special Chemicals,College of Chemistry and Chemical Engineering,Taiyuan University of Technology,Taiyuan 030024,China
2 College of Materials Science and Engineering,Taiyuan University of Technology,Taiyuan 030024,China
3 Shanxi Key Laboratory of Gas Energy Efficient and Clean Utilization,Taiyuan 030024,China
Keywords:Molecular sieves Adsorption CH4/N2 Separation Breakthrough
ABSTRACT Samples of methane molecules grade diameter channel CHA-type molecular sieves(Chabazite-K,SAPO-34 and SSZ-13)were investigated using the adsorption separation of CH4/N2 mixtures.The isotherms recorded for CH4 and N2 follow a typical type-Ι behavior,which were fitted well with the Sips model(R2 >0.999)and the selectivity was calculated using IAST theory.The results reveal that Chabazite-K has the highest selectivity(SCH4/N2=5.5).SSZ-13 has the largest capacity,which can adsorb up to a maximum of 30.957 cm3·g-1(STP)of CH4,due to it having the largest pore volume and surface area,but the lowest selectivity(SCH4/N2=2.5).From the breakthrough test,we can conclude that SSZ-13 may be a suitable candidate for the recovery of CH4 from low concentration methane(CH4 <20%)based on its larger pore volume and higher CH4 capacity.Chabazite-K is more suited to the separation of high concentration methane(CH4 >50%)due to its higher selectivity.
1.Introduction
There is a great interest today for the development of low-carbon clean energy to substitute conventional high-carbon fossil fuels,such as coal and petroleum[1].Natural gas with its lower atmospheric emissions and higher efficiency than coal and petroleum is considered as a cleaner-burning alternative fuel[2].As a matter of fact,unconventional natural gas,such as coal-bed methane,is discharged due to coal mining activities and mainly contains methane(<50%),nitrogen(>40%)and oxygen(>10%)[3].However,it is very difficult to utilize directly due to its low methane concentration,which usually results in it being released into the atmosphere for safety reasons[4].The large annual emission of coal-bed methane not only pollutes the environment,but is also a waste of resources.In particular,the removal of nitrogen from methane is one of the most challenging areas due to it having very similar physicochemical properties with methane[5].Currently,the commercial process used to separate CH4/N2mixtures utilizes cryogenic distillation,which is a highly energy intensive process and only suitable for large-scale separation[6].
With the development of new adsorbents and highly efficient separation cycle with low energy requirements,ease of handing and high efficiency,adsorption separation using various nanoporous adsorbents such as zeolites[7-10],carbon materials(activated carbon and carbon molecular sieves) [11-13] and metal-organic framework materials[14-17] have been reported as very attractive alternatives [18-21].Among these materials,zeolites with narrow and uniform pores,high surface area,adjustable hydrophobic and hydrophilic nature,ion exchange capacity, and high thermal and chemical stability have been widely used in industrial applications[22,23].
CHA-type molecular sieves with cage-like structure are an important class of small pore materials whose framework structures are characterized by a three-dimensional pore system confined by 8-membered ring openings. As shown in Fig. 1, one large ellipsoidal cavity of 0.73 nm×1.2 nm interconnected via 8-membered ring windows with pore apertures of 0.38 nm × 0.38 nm is called the chabazite cage,where the extraframework cations(K+,Na+and Ca2+)and water molecules be accessible,while the D6R and 4-ring windows are inaccessible to the adsorbates [24,25]. As the diameters of CHA-type molecular sieves (0.38 nm) are very close to the kinetic diameter of CH4(0.38 nm)and N2(0.36 nm),it is believed that CH4and N2molecules diffuse slowly into the pore of the CHA-type molecular sieves and may not facilitate the separation of CH4/N2mixtures.In fact,CHA-type molecular sieves with extraframework metal cation exhibit a very high adsorption potential towards CH4due to its methane molecules grade diameter channel and cage-like structure[26],and also have a higher selectivity than common type A and X zeolites,whose selectivity is usually below 3[27,28].Low-silica(Chabazite-K),high-silica(SSZ-13)zeolites and molecular sieve silicoaluminophosphate-34 (SAPO-34) are three of representative materials,which have widespread applications in catalysis[29]and gas separation[30,31].

Fig.1.Framework structure of CHA[32].
Over the years,various researchers[33-36]have studied the unary and binary adsorption of methane, carbon dioxide and nitrogen on CHA-type molecular sieves.However,most of these research studies have focused on CH4/CO2or N2/CO2mixtures,and quite a few involved the separation of CH4/N2mixtures due to the their diameters being very similar to the kinetic diameter of CH4and N2.In fact,an accurate prediction of the separation properties is not possible when only relying on the difference between the kinetic diameters of the adsorbate molecules and pore diameter of the adsorbent.The interactions between adsorbent and adsorbates,pore volume and surface area of the absorbents are also key parameters for its separation capability[37].
Thus,in this work we have investigated separation performance of CH4/N2mixtures on CHA-type molecular sieve(Chabazite-K,SAPO-34 and SSZ-13) to gain an improved understanding of the effects of the pore diameter,Si/Al ratios,surface area and extraframework metal cation on the adsorption separation of similar size light gases(i.e.CH4and N2).
2.Experimental
2.1.Materials
Chabazite-K used in this work was synthesized in our laboratory[38].SAPO-34 and SSZ-13 were purchased from Nankai University Catalyst Co.,Ltd.(Tianjin,China)and Beijing InnoChem Science&Technology Co.,Ltd.,respectively.All the purchased materials are in the form of powder and the purity of the gases used in this work was 99.99%,99.999%and 99.999%for CH4,N2and He,respectively.
2.2.Characterization
The X-ray diffraction patterns of the three molecular sieves(Chabazite-K, SAPO-34 and SSZ-13) were recorded on a Germany Bruker D8 ADVANCE X-ray diffractometer with Cu Kαradiation operated at 40 kV and 40 mA in the 2θ range of 5°-40°at 5(°)·min-1.
The surface areas,micropore volume and the total pore volume of the molecular sieves studied were calculated using the N2and CO2adsorption data recorded on a Micromeritics ASAP 2460 gas adsorption apparatus at 77 K and 273 K,respectively.Before the N2and CO2adsorption measurements, the samples were outgassed at 473 K and 1×10-6MPa for 12 h under a turbo molecular pump vacuum.
2.3.Pure component adsorption isotherm measurements
The methane and nitrogen adsorption isotherms measurements were performed at 298 K in the pressure range up to 0.1 MPa using Micromeritics ASAP 2460.Prior to the adsorption measurements,the samples were outgassed at 473 K under a high vacuum until no further weight loss was observed.
2.4.Dynamic breakthrough experiments
Breakthrough experiments were conducted to evaluate the separation performance of the CHA-type molecular sieves to CH4/N2mixtures.The experimental set-up and sample processing are described in detail in previous papers produced from our laboratory[39,40].Before each experiment,the sample pellets(4.8 g,4.6 g and 3.7 g of Chabazite-K,SAPO-34 and SSZ-13,respectively)were activated to remove moisture and other adsorbed species upon heating under vacuum at 473 K for at least 8 h.Then,helium gas was used to purge the whole set-up including the adsorption bed and pipelines until the adsorption bed was saturated with helium.Subsequently,the gas stream flowing through the adsorption bed was transferred from helium to the desired concentration of the CH4/N2mixtures(the volume fractions of methane in nitrogen were 20%and 50%,respectively).The breakthrough experiments were carried out at 298 K and 0.1 MPa with the flow maintained at 3 ml·min-1(STP) via the mass flow controllers. The gas flow at the outlet of the adsorption bed was measured online using a gas chromatograph (Shimadzu, GC-2014C, Japan) equipped with a molecular sieve 5A packed column and the temperature was 80°C and thermal conductivity detector(TCD).Helium was used as a carrier gas for the GC analysis.
3.Results and Discussion
3.1.Characterization
Fig.2 shows the XRD pattern of Chabazite-K,SAPO-34 and SSZ-13 compared with the standard XRD data.The main peak positions and relative diffraction intensity are similar to the reported values,and the crystallinity of SAPO-34 and SSZ-13 is higher than that of Chabazite-K.
The Si/Al values in Chabazite-K,SAPO-34 and SSZ-13 were 2.63,1 and 30,respectively,which are in agreement with the previous statement that Chabazite-K and SAPO-34 belong to the low-silica family of molecular sieves and SSZ-13 belongs to the high-silica family of molecular sieves.

Fig.2.XRD patterns of Chabazite-K,SAPO-34 and SSZ-13.
The isotherms recorded for the adsorption/desorption of N2at 77 K on Chabazite-K,SAPO-34 and SSZ-13 are shown in Fig.3,where the isotherms of SAPO-34 and SSZ-13 exhibit a typical Type-Ι behavior according to the International Union of Pure and Applied Chemistry(IUPAC)classification[41,42].SAPO-34 and SSZ-13 adsorbed significant volumes of N2at a relative pressure below 0.1,which demonstrates that there is a high volume-fraction of micropores contained in these molecular sieves.However,there is a very low nitrogen adsorption amount on Chabazite-K at a low relative pressure up until a relative pressure of 0.9.The phenomenon mainly stems from the existence of K+near the center of the 8-ring window,which limits the diffusion of N2molecule through the structure at 77 K[43].Thus,the BET model based on N2adsorption/desorption at 77 K is not suitable for measuring the surface area of Chabazite-K. We conducted CO2adsorption experiment on Chabazite-K at 273 K as a complementary method to measure the micropore surface area and micropore volume using the Dubinin-Astakhov (DA) method [44]. In order to compare with Chabazite-K objectively,the SAPO-34 and SSZ-13 samples were investigated using the CO2adsorption experiment under the same condition.The CO2adsorption isotherms recorded at 273 K and DA fitting lines are shown in Fig.4.A summary of the pore textural properties of the three adsorbents is listed in Table 1. Among the given materials, Chabazite-K shows the lowest CO2DA surface area and DA micropore volume,and SSZ-13 has the largest micropore pore volume and surface area.

Fig.3.N2 adsorption(solid symbols)/desorption(open symbols)isotherms at 77 K on Chabazite-K,●;SAPO-34,■and SSZ-13,▲.
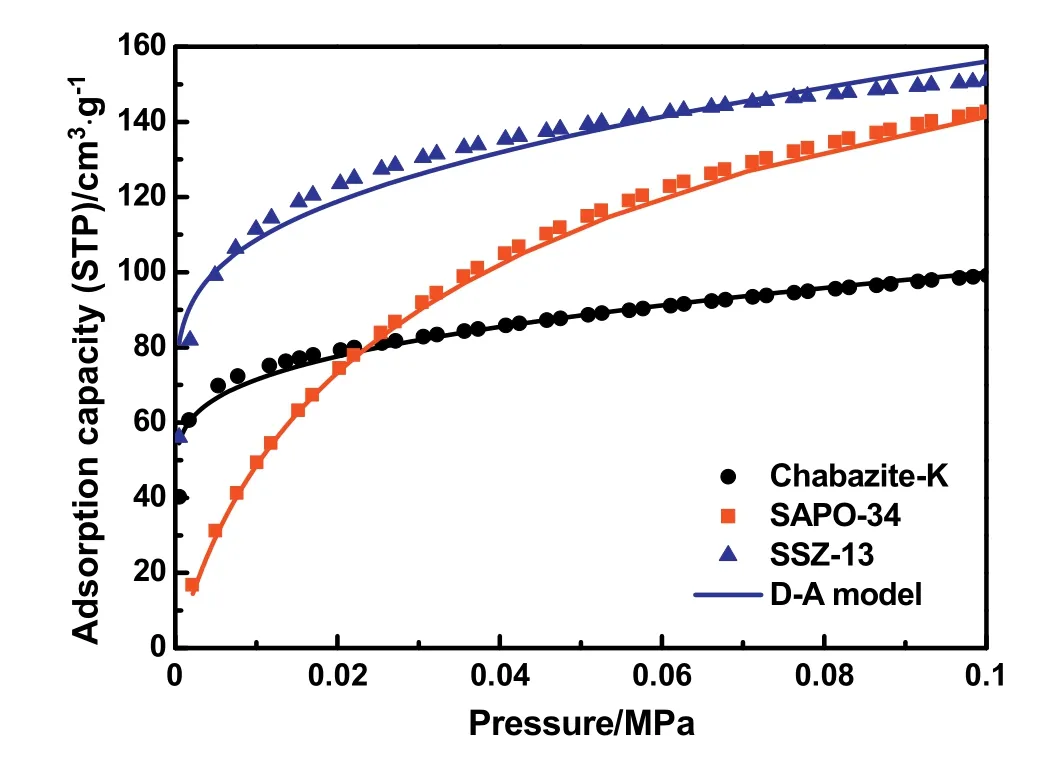
Fig.4.CO2 adsorption isotherms at 273 K on Chabazite-K,●;SAPO-34,■and SSZ-13,▲.The points are experimental data and the lines are fitted to the D-A model.
3.2.Pure component adsorption isotherms
Pure component adsorption isotherms of CH4and N2on Chabazite-K, SAPO-34 and SSZ-13 were measured at 298 K at pressures up to 0.1 MPa.The experiment and fitting results are shown in Fig.5.
It can be observed that the adsorption isotherms for CH4and N2can be classified as Type-Ι isotherms according to the International Union of Pure and Applied Chemistry(IUPAC)classification.The isotherms data were fitted with the Sips model[45],as shown in Eq.(1):

where i indicates component i (i.e. CH4or N2), qiand qmirepresent the absolute amount of component i adsorbed and the maximum amount of component i adsorbed, respectively, P is the pressure, biis the affinity constant and niis the heterogeneity parameter of the adsorbent-adsorbate system,which is bounded by 0 <n ≤1.The larger the deviation in n from 1,the stronger the non-uniformity of the surface in the adsorption system.Note that for n=1,the Sips model can be simplified to the Langmuir model.
It is clear that the fitting results are in good agreement over the pressure range studied.The fitting parameters and the square of correlation coefficients R2are summarized in Table 2.
For the studied molecular sieves,the adsorption of CH4was more favorable than N2,which can be seen from the higher CH4loading against N2over the total pressure range.This is mainly attributed to a stronger polarizability of CH4molecules than N2molecules, so N2molecules which only have a weak quadrupole moment and lower polarizability,are not adsorbed as much as CH4[46].Note that for CH4and N2,the loading at 0.1 MPa(the highest pressure used)and 298 K were lower on Chabazite-K and SAPO-34 than on SSZ-13.The CH4loading observed for SSZ-13(30.957 cm3·g-1,STP)was about 97%and 88%higher than those observed on Chabazite-K(15.680 cm3·g-1,STP)and SAPO-34(16.464 cm3·g-1,STP),respectively.Since SSZ-13 belongs to the highsilica family of zeolites,which have less balancing cations that make its framework electrically neutral and surface “smooth”, there are more adsorption site available for the CH4molecules rather than occupied by cations.To some extent,the pore volume also plays a key role in the adsorption CH4.The larger pore volume, the more adsorbates are adsorbed by the zeolite.As for Chabazite-K and SAPO-34,although the two molecular sieves have balancing cations,the size of K+is larger than that of H+,which cause the effect of the cations on the reduction of the pore space and adsorption loading to be more distinct in Chabazite-K,making the CH4loading the lowest in Chabazite-K.

Table 1 Textural properties of Chabazite-K,SAPO-34 and SSZ-13

Fig.5.Adsorption isotherms of CH4(a)and N2(b)at 298 K on Chabazite-K,●;SAPO-34,■and SSZ-13,▲.The points are experimental data and the lines are best fits to the Sips model.

Table 2 Isotherm model parameters for sorption of CH4 and N2 in Chabazite-K,SAPO-34 and SSZ-13
3.3.Separation selectivity
The separation selectivity of methane with respect to nitrogen is shown in Eq.(2):

where xiand yiare the molar fractions of component i in the adsorbed and gaseous phases at equilibrium,respectively.
The ideal adsorption solution theory(IAST)[47]has been successfully used to calculate separation selectivity based on pure component adsorption data.In this work,the separation selectivities were calculated for 20%and 50%CH4/N2mixtures(volume fraction)in the gas phase based on IAST.As shown in Fig.6,the CH4/N2separation selectivity was found to be in the order of:Chabazite-K >SAPO-34 >SSZ-13.For Chabazite-K,the selectivities are 5.5 and 6.1 for 20% and 50% CH4/N2mixtures at 0.1 MPa,respectively,which are the highest values among the three materials.The higher selectivity may be attributes to the existence of K+,which enhances the strength of interaction between Chabazite-K and CH4,which has a higher polarizability,and it is clear for SAPO-34 and SSZ-13 that the separation selectivity does not depend very strongly on the concentration in the external phase over a wide range of composition.
3.4.Dynamic breakthrough curve
Besides the adsorption isotherm,a dynamic breakthrough experiment is also significant in the evaluation of separation performance of the adsorbent.In this work,we tested the separation of the 20% and 50%CH4/N2mixtures(volume fraction)on an adsorption bed packed with Chabazite-K,SAPO-34 and SSZ-13,respectively.Fig.7 shows the experimental breakthrough curves measured at 298 K and 0.1 MPa in the terms of the outlet volume fraction of each component as a function of time. The dynamic information in regard to the penetration time,equilibrium time and retention time of CH4and N2as well as the separation efficiency are summarized in Table 3.
From the data listed in Table 3,we can quantify this separation performance.The equilibrium time is proportional to mass transfer limitation and the retention time reflects the whole separation performance.Meanwhile,we defined an index called separation efficiency to evaluate the validity of the separation process,as given by Eq.(3).Here,the penetration time refers to that of the more strongly adsorbed component i.e.CH4.

Both of the breakthrough curves clearly shown there is no signal for CH4and N2detected at the beginning, since both CH4and N2are retained in the adsorption bed until the first component N2elutes from the adsorption bed,which indicates N2is more weakly adsorbed.The N2profile is straighter than CH4,indicating that the mass transfer limitation is larger for CH4on the three molecular sieves,which can also be reflected by the equilibrium time of CH4being greater than the equilibrium time of N2. Among the materials studied, Chabazite-K,which has the highest selectivity and the lowest CH4adsorption capacity exhibits retention time analogous to that of SAPO-34 in both the 20%and 50%CH4/N2mixtures,but the penetration time of CH4and N2are longer on SAPO-34 than Chabazite-K during the separation of an equimolar CH4/N2mixtures.SSZ-13,which possesses the lowest selectivity and the highest CH4adsorption capacity,has the longest retention time(18 min) when compared with Chabazite-K (10 min) and SAPO-34(11 min),showing its superior separation performance for 20%CH4/N2mixtures. This may attributes to the high-silica nature of SSZ-13 making its framework electrically neutral and surface “smooth” and thus, its better separation performance for low concentration CH4/N2mixtures(CH4<20%),and separation performance of SSZ-13 is similar to that of Chabazite-K and SAPO-34 for an equimolar CH4/N2mixtures.However,Chabazite-K has the largest separation efficiency,which means that it can shorten the operation cycle effectively in industry manufacturing.From the actual manufacturing point of views,Chabazite-K is more suited to separation of high concentration methane(CH4>50%).

Fig.6.Adsorption selectivity of mixtures CH4/N2(20%and 50%)on Chabazite-K,●;SAPO-34,■and SSZ-13,▲.

Fig.7.Breakthrough data of Chabazite-K(a,b),SAPO-34(c,d),and SSZ-13(e,f)for mixtures CH4/N2(20%and 50%)at 298 K and 0.1 MPa.
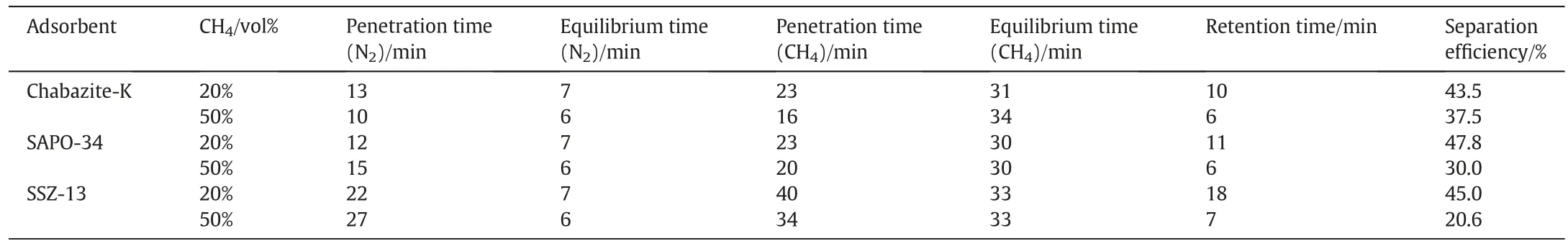
Table 3 Comparison of dynamic performance on three molecular sieves
4.Conclusions
Our results indicate that Chabazite-K,which has the smallest pore volume and surface area among the molecular sieves studied adsorbs the lowest quantity of CH4,but possess the highest CH4/N2selectivity.SAPO-34,which has the 2nd largest surface area and the 2nd largest micropore volume among the series captures the 2nd largest quantity of N2and similar quantity of CH4to Chabazite-K at 1 bar,and shows the 2nd largest selectivity.As for SSZ-13,which has the largest micropore volume and the largest surface area among the three materials,can hold the largest quantity of CH4and N2,but possess the lowest selectivity.A good relationship between the Si/Al ratio,pore volume and uptake of CH4and N2was observed when comparing SAPO-34 and SSZ-13.A high Si/Al ratio usually leads to a higher pore volume and adsorption capacity,which is due to its structure with less charge-balancing cations. While in the case of Chabazite-K and SAPO-34 with similar Si/Al ratio,it is the extraframework metal cation that determines the adsorption capacity and selectivity of the materials.Due to the existence of metal cation,the size of the openings were narrowed and result in a decrease in the adsorption capacity of CH4and enhanced the interactions with CH4due to its higher polarizability,causing an increase in the selectivity.In the low concentration CH4/N2mixtures(i.e.20%/80%),the separation performance was found to be in the order of:SSZ-13 >Chabazite-K ≈SAPO-34.While in the equimolar CH4/N2mixtures, the separation performance of the three molecular sieves was similar,but Chabazite-K had the largest separation efficiency,which is more competitive in industry manufacturing.Thus,in high concentration methane,the separation performance may be ranked by the separation efficiency,which was found to be in the order of:Chabazite-K >SAPO-34 >SSZ-13.In other words,SSZ-13 may be a suitable candidate for the recovery of CH4from low concentration methane due to its larger pore volume,and Chabazite-K is more suited to the separation of high concentration methane due to its higher selectivity.Upon comparing the above results,it can be concluded that the zeolite framework with electrically neutral“smooth surface”in the higher Si/Al ratio zeolites contributes to the separation of low concentration methane.
Acknowledgment
We acknowledge financial support from the National Natural Science Foundation of China(Nos.51672186,21676175).
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Assessment of the TFM in predicting the onset of turbulent fluidization☆
- CFD study on double-to single-loop flow pattern transition and its influence on macro mixing efficiency in fully baffled tank stirred by a Rushton turbine☆
- Simulation of drop breakage in liquid-liquid system by coupling of CFD and PBM:Comparison of breakage kernels and effects of agitator configurations☆
- Heat transfer characteristics of molten plastics in a vertical falling film reactor☆
- Stabilizing silica nanoparticles in high saline water by using polyvinylpyrrolidone for reduction of asphaltene precipitation damage under dynamic condition
- Numerical simulation and experimental study on dissolving characteristics of layered salt rocks
