On the Generic Taxonomy of Opisthotropis balteata (Cope, 1895)(Squamata: Colubridae: Natricinae): Taxonomic Revision of Two Natricine Genera
2019-06-26JinlongRENKaiWANGPengGUOYingyongWANGTaoThienNGUYENandJiatangLI
Jinlong REN, Kai WANG, Peng GUO, Yingyong WANG, Tao Thien NGUYEN and Jiatang LI
1 CAS Key Laboratory of Mountain Ecological Restoration and Bioresource Utilization and Ecological Restoration and Biodiversity Conservation Key Laboratory of Sichuan Province, Chengdu Institute of Biology, Chinese Academy of Sciences, Chengdu, Sichuan 610041, China
2 Center for Excellence in Animal Evolution and Genetics, Chinese Academy of Sciences, Kunming, Yunnan 650223,China
3 University of Chinese Academy of Sciences, Beijing 100049, China
4 Sam Noble Oklahoma Museum of Natural History and Department of Biology, University of Oklahoma, Norman,Oklahoma 73019, USA
5 College of Life Sciences and Food Engineering, Yibin University, Yibin, Sichuan 644007, China
6 State Key Laboratory of Biocontrol / The Museum of Biology, School of Life Sciences, Sun Yat-sen University,Guangzhou, Guangdong 510275, China
7 Vietnam National Museum of Nature, Vietnam Academy of Science and Technology, 18 Hoang Quoc Viet Road, Hanoi,Vietnam
8 Graduate University of Science and Technology, Vietnam Academy of Science and Technology, 18 Hoang Quoc Viet,Cau Giay, Hanoi, Vietnam
9 Southeast Asia Biodiversity Research Institute, Chinese Academy of Sciences, Yezin, Nay Pyi Taw 05282, Myanmar
Abstract The single prefrontal configuration has historically been used as an important diagnostic character for many natricine taxa. For example, the genus Trimerodytes Cope, 1895 was long been regarded as a junior synonym of Opisthotropis Günther, 1872 for their similar prefrontal configurations and the type species, T. balteatus Cope,1895, has been assigned to the genus Opisthotropis. However, as the number and arrangement of prefrontal vary frequently both at species and generic level, it is questionable whether the synonymization of Trimerodytes reflects their evolutionary relationships. On the basis of recently collected specimens of O. balteata, the generic status of the species was assessed using both molecular and morphological data. Opisthotropis was recovered as polyphyletic with reference to O. balteata, because O. balteata is nested within the genus Sinonatrix Rossman and Eberle, 1977 and is the sister species of the type species of Sinonatrix. Consequently, we herein resurrect the long-overlooked synonym Trimerodytes from Opisthotropis and synonymize the junior generic nomen Sinonatrix with Trimerodytes. In addition, based on morphological similarities between the monotypic genus Paratapinophis Angel, 1929 and Trimerodytes, we doubt about the validity of Paratapinophis. Following taxonomic changes in this work, the taxonomic account of the genus Trimerodytes, updated descriptions of its type species, and diagnostic key to Trimerodytes species are provided.
Keywords Paratapinophis, prefrontal scales, Sinonatrix, taxonomic revision, Trimerodytes
1. Introduction
The family Colubridae includes more than half of the known snake species of the world, with eight subfamilies recognized to date (Figueroa et al., 2016). This family represents the most morphologically diverse and widely distributed group among all squamates (Uetz et al., 2018;Zhao et al., 1998). Within the family, numbers of cephalic scales and their arrangements represent one of the most important diagnostic characters (Boulenger, 1893;Smith, 1943; Zhao, 2006). Regarding these head scales,most colubrid snakes have nine enlarged dorsal side of head scales, including paired internasals, prefrontals,supraoculars, parietal scales, and a single frontal scale.Such set of head scales and their configurations are known as the typical colubrid configuration or “colubridelapid dorsal nine-scute arrangement” (David et al., 2015;O’shea et al., 2018).
Despite the fact that such typical configuration of head scales is common, a great variation exits among the keelback snakes of the subfamily Natricinae, particularly for the prefrontal scales (Bourret, 1934; David et al.,2015; Smith, 1943; Zhao and Jiang, 1981). Since the presence of paired prefrontals is considered to be the typical configuration, any variation from this condition,such as a single prefrontal, is regarded as an important diagnostic character in taxonomic delimitations,particularly at the generic level (Angel, 1929; Bourret,1934; David et al., 2015; Taylor and Elbel, 1958).
The single prefrontal configuration is found in many natricine groups, including Opisthotropis Günther, 1872,Paratapinophis Angel, 1929, Isanophis David, Pauwels,Nguyen and Vogel, 2015, Hebius Thompson, 1913,Trachischium Günther, 1858, and Rhabdops Boulenger,1893 (Angel, 1929; Bourret, 1934; Kizirian et al., 2018;Smith, 1943; Taylor and Elbel, 1958; David et al., 2015).Furthermore, the morphology-based taxonomy that relies on the single prefrontal has resulted in taxonomic confusions, in which species were incorrectly assigned to a genus based on their similar prefrontal configurations(Brown and Leviton, 1961; Pope, 1935; Smith, 1943;Taylor and Elbel, 1958).
Erected as a monotypic genus, with Trimerodytes balteatus Cope, 1895 as the sole included species,the genus Trimerodytes was later synonymized with Opisthotropis on the basis of morphological similarities,including the presence of a single prefrontal scale (Pope,1935). Most later authors followed Pope (1935) and considered Trimerodytes to be a junior synonym of Opisthotropis and its type species, T. balteatus, has been assigned to Opisthotropis (Brown and Leviton, 1961;Nguyen et al., 2009; Smith, 1943; Zhao, 2006; Zhao et al., 1998; Zheng, 1992). Although there have been recent phylogenetic studies on Opisthotropis, none of them included O. balteata in their analyses (Ren et al.,2017; Wang et al., 2017b; Ziegler et al., 2017; 2018).Therefore, the generic assignment of O. balteata has not been confirmed by molecular data. Consequently, both the validity of Trimerodytes and the phylogenetic placement of its type species have remained unknown (Cope, 1895;David et al., 2011).
On the basis of newly collected genetic tissues and specimens of O. balteata, in this study we assessed the taxonomic validity of Trimerodytes and the phylogenetic position of O. balteata. Furthermore, we examined the usefulness of the prefrontal configuration as the generic diagnosis among natricine genera. Our results indicate that the genus Opisthotropis is polyphyletic with respect to O. balteata, which is nested within the genus Sinonatrix Rossman and Eberle, 1977 and is sister to Sinonatrix annularis (Hallowell, 1856), the type species of the latter genus (Rossman and Eberle, 1977). As a consequence, we resurrect the long-overlooked synonym Trimerodytes from Opisthotropis and synonymized the junior name Sinonatrix with Trimerodytes. In addition, we comment on the similarities in morphological characters between the monotypic genus Paratapinophis and Trimerodytes. Lastly, we provide a taxonomic account of Trimerodytes, the updated description of its type species,and a diagnostic key to this genus.
2. Materials and Methods
2.1. SamplingSpecimens were collected during field surveys from June 2015 to September 2018. After being euthanized, fresh liver tissues were taken and preserved in 95% ethanol, and then stored at –20°C for DNA extraction. Specimens were preserved in 10% formalin in the field and transferred to 75% ethanol for permanent storage after fieldwork. All of these recently collected specimens were deposited in major collections in China,including the Museum of Herpetology, Chengdu Institute of Biology, Chinese Academy of Sciences, Chengdu,China (CIB); the Museum of Biology, Sun Yat-sen University, Guangzhou, China (SYS); and College of Life Sciences and Food Engineering, Yibin University, Yibin,China (YBU). In addition, specimens were examined in the following collections: Institute of Ecology and Biological Resources, Vietnam Academy of Science and Technology, Hanoi, Vietnam (IEBR); Museum of Kunming Institute of Zoology, Chinese Academy of Sciences, Kunming, China (KIZ); Vietnam National Museum of Nature, Vietnam Academy of Science and Technology, Hanoi, Vietnam (VNMN); and Zoological Museum, Vietnam National University, Hanoi, Vietnam(VNUH). The topographic map was created from MAP WORLD, http://www.tianditu.com.
2.2. Molecular analysesGenomic DNA was extracted from macerated liver tissue samples using an Ezup Column Animal Genomic DNA Purification Kit(Sangon Biotech, China), according to the protocols of the manufacturer. A total of 1 060 bp of the mitochondrial gene cytochrome b (cyt b) was targeted and amplified via the polymerase chain reaction(PCR), using the following primer pairs: L14919 (5'–AACCACCGTTGTTATTCAACT–3')/L14910 (5'–GACCTGTGATMTGAAAACCAYCGTTGT–3'), and H16064 (5'–CTTTGGTTTACAAGAACAATGCT TTA–3') (Burbrink et al., 2000; Guo et al., 2012; Wang et al., 2017b), PCR protocols as described by Ren et al.(2017). The PCR products were purified and sequenced in both directions using an ABI 3730xL sequencer by Sangon Biotech Co., Ltd (Chengdu, China). All newly generated sequences of this work were deposited in GenBank, and additional sequences of natricine species were also obtained from GenBank (Table 1). Outgrouptaxa were selected based on previous phylogenetic studies (Figueroa et al., 2016; Pyron et al., 2013), which include Hebius johannis (Boulenger, 1908), Natrix natrix(Linnaeus, 1758), and Rhabdophis leonardi (Wall, 1923).The DNA sequences of cyt b dataset were aligned using the FasParser software package (Sun, 2017) and checked visually for minor manual justifications. Phylogenetic relationships were estimated using two methods,Bayesian inference (BI) and maximum likelihood (ML),both with partitions based on codon position, which were selected in PartitionFinder 2 (Lanfear et al., 2017). The most optimum substitution model of sequence evolution(GTR+Γ for all codon positions) was selected under the Akaike Information Criterion (AIC). BI analyses were implemented by MrBayes v3.1.2 (Ronquist and Huelsenbeck, 2003), two runs were performed simultaneously with four Markov chains and the dataset was conducted with 10 000 000 replicates. Starting trees were random and the first 25% trees were discarded as“burn-in”, sampling one tree per 100 replications for each run. Bayesian posterior probability (BPP) was determined to test the confidence of tree topology, nodes in the trees were considered to be significantly supported when BPP≥ 0.95. Tracer 1.6 (Rambaut et al., 2013) was performed to investigated the convergence of sampled trees.

Table 1 Information of samples, voucher numbers, sequences GenBank accession numbers and collection localities used in molecular analysis. Voucher abbreviations: SYS=Museum of Biology, Sun Yat-sen University, Guangzhou, China; CIB=Chengdu Institute of Biology, Chengdu, China; YBU=Yibin University, Yibin, China; IEBR=Institute of Ecology and Biological Resources, Hanoi, Vietnam;ZFMK=Zoologisches Forschungsmuseum Alexander Koenig, Bonn, Germany; VNMN=Vietnam National Museum of Nature, Hanoi,Vietnam; GP=Dr. Guo Peng’s personal catalogue numbers; CAS=California Academy of Science, San Francisco, USA; MTD T=Museum of Zoology, Senckenberg Dresden (Tissue Collection), Dresden, Germany; SCUM=Sichuan University Museum, Chengdu, China.
The partitioned ML analyses were conducted using the most complex model GTR+Γ by through 100 different runs in RAxML 8.2.10 (Stamatakis, 2014), bootstrap proportions (BSP) were assessed to test the node support,using the rapid-bootstrapping algorithm with 1000 nonparametric bootstrap replicates, where nodes in the trees were considered to be strongly supported when BSP ≥ 70.
In addition to the phylogenetic analyses, uncorrected pairwise distances (p-distances) of the cyt b dataset among species of Sinonatrix and Opisthotropis were calculated using MEGA 6 (Tamura et al., 2013).
2.3. Morphological dataMeasurements were taken with a digital slide-caliper to the nearest 0.1 mm, except the total length, snout-vent length, and tail length, which were measured using a measuring tape to the nearest 1 mm.Measurement methods and their definitions followed Zhao(2006) and Ren et al. (2018), and included: total length(TL), snout-vent length (SVL), tail length (TaL), head length (HL), and head width (HW), rostral length (RL),rostral width (RW), interorbital distance (IOD), eye width(EW), distance between the lower margins of eye and of lip (SoL), maximum loreal length (LoL), maximum loreal depth (LoD), maximum anterior temporal length (ToL),and maximum anterior temporal depth (ToD). In addition,to remove covariance of raw measurements for better comparisons, the following ratios were also obtained from raw measurements, including TaL/TL, HL/HW, SL/SW,IOD/HW, EW/SoL, LoL/LoD, and ToL/ToD.
Definitions of pholidosis characters and their counting methods followed Zhao (2006) and Ren et al. (2018),which include internasal counts (IN), prefrontal counts(PrF), frontal counts (F), parietal counts (P), loreal counts (L), preocular counts (PrO), postocular counts(PtO), supraocular counts (SpO), subocular counts(SbO), supralabial counts (SpL), infralabial counts (IfL),temporal counts (TEM), chin shield counts (CS), dorsal scale row counts (DSR), ventral counts (VEN), and subcaudal counts (SC). Dorsal scale rows were taken at one head length behind head, at midbody, and at one head length before cloaca, respectively.
In addition to the scale counts, the following characters were also used, including: SpL-orbit: supralabial enter orbit; aTEM: anterior temporal format; pTEM: posterior temporal format; HdN: distinction between head and neck; DoN: direction of nostrils; MT: maxillary teeth counts; and the numbers of annulated markings present along both trunk and tail were formulated as trunk+tail(eg. 42+13). Symmetric characters were given as left/right, and averages were used in the comparisons, except for maxillary teeth and the number of annulated markings along lateral body, which were only counted on the left side.
Morphological and ecological data of related taxa were based on specimens examined (Appendix 1) and from the literature (Brown and Leviton, 1961; Chuaynkern et al.,2014; David et al., 2011; Günther, 1872; Huang et al.,1990; Iskandar and Kamsi, 2009; Le et al., 2015; Murphy et al., 2008; Noonloy et al., 2018; Okada and Takara,1958; Orlov et al., 1998; Ota, 2004; Pauwels et al., 2009;Pope, 1935; Rao and Yang, 1998; Rasmussen, 1982; Ren et al., 2017; Rossman and Eberle, 1977; Smith, 1943;Stuart and Heatwole, 2008; Teynié et al., 2014; Toyama,1983; Vogel et al., 2004; Wang et al., 2017a, 2017b; Wu et al., 1985; Yang et al., 2011, 2013; Zhao, 2006; Zhao et al., 1998; Zheng, 1992; Ziegler et al., 2017, 2018).
3. Results
3.1. Molecular resultsA total of 1 060 base pairs were aligned for the dataset of 44 sequences, which represented all available 15 Opisthotropis species and four currently recognized Sinonatrix species, as well as three outgroup taxa. The BI and ML analyses of dataset yielded identical topologies, where the genera Opisthotropis and Sinonatrix were recovered as polyphyletic and paraphyletic groups with regard to O. balteata, respectively (Figure 1). With the exclusion of O. balteata, all remaining species of Opisthotropis formed a monophyletic clade (clade A,BPP=0.99, BSP=41), whereas all species of the genus Sinonatrix and O. balteata formed another well-supported clade, clade B (BPP=1.00, BSP=100). Within clade B, the previously unassigned species S. yunnanensis Rao and Yang, 1998 was strongly supported as the sister taxon to S. aequifasciata (Barbour, 1908) (BPP=0.90, BSP=79).Opisthotropis balteata was nested within the genus Sinonatrix and sister to the type species of the genus Sinonatrix, S. annularis, with strong statistical support(clade B, BPP=1.00, BSP=99).
The infraspecific uncorrected genetic p-distances of O. balteata ranged from 0–0.2% (Table 2). The p-distances varied from 8.8% to 12.4% between O. balteata and all analyzed Sinonatrix species [minimum distance to S. annularis, which was recovered as sister group of O. balteata (Figure 1); maximum distance to S. aequifasciata (Table 2)] and from 14.7% to 18.6%between O. balteata and all other sampled species of Opisthotropis (minimum to O. jacobi; maximum to O. tamdaoensis).
3.2. Morphological resultsMorphological characters of our newly collected specimens of O. balteata matched the original description by Cope (1895) and largely agreed with previous descriptions (Fan, 1931; Peracca, 1904;Steindachner, 1906; Zhao, 2006; Zhao et al., 1998),except that the slightly lower number of maxillary teeth(16–17 vs. 17–18, 25 by Fan, 1931) and slightly fewer ventral scales (187–196 vs. 194–205) (Table 3).
Opisthotropis balteata possesses a suite of morphological characters that differentiate itself from remaining congeners of the genus Opisthotropis,including having (1) a relatively larger body size, TL 604–1 021 mm (vs. TL mostly lower than 600 mm); (2) a head moderately distinct from the neck (vs. barely distinct);(3) dorsolaterally directed nostrils (vs. dorsally); (4) a relatively larger eye size (vs. overwhelming small); (5)large maxillary teeth, which are enlarged posteriorly (vs.small and subequal); (6) a higher number of postocular[3 (rarely 2, 4, 5) vs. 1–2 (3 occur in O. kuatunensis and O. laui only)]; (7) the presence of annulated markings (vs.color uniform, with longitudinal lines, crossbars or spots);(8) the presence of ventral black bands (vs. uniform color). Although O. balteata has a single prefrontal similar to all species of Opisthotropis, the prefrontal of O. balteata differs in shape (prefrontal spindle-shaped vs.tricuspid or subrectangular) (Table 4; Figures 2–3).

Figure 1 Bayesian inference (BI) phylogenetic tree estimated from partial mtDNA cyt b sequences, depicting phylogenetic relationships of two sister genera, Opisthotropis and Sinonatrix, of the subfamily Natricinae. Numbers above branches are bayesian posterior probability and bootstrap proportions (BPP/BSP), respectively. Branches in red showing the nominal taxa of Opisthotropis. Exemplar dorsal side of head drawings showing variation in the number of prefrontals, which are the two conditions that species classified by, i.e. (1) single prefrontal (solid black) and (2) paired prefrontals (outlined in black). Photographs and line drawings by Jinlong REN.

21 20 13.4%Table 2 Uncorrected pairwise sequence divergence among 1 060 bp fragments of mtDNA cyt b sequences of clade A and clade B as shown in phylogenetic tree (see Figure 2).19 14.3%15.1%18 13.2%13.7%15.6%17 11.1%14.0%14.0%14.0%16 14.8%15.1%15.5%12.7%15.5%15 15.3%11.1%13.0%12.5%12.1%15.0%14 15.3%16.1%15.0%15.8%15.3%13.5%5.9%13 15.6%12.9%16.4%13.2%13.0%5.9%14.2%15.1%12 14.7%12.4%16.0%14.5%15.3%17.3%15.3%13.5%13.7%11 14.3%16.3%14.2%13.4%12.4%14.7%14.7%16.3%8.5%13.0%10 12.4%15.8%16.0%14.0%15.5%13.5%15.3%14.5%16.3%12.2%13.7%9 14.2%14.0%12.4%15.5%5.2%14.0%16.9%15.3%16.1%15.5%13.2%5.2%8 15.8%14.0%15.0%16.4%12.7%15.8%11.2%15.5%11.1%11.1%13.0%14.7%15.0%7 18.1%16.9%16.4%16.4%16.8%16.8%15.5%18.2%16.1%18.7%18.2%18.1%16.6%16.1%6 8.5%17.8%16.0%16.8%13.7%15.6%16.4%16.0%17.1%15.0%17.6%17.8%16.9%15.5%15.6%5 9.3%10.7%16.0%16.1%15.6%15.0%16.1%16.8%15.5%15.8%15.0%16.9%17.4%17.3%15.0%15.8%4 10.3%10.7%11.1%17.4%16.9%17.4%15.6%16.4%19.1%15.6%16.4%15.1%18.2%18.1%17.9%15.6%16.9%3 8.8%9.9%11.6%12.4%16.9%17.6%16.6%14.8%17.8%17.9%17.3%17.3%16.9%17.8%17.6%18.6%16.0%17.1%2 0.2%8.8%10.1%11.7%12.2%16.8%17.4%16.4%14.7%17.9%17.8%17.1%17.1%17.1%17.9%17.4%18.4%16.1%16.9%1 0.0%0.2%8.8%10.1%11.7%12.2%16.8%17.4%16.4%14.7%17.9%17.8%17.1%17.1%17.1%17.9%17.4%18.4%16.1%16.9%Voucher GP 746 Species balteata CIB 109017 Opisthotropis balteata CIB 109018 Opisthotropis balteata YBU 16109 Opisthotropis annularis CIB 109020 Sinonatrix percarinata CIB 109022 Sinonatrix yunnanensis CAS 224485 Sinonatrix andersonii SYS r001020 aequifasciata CIB 109019 Sinonatrix Opisthotropis cheni YBU 071040 Opisthotropis Opisthotropis jacobi IEBR 4329 guangxiensis Opisthotropis kuatunensis SYS r001008 Opisthotropis lateralis SYS r000951 Opisthotropis latouchii SYS r000670 Opisthotropis laui SYS r001161 Opisthotropis maculosa SYS r000946 Opisthotropis maxwelli SYS r001053 Opisthotropis tamdaoensis IEBR A.2016.33 voquyi IEBR 4327 Opisthotropis shenzhenensis SYS r001018 Opisthotropis Opisthotropis zhaoermii CIB 109999 Opisthotropis Clade 1B 2B 3B 4B 5B 6B 7B 8A 9A 10A 11A 12A 13A 14A 15A 16A 17A 18A 19A 20A 21A
With the exclusion of O. balteata, members of the genus Opisthotropis differs from Sinonatrix in the following distinct morphological characters, including having (1) a relatively smaller body size, slenderly built (vs. larger, strongly built and robust); (2) a barely distinct head from the neck (vs. moderately or clearly distinct); (3) dorsally directed nostrils (vs. dorsolaterally);(4) a relatively smaller eye size (large or moderate);(5) maxillary teeth small, subequal (vs. large, enlarged posteriorly); (6) prefrontal single (vs. paired) (Tables 4–5;Figures 2–3).
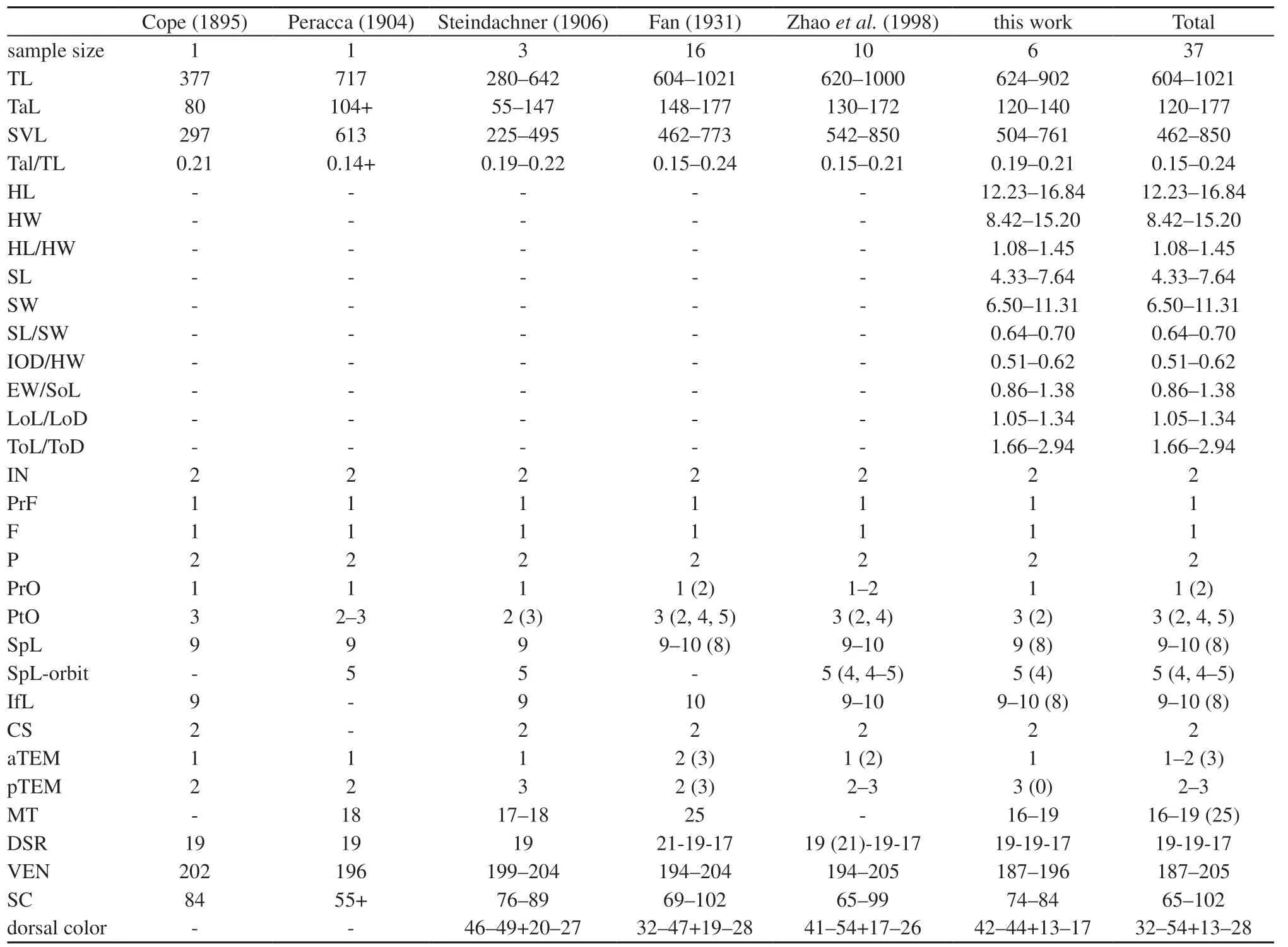
Table 3 Morphological and pholidosis characters of Trimerodytes balteatus obtained from the literature and this work.
In contrast, O. balteata possesses a combination of morphological characters shared with the members of the genus Sinonatrix, including the head moderately or clearly distinct from the neck, large or moderate eyes, dorsolaterally directed nostrils, enlarged posterior maxillary teeth, and annulated color patterns (Table 4; Figures 2–3). Additionally, O. balteata is similar to Sinonatrix by having 19-19-17 dorsal scale rows, which is a morphological character shared among all the four species of Sinonatrix (Le et al., 2015; Rao and Yang,1998), whereas it is unknown in any Opisthotropis species(Table 4).
Furthermore, the ecology of O. balteata also matches the members of the genus Sinonatrix and differs from congeners of Opisthotropis, including diet preference(mainly fishes for O. balteata and Sinonatrix species vs. worms, small shrimps, crabs, or tadpoles for Opisthotropis species) and activity patterns (often diurnal for O. balteata and Sinonatrix species vs. strictly nocturnal for most Opisthotropis) (Karsen et al., 1986;Karsen et al., 1998; Le et al., 2015; Noonloy et al., 2018;Ota, 2004; Pauwels et al., 2009; Pope, 1935; Zhao, 2006;Zhao et al., 1998).
4. Discussion
4.1. Taxonomic assignment of O. balteataDespite the long assignment of O. balteata to the genus Opisthotropis(Nguyen et al., 2009; Smith, 1943; Zhao, 2006; Zhao et al., 1998; Zheng, 1992), one study has previously suggested that this generic assignment might be questionable. While recording O. balteata from Luoxiang,Guangxi Zhuang Autonomous Region, China (considered to be O. multicinctata at the time, later synonymized to O. balteata), Fan (1931) suggested that the species was “rather isolated” from other congeners of the genus Opisthotropis in terms of external morphology.
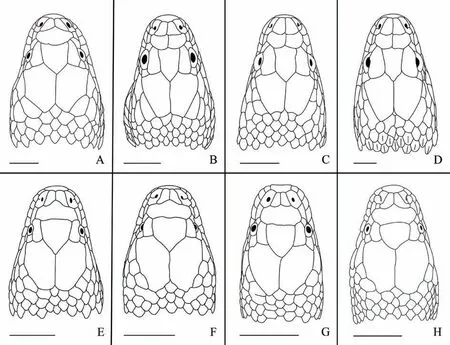
Figure 2 Comparisons of head in dorsal view among species of Trimerodytes (upper row) and Opisthotropis (lower row). (A) Trimerodytes balteatus (CIB 109017; adult female; Guangxi, China); (B) T. annularis (CIB 11461; adult male; Wuyishan, Fujian, China); (C) T.aequifasciatus (CIB 11436; subadult female; Yizhang, Hunan, China); (D) T. percarinatus (CIB 109023; adult female; Wuyishan, Fujian,China); (E) O. cheni (CIB 78149; adult male; Yizhang, Hunan, China); (F) O. shenzhenensis (SYS r001018; holotype; adult male; Shenzhen,Guangdong, China); (G) O. guangxiensis (SYS r001739; adult male; Nanning, Guangxi, China); (H) O. cucae (IEBR A.0924; holotype; adult female; Sa Thay, Kon Tum, Vietnam). Scale bar 5 mm. Line drawings by Jinlong REN.
Our morphological and molecular evidences agree with those of Fan (1931) and show that the genus Opisthotropis is polyphyletic with respect to O. balteata and species of Sinonatrix, and the continuous recognition of O. balteata in the genus Opisthotropis will preclude the monophyly of both genera Opisthotropis and Sinonatrix (Figure 1).In combining evidence from external morphology and ecology between O. balteata and members of Sinonatrix,therefore, and in order to stabilize the generic taxonomy and best reflect the evolutionary relationships of these snakes, O. balteata should be removed from Opisthotropis and placed into the genus currently represented by Sinonatrix (clade B).
However, with the inclusion of O. balteata, the generic name of the lineage (clade B) that is currently represented by Sinonatrix needs further justification. Clade B includes both the type species (i.e. Sinonatrix annularis)and other members of the genus currently known as Sinonatrix, plus “Opisthotropis” balteata. This latter species, “Opisthotropis” balteata is the type species by monotypy of Trimerodytes, which is currently considered to be a junior synonym of the genus Opisthotropis. As a consequence, Trimerodytes Cope, 1895 is distinct from Opisthotropis, and Sinonatrix Rossman and Eberle, 1977 is a junior synonym of Trimerodytes, a generic nomen which has hence priority.
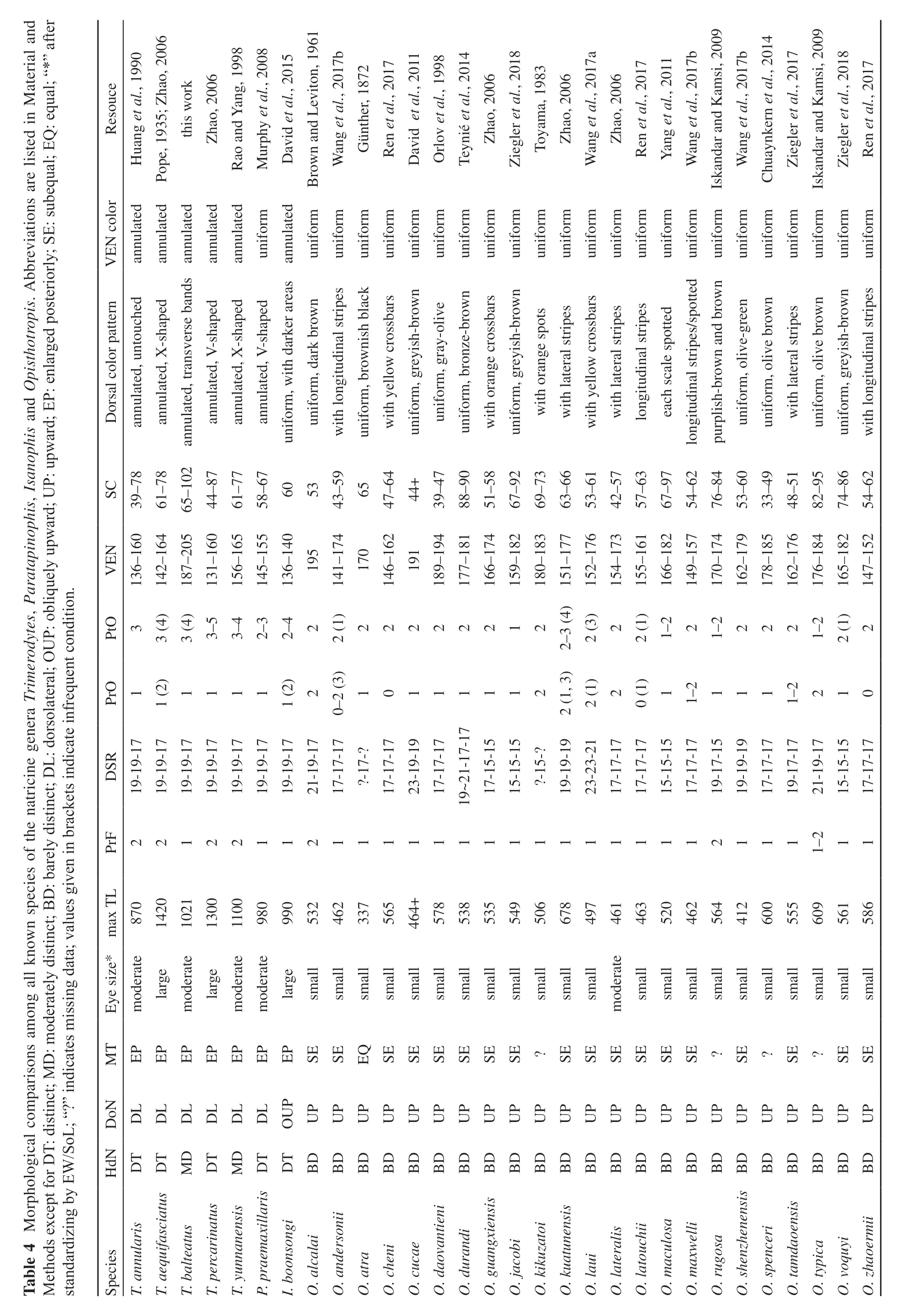
Table 4 Morphological comparisons among all known species of the natricine genera Trimerodytes, Paratapinophis, Isanophis and Opisthotropis. Abbreviations are listed in Material and Methods except for DT: distinct; MD: moderately distinct; BD: barely distinct; DL: dorsolateral; OUP: obliquely upward; UP: upward; EP: enlarged posteriorly; SE: subequal; EQ: equal; “*” after standardizing by EW/SoL; “?” indicates missing data; values given in brackets indicate infrequent condition.Resouce Huang et al., 1990 Pope, 1935; Zhao, 2006 this work Zhao, 2006 Rao and Yang, 1998 Murphy et al., 2008 David et al., 2015 Brown and Leviton, 1961 Wang et al., 2017b Günther, 1872 Ren et al., 2017 David et al., 2011 Orlov et al., 1998 Teynié et al., 2014 Zhao, 2006 Ziegler et al., 2018 Toyama, 1983 Zhao, 2006 Wang et al., 2017a Zhao, 2006 Ren et al., 2017 Yang et al., 2011 Wang et al., 2017b Iskandar and Kamsi, 2009 Wang et al., 2017b Chuaynkern et al., 2014 Ziegler et al., 2017 Iskandar and Kamsi, 2009 Ziegler et al., 2018 Ren et al., 2017 annulated annulated annulated annulated annulated uniform annulated uniform uniform uniform uniform uniform uniform uniform uniform uniform uniform uniform uniform uniform uniform uniform uniform uniform uniform uniform uniform uniform uniform uniform VEN color Dorsal color pattern annulated, untouched annulated, X-shaped annulated, transverse bands annulated, V-shaped annulated, X-shaped annulated, V-shaped uniform, with darker areas uniform, dark brown with longitudinal stripes uniform, brownish black with yellow crossbars uniform, greyish-brown uniform, gray-olive uniform, bronze-brown with orange crossbars uniform, greyish-brown with orange spots with lateral stripes with yellow crossbars with lateral stripes longitudinal stripes each scale spotted longitudinal stripes/spotted purplish-brown and brown uniform, olive-green uniform, olive brown with lateral stripes uniform, olive brown uniform, greyish-brown with longitudinal stripes SC 39–78 61–78 65–102 44–87 61–77 58–67 6053 43–59 65 47–64 44+39–47 88–90 51–58 67–92 69–73 63–66 53–61 42–57 57–63 67–97 54–62 76–84 53–60 33–49 48–51 82–95 74–86 54–62 VEN 136–160 142–164 187–205 131–160 156–165 145–155 136–140 195 141–174 170 146–162 191 189–194 177–181 166–174 159–182 180–183 151–177 152–176 154–173 155–161 166–182 149–157 170–174 162–179 178–185 162–176 176–184 165–182 147–152 PtO3 3 (4)3 (4)3–5 3–4 2–3 2–42 2 (1)22222212 2–3 (4)2 (3)2 2 (1)1–2 2 1–2 222 1–2 2 (1)2 PrO1 1 (2)1111 1 (2)2 0–2 (3)10111112 2 (1, 3)2 (1)2 0 (1)1 1–2 111 1–2210 DSR 19-19-17 19-19-17 19-19-17 19-19-17 19-19-17 19-19-17 19-19-17 21-19-17 17-17-17?-17-?17-17-17 23-19-19 17-17-17 17-15-15 15-15-15?-15-?19-19-19 23-23-21 17-17-17 17-17-17 15-15-15 17-17-17 19-17-15 19-19-19 17-17-17 19-17-17 21-19-17 15-15-15 17-17-17 19~21-17-17 PrF221221121111111111111112111 1–2 11 max TL 870 1420 1021 1300 1100 980 990 532 462 337 565 464+578 538 535 549 506 678 497 461 463 520 462 564 412 600 555 609 561 586 Eye size*moderate large moderate large moderate moderate large small small small small small small small small small small small small moderate small small small small small small small small small small MT EP EP EP EP EP EP EP SE SE EQ SE SE SE SE SE SE?SE SE SE SE SE SE?SE?SE?SE SE DoN DL DL DL DL DL DL OUP UP UP UP UP UP UP UP UP UP UP UP UP UP UP UP UP UP UP UP UP UP UP UP HdN DT DT MD DT MD DT DT BD BD BD BD BD BD BD BD BD BD BD BD BD BD BD BD BD BD BD BD BD BD Species T. annularis T. aequifasciatus T. balteatus T. percarinatus T. yunnanensis P. praemaxillaris I. boonsongi O. alcalai O. andersonii O. atra O. cheni O. cucae O. daovantieni O. durandi O. guangxiensis O. jacobi O. kikuzatoi O. kuatunensis O. laui O. lateralis O. latouchii O. maculosa O. maxwelli O. rugosa O. shenzhenensis BD O. spenceri O. tamdaoensis O. typica O. voquyi O. zhaoermii
Could Trimerodytes be considered to be a nomen oblitum? The possibility of a “reversal of precedence”is addressed by the International Code of Zoological Nomenclature (ICZN, 1999), Art. 23.9. Although the taxon Trimerodytes satisfies Art. 23.9.1.2, the necessary condition included in Art. 23.9.1.1 is not met in the present case since Trimerodytes was used as a valid generic name by Mell (1931: 206) and Schmidt (1927:438), so after 1899. As a consequence, the reversal of precedence cannot be applied here. Hence, we resurrect Trimerodytes Cope, 1895 from Opisthotropis Günther,1872 as a valid genus, and place Sinonatrix Rossman and Eberle, 1977 in its junior synonymy.

Figure 3 Lateral head (column 1), dorsal side of head (column 2), ventral head (column 3), dorsal color pattern (column 4), and ventral color pattern (column 5) comparisons of species of Trimerodytes and Opisthotropis. (A) T. percarinatus (CIB 109023); (B) T. aequifasciatus (CIB 11436); (C) T. annularis (CIB 109020; adult male; Guangxi, China); (D) T. balteatus (CIB 109017); (E) O. cheni (CIB 78148; adult male;Yizhang, Hunan, China); (F) O. tamdaoensis (VNMN 06217; adult female; Tamdao, Vinh Phuc, Vietnam); (G) O. jacobi (VNMN 06220;adult female; Tamdao, Vinh Phuc, Vietnam); (H) O. cf. maculosa (SYS r001525; adult female; Zhaoqing, Guangdong, China). Photographs by Jinlong REN.
Following systematic implications as presented above, we present a new classification scheme of genus
Trimerodytes.
Taxonomic account
Trimerodytes Cope, 1895 (Figures 4–5)
Liparophis M. G. Peracca, 1904, Rev. Suisse Zool.,Geneva, 12: 664. Type species: Liparophis bedoti M. G.Peracca, 1904.
Sinonatrix D. A. Rossman and W. G. Eberle, 1977,Herpetologica, Lawrence, 33: 42. Type species:Tropidonotus annularis E. Hallowell, 1857.
Type speciesTrimerodytes balteatus Cope, 1895
Diagnosis(1) body size relatively large; (2) head
moderately distinct from neck; (3) nostrils situated and directed dorsolaterally; (4) eye size relatively large or moderate; (5) maxillary teeth large, enlarged posteriorly;(6) dorsal scale rows 19-19-17, keeled at least in the posterior part of body; (7) prefrontal single or paired;(8) a higher number of postoculars, usually 3–5; (9)hemipenis bilobed, spinous, sulcus spermaticus not forked; (10) body with annulated, V-shaped, or X-shaped markings, become darker or vaguer with age; (11) ventral surface with black bands or uniformly colored.

Table 5 Differences between the genus Trimerodytes and Opisthotropis.

Figure 4 Adult female specimen of Trimerodytes balteatus (CIB 109017; Guangxi, China) in preservative. (A) Dorsal view; (B) Ventral view; (C) Dorsal side of head view; (D) Ventral head view; (E) Lateral head view, left side; (F) Ventral cloacal region and ventral tail view.Scale bar 5 mm. Photographs by Jinlong REN.

Figure 5 General view comparisons of Trimerodytes species in life, showing diagnostic character of annulated color patterns of this genus.(A) T. balteatus (CIB 109017; adult female; Guangxi, China); (B) T. balteatus (CIB 109017), showing coloration before shedding; (C) T.percarinatus (adult male; K Bang, Gia Lai, Vietnam); (D) T. annularis (CIB 109020; subadult male; Guangxi, China); (E) T. aequifasciatus(IEBR 3630; adult female; Bac Giang, Vietnam; from Le et al., 2015); (F) T. yunnanensis (IEBR A.2015.12; adult male; Dien Bien, Vietnam;from Le et al., 2015). Photographs A, B, C, D by Jinlong REN; E and F from Le et al. (2015), respectively.
EtymologyThe generic nomen of Trimerodytes is a noun made of three parts: (1) the Ancient Greek τρία“tría”, meaning “three”; (2) the Ancient Greek μέρoς“méros”, meaning part, component, or region; and (3)the Greek “dytēs”, meaning diver or swimmer. The generic nomen refers to the three-parted (black-yellowblack) annulated markings and the aquatic life of the type species T. balteatus (Figures 3D, 4, 5A–B). The gender of this generic name is masculine. We suggest “Annulate Keelback” as its English common name, and Huan You She Shu (环游蛇属) as its Chinese common name.
ContentFive species are currently included (listed below in order of: scientific name; English common name; and Chinese common name):
Trimerodytes aequifasciatus (Barbour, 1908) comb.nov.; Asiatic Annulate Keelback; “Huan Wen Hua You She” (环纹华游蛇)
Trimerodytes annularis (Hallowell, 1856) comb. nov.;Red-bellied Annulate Keelback; “Chi Lian Hua You She”(赤链华游蛇)
Trimerodytes balteatus Cope, 1895; Banded Annulate Keelback; “Heng Wen Huan You She” (横纹环游蛇)
Trimerodytes percarinatus (Boulenger, 1899) comb.nov.; Olive Annulate Keelback; “Wu Hua You She” (乌华游蛇)
Trimerodytes yunnanensis (Rao and Yang, 1998)comb. nov.; Yunnan Annulate Keelback; “Yun Nan Hua You She” (云南华游蛇)
DistributionMembers of the genus distribute across southern and eastern China (including Taiwan and Hainan) and northern Southeast Asia (Vietnam, Laos,Thailand, Myanmar, and northeast India).
Key to species of Trimerodytes(after Rao and Yang,1998 and Zhao, 2006)
1 Prefrontal single; ventrals 187–205...............T. balteatus- Prefrontal paired; ventrals fewer than 170.......................2
2 Dorsal transverse lines thick, crossed, X-shaped, less than 29 markings on trunk.................................................3- Dorsal transverse lines thin, not crossed, shape not as such, more than 30 markings on trunk..............................4
3 Ventrals 156–165; maxillary teeth 31–35 .............................................................................................T. yunnanensis- Ventrals 142–154; maxillary teeth 22–28 ..........................................................................................T. aequifasciatus
4 Dorsal transverse bands in contact, V-shaped; ventral background color grayish white; labial sutures pale,indistinct.....................................................T. percarinatus- Dorsal transverse bands not in contact, bar-like; ventral background color redish orange; labial sutures black,distinct.............................................................T. annularis
Trimerodytes balteatusCope, 1895 (Figures 2A, 3D, 4,5A–B)
Trimerodytes balteatus E. D. Cope, 1895, Proc. Acad.Nat. Sci. Philadelphia, 46: 426. Type locality: Nodoa(=Nada), Hainan Island, Hainan Prov., China.
Opisthotropis balteata: C. H. Pope, 1935, Rept. China,New York: 167.
Liparophis bedoti M. G. Peracca, 1904, Rev. Suisse Zool., Geneva, 12: 664. Type locality: China.
Opisthotropis multicinctata T.-H. Fan, 1931, Bull.Dept. Biol. Coil. Sci. Sun Yat-sen Univ., Canton, 11:82. Type locality: Loshiang (=Luoxiang), Kwangsi(=Guangxi Zhuang Autonomous Region), China.
DiagnosisTrimerodytes balteatus can be diagnosed from other morphologically similar species by a combination of the following characters: (1) body cylindrical, TL 604–1 021 mm; (2) tail relatively moderate, TaL/TL 0.15–0.24; (3) head moderately distinct from neck; (4)nostrils directed dorsolaterally; (5) eyes moderate, EW/SoL 0.86–1.38; (6) maxillary teeth mostly 16–19, slightly enlarged posteriorly, without diastema; (7) dorsal scale rows 19-19-17; (8) prefrontal single, spindle-shaped; (9)supralabials 9–10, usually 4th or 5th entering orbit; (10)ventrals 187–205; (11) dorsal scales smooth anteriorly,smooth or feebly keeled at midbody, tending to be moderately keeled rear body and on tail, outer most three or four dorsal scale rows along both sides of body smooth entirely before cloaca; (12) body brownish-red or brownish-yellow, encircled by 32–54 black bands with yellow centers, 13–28 present on tail; (13) venter yellowish-beige, with annulated or alternating black bands (Figures 2A, 3D, 4, 5A–B; Tables 3–4).
DescriptionBody cylindrical and elongate, slenderly built, size relative large, TL 604–1 021 mm; tail relatively moderate, TaL/TL 0.15–0.24; head moderately distinct from neck, not dorsally depressed; snout broad and short;eye moderate, EW/SoL 0.86–1.38; pupil round; nostril large, elliptic or round, piercing in the middle of the nasal,directed dorsolaterally. Maxillary teeth mostly 16–19,count for 25 in Luoxiang population, Guangxi, China(Fan, 1931), progressively increasing in size, without diastema.
Dorsal scale rows 19-19-17, relatively enlarged, about equal in size, without apical pits; smooth anteriorly,smooth or weekly keeled at midbody, tending to be moderately keeled posteriorly, outer most three or four dorsal scale rows smooth entirely before cloaca.
Dorsal scale row reductions:

Ventrals 187–205; precloacal not divided; cloacal plate divided, subcaudals 65–102, paired, with single terminal rigid tip.
Rostral broad, width approximately twice as long as high, visible from above; nasals subrectangular, semidivided; furrow from lower edge of nostrils to lower edge of nasals, in contact with supralabials 1–2 or 1–3 ventrally; internasals paired, subtriangular, in broad contact, truncated anteriorly, curved outwards, not in contact with loreal; prefrontal single in all specimens,spindle-shaped, much broader than long, penetrate into internasals anteriorly, both sides curved backwards;frontal pentagonal or bell-shaped, longer than wide,shorter than snout length, length approximately equal to parietal sutures, penetrate into parietals posteriorly;parietals paired, subhexagonal, width equal to parietal sutures; supraoculars 1/1, narrowed anteriorly, nearly twice as long as wide. Loreal 1/1, slightly longer than depth, LoL/LoD 1.03–1.34, not entering orbit;preocular 1/1 (2/2 in No. 2583 only, by Fan, 1931), much higher than long; postoculars usually 3, variable and asymmetric, 2, 4, 5 occasionally occur on single side of head, the lowest ones situated at infero-posterior corner of eye; subocular absent, in some cases the lower most postoculars encompass forwards more than three fourths length of lower margins of eye, nearly in contact with preocular, which defined as postoculars only rather than subocular or postsubocular. Supralabials 9–10 (rarely 8 by Fan, 1931), usually 4th or 5th entering orbit (rarely both 4th and 5th entering orbit); infralabials 9–10 (8 on single side of YBU 16060 and SYS r001677 only),first five in contact with anterior chin shields; anterior temporals 1–2 (rarely 3), longer than depth, ToL/ToD 1.66–2.94; posterior temporals 2–3 (absent on right side of CIB 9971), usually asymmetric; chin shields two pairs,anterior chin shield distinctly shorter than posterior ones,posterior chin shields separated from each other by 1+2 or 1+3 scales; minute granular tubercles absent.
Coloration in lifeIn life, the background color of dorsal side of the head is yellowish-beige, and dorsal side of head scales are densely covered by large and irregular black blotches. These large clusters of blotches may fuse together, forming several transverse belts across the dorsal side of the head. The intervals of these black belts are orangish-yellow to brownish-red, especially on anterior dorsal side of head, composing continuous or discontinuous narrow pale streaks. The pale streaks just after the posterior margins of parietals are always present and continuous, which also extend to lateral and ventral surface of head. Parietals are almost covered with black,but a pair of elliptic orangish spots are symmetrically present on inner sides of corresponding scales, just separated by parietal sutures; similar paired spots are also observed on internasals in some specimens. The color pattern of lateral parts of head is simpler and paler, only parts of dark blotches extend onto the supralabials, black and blurry labial sutures present on both supralabials and infralabials (Figure 5A). Ventral surfaces of head are uniformly yellowish-beige, while the black infralabial sutures are clear and striking. The first small scale that is located just after the first chin shield is black, connecting sometimes with a thick cross band posteriorly. If connected, such band forms a distinct black arrowhead or large triangular pattern on ventral head.
The ground color of lateral body is brownish-red dorsally and orangish-yellow ventrally, color transitional zones are observed on dorsal scales of row 2–3 of both sides without clear boundaries. Body is encircled by 32–54 annulated markings, each marking is a broad black transverse band with yellow center, which covers one third to one and a half the length of dorsal scale. The yellow center of transverse band expands on the lowest two or three dorsal scale rows and sometimes to the outer margins of ventrals. Each yellow center is bordered by two thick black streaks along each edge of the center, and these black streaks are thicker than the yellow center and occupy one to two the length of dorsal scale. Together,the yellow centers and the black streaks form a strong contrast color pattern along the whole body. Sometimes the two halves of annulated markings are not evenly connected along the vertebral column, thus the broad bands along both sides of body could be in the stagger arrangement. Dorsal markings are darker and more indistinct in larger specimens. Coloration on tail is similar to trunk, with 13–28 annulated markings. These lateral markings decreasing in width towards the ventral scales,until the midline of venter, which only about one or two ventral scale width, forming annulated or alternating black bands on yellowish-beige ventral surface (Figure 5A).
The coloration of individuals turns to a dull purplishsilver hue before shedding, especially for the posterior body (Figure 5B). In juveniles, the black annulated markings are thicker with clear boundaries, in strong contrast to the background color; the yellow centers of marking are absent or small spots only present near the vertebral column.
Coloration in preservativeThe pattern of the recently preserved specimens resembles the coloration of live animals, but the light coloration on the dorsal side of head fades to an yellowish hue, dorsal ornamentation becomes darker (Figures 3D, 4).
ComparisonTrimerodytes balteatus can be readily distinguished from other species of Trimerodytes by having a single prefrontal (vs. paired prefrontals), and a higher number of ventral scale count (187–205 vs.less than 170). In addition, T. balteatus differs from T.yunnanensis by having a lower number of maxillary teeth [16–19 (25?) vs. 31–35] and a different dorsal color pattern (body annulated by transverse markings vs.crossed by X-shaped markings); from T. aequifasciatus by having a moderately distinct head (vs. distinct) and a higher number of trunk markings (32–54 vs. 22–28); from T. percarinatus by having moderately keeled dorsal scales at midbody (vs. strongly keeled throughout) and the presence of labial sutures (vs. absent). Lastly, T. balteatus differs from its sister species, T. annularis, in having a different head color pattern (black streaks present on dorsal surface vs. absent) and a different background color of ventral surface (uniformly yellowish vs. red or reddish-orange).
From other natricine taxa that have single prefrontal,T. balteatus differs from the genus Isanophis by its head moderately distinct from neck (vs. well distinct),eye size moderate (vs. large), and moderately keeled posterior dorsal scales (vs. strongly keeled throughout);from the genus Opisthotropis see above; from the genus
Trachischium by having a different dorsal scale formula(19-19-17 vs. 13 or 15 throughout), a different maxillary condition (maxillary teeth enlarged posteriorly vs.subequal), and head moderately distinct from neck (vs.indistinct); from Hebius annamensis (Bourret, 1934) by having 19 rows of dorsal scales at the midbody (vs. 15 or 17), a lower number of subcaudals (65–102 vs. 116–146),and a higher number of ventrals (187–205 vs. 158–172);from Rhabdops bicolor by having paired internasals (vs.single), and a higher number of supralabials (9–10 vs. 5)(Table 6).
HemipenisMaterial not available in this work. Pope(1935) provided hemipenial descriptions based on single topotypic specimens as follows: The hemipenis extends to the ninth subcaudal plate and is spinous, the proximal spines being the largest. There are three much enlarged basal spines or hooks set in a compact, longitudinal row,the distal hook larger than the other two. The lips of the sulcus are spinous and are most conspicuous proximally.
DistributionTrimerodytes balteatus is currently known from southern China [including Hainan (Danzhou),Guangxi (Huanjiang, Jinxiu, Longsheng, Wuzhou, and Yishan), Guangdong (Luoding, Gaoyao, Zhaoqing, and Zhuhai), and Hong Kong), and Vietnam (Hai Phong(Cat Ba Island), and Bac Giang (Tay Yen Tu Nature Reserve)] (Figure 6). The previously reported record from Cambodia is rejected here (see discussion below).
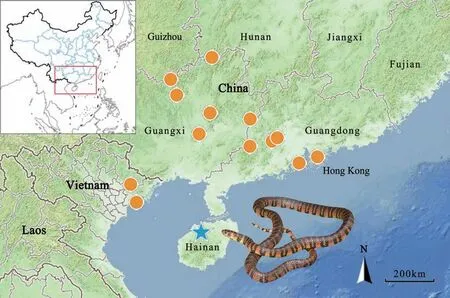
Figure 6 Distribution records of Trimerodytes balteatus based on the records from literature, museum collections, and this study. Star represents the type locality (Nodoa, now Nada, Danzhou, Hainan, China).
Natural historyTrimerodytes balteatus is an aquatic species that inhabits torrents and mountain streams at low to moderate elevations. This diurnal species often hides under rocks in the water (Karsen et al., 1998;Pope, 1935). Individuals were seen foraging in streams or drainage ditches near villages during fieldworks at daytime, and two adults were encountered competing for a single loach, where each snake bit on one end of the fish. Individuals are not aggressive, and no striking attempts were observed, but instead all snakes released a strong musk when captured. In addition, tail autotomy was also observed (see discussion below).
EtymologyThe specific epithet, balteatus, is a Latin adjective that means girded or belted. The term may be used to describe the distinctive annulated markings of this species.
4.2. Variation, distribution, and behavior of Trimerodytes balteatusVariation of the maxillary teeth count are evident in T. balteatus. Fan (1931) firstly recorded 25 maxillary teeth for the population from Luoxiang, Guangxi, China, which was distinctly higher than the maxillary teeth counts provided by later authors(vs. 16–21) (Peracca, 1904; Pope, 1935; Steindachner,1906; this work, Table 3). Additionally, Pope (1935)recorded the maxillary teeth count as 20–21 based on topotypic specimens from Hainan Island, China, thus the maxillary teeth counts of Luoxiang Population provided by Fan (1931) is questionable pending further confirmation. Nevertheless, considering all published data to date, maxillary teeth of T. balteatus vary from 16–25 among populations (Fan, 1931; Peracca, 1904;Steindachner, 1906). Future studies are needed to gain a better understanding on the population variation of this species.
Regarding the distribution of T. balteatus, an isolated record from Cambodia is likely erroneous. Steindachner(1906) first reported a country record of T. balteatus from Cambodia based on three specimens purchased by Herrn Fruhstorfer. Such record was accepted by most later publications (Nguyen et al., 2009; Zhao, 2006; Zhao and Adler, 1993; Zhao et al., 1998). However, Saint-Girons (1972) indicated that all specimens alleged to be from Cambodia were actually collected in Vietnam.In addition, despite continuous fieldworks in Southeast Asia for decades, specimens of T. balteatus were only collected from southern China and northern Vietnam,and no other specimens were collected from any of the regions/countries that border Cambodia to date, including southern Vietnam, Thailand, and Laos. Therefore, we reject the Cambodian record of the species.
As for the distribution records of T. balteatus within China, Ye and Deng (1997) reported the new provincial records of this species from central and southern Hunan Province (Mt. Mangshan, Yizhang County and Nanyue District, Hengyang City). These record are also erroneous as their specimens distinctly differ from T. balteatus by a combination of morphological characters: a smaller body size (320–490 mm vs. 604–1 021 mm), a lower number of ventrals (156–163 vs. 187–205), subcaudals (56–63 vs. 65–102), and dorsal scale rows at midbody (17 vs.19), which probably represent Opisthotropis cheni Zhao,1999. Shen et al. (2014) also recorded the species in“southern Hunan Province”, likely from the area near the boundaries between Hunan Province and Guangxi Zhuang Autonomous Region (Figure 6). However, no detailed description or vouchered specimen was associated with this geographic distribution within Hunan Province. This record, if true, would represent the most northern range of the species. Further confirmations are needed to better understand the distribution and ecology of this species.
Trimerodytes balteatus was recorded to display tail autotomy, which is rare in snakes (Hoogmoed and Avila-Pires, 2011). Fan (1931) firstly wrote that individuals of T. balteatus would “throw down its tail and escape like gekkoes”. Similarly, such tail autotomy behavior was also observed in other Trimerodytes species. During our fieldwork in May, 2016, two individuals of T.percarinatus (a juvenile and an adult female) autotomized their tails without being touched, and the autotomized part of the tails wiggled actively on the ground. Segmented myomeres were observed, and no signs of bleeding were found from the autotomized tail. Interestingly, such tail autotomy was only observed for certain populations of T.percarinatus in southern Guangxi Zhuang Autonomous Region, China, and such behavior has not been observed by authors or documented in other populations from Fujian, Hainan or Yunnan Provinces of China or Vietnam.Future studies are needed to understand the evolutionary mechanisms behind such physiological variation.
4.3. The validity of ParatapinophisThe monotypic genus Paratapinophis was established by Angel(1929) based on two neonate specimens of the type species, P. praemaxillaris Angel, 1929, collected in northern Laos. The genus was diagnosed by having a short, external process that extends anteriorly the premaxillary bone. Later, Pope (1935) recognized that the previously proposed diagnostic character of Paratapinophis was actually egg tooth of neonates, and he considered Paratapinophis to be a junior synonym of Opisthotropis. However, Pope (1935) also presented distinct morphological differences that differentiate P.praemaxillaris from Opisthotropis, including having well-developed posterior chin shields, smooth scales, and the large body size (Pope, 1935: 164). Nevertheless, this synonymization of Paratapinophis was accepted by later authors and has not been re-evaluated for a long time(Brown and Leviton, 1961; Cai et al., 2015; Chan-Ard et al., 2015; Mo et al., 1984; Rasmussen, 1982; Smith,1943; Zhao, 2006; Zhao et al., 1998).

Table 6 Morphological comparisons among natricine genera and species with untypical prefrontals (after David et al., 2015 and this work). “*” after standardizing by EW/SoL; “?” indicates missing data; values given in brackets indicate infrequent condition.DSR keeling 15–23 smooth or moderately keeled, more strongly keeled posteriorly 19 smooth; tuberculate posteriorly Maxillary teeth DSR at midbody subequal Eye size*small moderate last 3 or 4 slightly enlarged Direction of nostrils upward dorsolateral dorsal dorsolateral Head/neck Position of nostrils barely distinct distinct Internasal paired paired Prefrontal shape tricuspid Prefrontal mostly single tricuspid or subrectangular single Taxa Opisthotropis Paratapinophis 19 strongly keeled throughout 19 smooth anteriorly, moderately keeled posteriorly 15–17 moderately anteriorly, strongly posteriorly large last 3 distinctly enlarged enlarged posteriorly moderate moderate or small last 2 moderately enlarged obliquely upward dorsolateral dorsolateral dorsolateral dorsolateral dorsolateral clearly distinct paired paired moderately distinct paired moderately distinct tricuspid spindle-shaped subrectangular single single single or paired Isanophis Trimerodytes balteatus Hebius annamensis 13 obtusely keeled posteriorly subequal small dorsolateral dorsolateral indistinct paired?usually single Trachischium fuscum 13 obtusely keeled posteriorly subequal small dorsolateral dorsolateral indistinct paired usually single bicuspid posteriorly Trachischium guentheri smooth throughout 17 subequal small slightly upward dorsolateral barely distinct single tricuspid single (3)Rhabdops bicolor feebly keeled 19 23 strongly keeled throughout enlarged posteriorly moderate large last 2 distinctly enlarged dorsolateral lateral dorsolateral lateral 2–3 moderately distinct clearly distinct paired subrectangular subrectangular 2–4 3–4 Atretium yunnanensis Pseudagkistrodon rudis multiprefrontalis
Recently, Murphy et al. (2008) resurrected the genus Paratapinophis from Opisthotropis as a valid monotypic genus on the basis of morphological characters from newly collected specimens. Murphy et al. (2008)stated that Paratapinophis differs from Opisthotropis in possessing a distinct head from neck, with keeled tubercles in the middle of dorsal scales, sexually dimorphic scale ornamentation and coloration, a large and robust body, a lower number of maxillary teeth, and two pairs of posterior chin shields. Later David et al. (2015)accepted this revalidation, regarding Paratapinophis as a valid, monotypic genus. However, David et al. (2015)only compared the resurrected Paratapinophis with natricine taxa that have a single prefrontal.
Based on our survey of reported morphological characters, we found that Paratapinophis shows a high level of similarities with respect to Trimerodytes(Table 4). The previously proposed generic diagnoses of Paratapinophis and Trimerodytes are identical in having: (1) head distinct from neck, (2) dorsolaterally directed nostrils, (3) large or moderate relative eye size (after standardizing by EW/SoL, see method),(4) posteriorly enlarged maxillary teeth, (5) large and robust body, (6) same number of dorsal scale rows(19-19-17), (7) same numbers of pre- and postoculars(mostly 1 and 3, respectively; the lowest postocular was construed to be subocular by Murphy et al., 2008), (8)overlapping ventral and subcaudal scale counts, and(9) similar V-shaped markings of dorsal color patterns(Table 4).
In addition, the remaining diagnostic characters of Paratapinophis provided by Murphy et al. (2008) were believed to be not characteristic in Paratapinophis.Firstly, the “small tubercles in the middle of the scale”were in fact a specialized keel, which were also shared in other genus (i.e. Opisthotropis andersonii; unpublished data). Secondly, the mention of “two pairs of posterior chin shields” is doubtful, because such character was never mentioned by any of the previous authors, including the original description and its illustration (Angel, 1929:77, figure B). In addition, Pope (1935: 164) also failed to recognize the two pairs of posterior chin shields, whereas in Trimerodytes species, the posterior chin shields usually separated from each other by two scales only (Figure 3).
Thus the “inner posterior pair” chin shields described by Murphy et al. (2008) are likely the smaller scales between posterior chin shields, as a universal condition in colubrids rather than “two pairs of posterior chin shields”.
Therefore, given the high level of similarities between Paratapinophis and Trimerodytes, and the questionable morphological diagnosis of the former genus in recent literature, Paratapinophis is most likely a junior synonym of Trimerodytes, and its type species, P. praemaxillaris,is likely a member of Trimerodytes with single prefrontal,which is similar to T. balteatus. We recommend future phylogenetic studies on Paratapinophis to confirm its taxonomic status and resolve its relationship with respect to Trimerodytes.
4.4. Prefrontal variations of natricine snakesVariations of prefrontal scales historically play an important role in natricine snake taxonomy, particularly at generic level (Angel, 1929; Bourret, 1934; David et al.,2015). However, different genera often share the same untypical prefrontal configuration (e.g. single prefrontal in Opisthotropis, Trimerodytes, Paratapinophis, and Isanophis) (David et al., 2015; this work). Furthermore,variations from the standard configuration are also common among congeners (e.g. genus Trimerodytes,Hebius, and Trachischium), and even among populations or individuals (e.g. Pseudagkistrodon rudis multiprefrontalis and Hebius annamensis) (David et al.,2015; Kizirian et al., 2018; Smith, 1943; Zhao and Jiang,1981; Zhao et al., 1998) (Figures 1, 7; Table 6).
As results, the historical overemphasis on the diagnostic value of prefrontal scales has resulted in misleading taxonomic hypotheses, including the mis-assignment of Trimerodytes balteatus and the establishment of synonym such as Parahelicops (Kizirian et al., 2018; Ren et al., 2018). Given the high level and high frequency of variation in number and configuration of prefrontal scales in the subfamily Natricinae (Figures 1, 7; Table 6), we argue that their diagnostic importance should not be overemphasized, and we call for future studies on the scale evolution among snakes to better understand the evolutionary and taxonomic significance of different groups of scales.
AcknowledgementsWe thank Chung Van HOANG, Hoa Thi NINH, Hieu Minh TRAN, Devon P. HUMPHREYS for their help in the field; Yuezhao WANG, Ke LV, Shuo LIU, Truong Quang NGUYEN and Zhiyong YUAN for giving us access to examine specimens under their care; Ke JIANG, Yulin XIE, Ping WANG, Xinhong XIE, Shaobing HOU, Jian WANG, Zhitong LYU and Honghui CHEN for their kind help in morphological data collection. This study was supported by the National Key Research and Development Program of China(2017YFC0505202); Strategic Priority Research Program of Chinese Academy of Sciences (XDB31000000);the National Natural Science Foundation of China(31722049, 31772434); Key Research Program of Frontier Sciences, CAS (QYZDB-SSW-SMC058);the Southeast Asia Biodiversity Research Institute,Chinese Academy of Science (Y4ZK111B01); the Youth Innovation Promotion Association of CAS; the CAS
“Light of West China” Program (2018XBZG_JCTD_001)and Talent Program from Organization Department of Sichuan Provincial Committee, and partially supported by project BSTMV.08/16-19 to NTT.

Figure 7 Comparisons of head in dorsal view among species of subfamily Natricinae, showing the variations in the number of prefrontals among different groups. (A) Opisthotropis latouchii (from Wu et al., 1985); (B) Paratapinophis praemaxillaris (neonate; from Angel,1929); (C) Trimerodytes balteatus (from Zhao et al., 1998); (D) Hebius annamensis (from Bourret, 1936); (E) Trachischium guentheri (from Boulenger, 1893); (F) Rhabdops bicolor (from Smith, 1943); (G) Atretium yunnanensis (from Zhao et al., 1998); (H) Pseudagkistrodon rudis muiltiprefrontalis (from Zhao and Jiang, 1981).
猜你喜欢
杂志排行
Asian Herpetological Research的其它文章
- Genetic Structure of the Red-spotted Tokay Gecko, Gekko gecko(Linnaeus, 1758) (Squamata: Gekkonidae) from Mainland Southeast Asia
- Comparative Skin Histology of Frogs Reveals High-elevation Adaptation of the Tibetan Nanorana parkeri
- Molecular Phylogeny and Evolution of Two Rhacophorus Species Endemic to Mainland Japan
- Evaluating the Importance of Environmental Variables on Spatial Distribution of Caspian cobra Naja oxiana (Eichwald,1831) in Iran
- Appendix 1 Specimens examined.
