S3307对始花期和始粒期淹水绿豆光合作用及产量的影响
2019-06-25冯乃杰王诗雅左官强郑殿峰
于 奇 冯乃杰 王诗雅 左官强 郑殿峰,2,*
S3307对始花期和始粒期淹水绿豆光合作用及产量的影响
于 奇1冯乃杰1王诗雅1左官强1郑殿峰1,2,*
1黑龙江八一农垦大学农学院, 黑龙江大庆 163319;2黑龙江八一农垦大学国家杂粮工程技术研究中心, 黑龙江大庆 163319
淹水胁迫是作物生长发育过程中遭受的主要非生物胁迫之一, 探究提高绿豆耐淹性的机制对绿豆抗涝栽培具有重要意义。本文在2017—2018年以耐淹性不同的绿豆品种绿丰2号和绿丰5号为试验材料, 采用盆栽方式探究了烯效唑(S3307)对淹水胁迫下绿豆叶片生理、光合作用及产量的影响。结果表明, 在不同生育时期淹水胁迫下, 绿豆叶片的叶绿素含量(SPAD)及光合特性参数均显著下降, 丙二醛(MDA)含量显著增加, 始花期(R1期)淹水胁迫下绿豆的减产率为24.70%~33.63%, 始粒期(R5期)减产率为18.07%~28.87%。2个绿豆品种均表现为R1期受淹水胁迫危害程度大于R5期, 绿丰2号耐淹性强于绿丰5号。喷施S3307后显著提高淹水胁迫下绿豆叶片的SPAD、净光合速率(n)、蒸腾速率(r)和气孔导度(s), 并显著降低了MDA含量。绿豆在R1期淹水胁迫下的缓解率为28.91%~52.34%, R5期缓解率为13.77%~27.36%。表明叶面喷施S3307可有效提高淹水胁迫下绿豆叶片的生理功能及光合能力, 进而降低减产幅度, 但不同淹水时期和绿豆品种对S3307的调控响应存在差异。
绿豆; 烯效唑(S3307); 淹水胁迫; 光合作用; 产量
根据联合国食品与农业组织统计, 世界范围内的绿豆产量从1990年到2014年增加了4倍, 达到2140万吨, 其中亚洲约占85.4%[1]。绿豆是中国重要的豆科作物之一, 具有生育期短、播种范围广和适应性强等特点, 成为国际市场的高附加值产品[2-3]。同时绿豆通过根瘤菌的大气固氮作用在恢复土壤肥力方面起到了关键作用, 有利于可持续农业生产[4]。淹水胁迫是农业生产中的主要非生物胁迫之一, 制约作物生长、降低产量, 成为主要农业制约因素[5-6]。据估计, 全球范围内12%的作物区受淹水胁迫影响[7]。淹水胁迫造成了约40%~80%的作物产量损失, 面积超过1700万公顷[8-9]。中国绿豆产区主要在黄淮河流域及东北地区, 该地区持续性强降雨大多发生在6月中旬至7月中旬, 正值绿豆生殖生长期, 极易造成淹水危害[10-11]。
淹水胁迫影响植物生长, 降低叶片光合作用效率, 最终降低作物产量, 影响农业生产[12]。净光合速率(n)、蒸腾速率(r)、气孔导度(s)和胞间CO2浓度(i)是植物在淹水胁迫下光合生理响应的重要指标[13]。淹水胁迫会影响北美鹅掌楸[14]、灰化苔草幼苗[15]和玉米[16]的正常生长, 降低叶绿素含量和光合效率, 并使细胞膜质过氧化程度加深, 丙二醛(MDA)含量增加。研究表明, 小麦淹水超过5 d会使其乳熟时间延后并降低产量, 玉米苗期受淹水胁迫3~7 d后其产量下降58.8%~69.8%, 印度木豆受淹后会使单株产量下降27.4%~61.5%[17-19]。通过化控技术提高作物的光合效率、调控作物的生理代谢、增加作物产量[20]。烯效唑(S3307)是一种新型高效三唑类植物生长延缓剂, 具有前控后促的作用, 在抗倒伏和抗逆等方面均有明显效果[21-22]。喷施S3307能缓解淹水胁迫下大豆叶片净光合速率(n)、蒸腾速率(r)、气孔导度(s)和胞间CO2浓度(i)的下降, 且在恢复正常水分管理后能促进光合能力的快速恢复[23]。叶喷S3307或S3307浸种后可提高大豆和绿豆的光合速率, 促进生育后期植物叶片的水分利用效率[24-26]。Yan等[27]、曾红等[28]、杨文钰等[29]和Gawad等[30]研究表明, 在大豆、玉米和小麦等作物上应用S3307后均有显著的增产效应。然而关于淹水胁迫对植物影响的研究多集中于大豆、棉花、玉米等作物, 较少涉及绿豆, 且关于S3307缓解绿豆淹水伤害的相关研究也较少。本试验探究不同淹水时期对耐淹性不同的2个绿豆品种光合作用及产量的影响以及S3307在始花期(R1期)和始粒期(R5期)淹水胁迫过程中对绿豆生长、光合特性参数及产量的影响, 以期为绿豆抗涝栽培奠定基础。
1 材料与方法
1.1 试验材料
供试绿豆品种为绿丰2号和绿丰5号, 由国家杂粮工程技术研究中心种植资源室提供。供试50 mgL–1烯效唑母液由黑龙江八一农垦大学农学院化控研究室提供。
1.2 试验设计
本试验于2017—2018年在黑龙江八一农垦大学国家杂粮工程技术研究中心盆栽基地, 采用盆栽方式, 自然光照培养绿豆绿丰2号和绿丰5号。树脂花盆上口径∶底径∶高 = 32 cm∶23 cm∶31 cm, 下部带有排水口, 底部有隔水层。试验用土按过筛栽培土∶腐殖土∶酒糟土= 6∶1∶1比例均匀混合组成, 每盆装土17 kg, 施肥量为纯N 0.125 g kg–1, P2O50.25 g kg–1, K2O 0.1 g kg–1。挑选大小一致饱满的绿豆种子, 用10%次氯酸钠消毒15 min, 蒸馏水反复冲洗干净并晾干后用于播种。每盆等距播种15粒, 于真叶期定苗5株, 试验期间用自动定时滴灌装置浇水, 使每盆土壤水分一致并保持在田间最大持水量的80%左右, 定期除草并喷洒农药防治病虫害。采用完全随机区组设计, 培养至始花期(R1期)和始粒期(R5期)开始处理, 每个品种设R1期对照(R1期喷施蒸馏水后正常水分管理; R1CK)、R1期淹水处理(R1期喷施蒸馏水后淹水胁迫处理, 淹水时间持续5 d; R1W)、R1期S3307+淹水处理(R1期喷施S3307后淹水胁迫处理, 淹水时间持续5 d; R1W+S)、R5期对照(R5期喷施蒸馏水后正常水分管理; R5CK)、R5期淹水处理(R5期喷施蒸馏水后淹水胁迫处理, 淹水时间持续5 d; R5W)、R5期S3307+淹水处理(R5期喷施S3307后淹水胁迫处理, 淹水时间持续5 d; R5W+S) 6个处理。在R1期和R5期喷施等量的蒸馏水或S3307于绿豆叶片的正反面, 直至绿豆叶尖滴水时停止喷施, S3307喷施浓度为50 mg L–1。于喷药5 d 后同一时间点套盆进行淹水处理(以水面高于土面3 cm为准), 淹水时间持续5 d, 5 d后放水恢复正常水分管理直至收获。分别于R1期(R1-0, 即叶面喷施蒸馏水或S3307)、R1期后5 d (R1-5, 即喷药5 d后)、R1期后10 d (R1-10, 即淹水5 d后)、R1期后15 d (R1-15, 即淹水胁迫解除5 d后)及R5期(R5-0)、R5期后5 d (R5-5)、R5期后10 d (R5-10)、R5期后15 d (R5-15)测定各个处理的叶绿素相对含量(SPAD)、丙二醛(MDA)含量及光合特性参数等指标, 测定完成后随机选取每品种每处理长势一致的4盆绿豆植株正常水分管理直至收获, 用于后期测定产量。
1.3 测定项目与方法
1.3.1 叶绿素相对含量(SPAD)测定 选取每处理4株长势一致的植株, 采用SPAD-502型叶绿素仪(Japan)测定每株绿豆主茎顶部倒数第3片复叶中间小叶上、中、下部的SPAD值, 取平均值。
1.3.2 丙二醛(MDA)含量测定 取绿豆主茎顶部倒数第3片复叶, 液氮速冻30 min后置–40℃冰箱中保存,待样品全部收集完毕后统一测定。取0.5 g混合后的新鲜叶片置预冷的研钵中, 分3次加入10 mL 50 mmol L–1预冷的磷酸缓冲液(pH 7.8)在冰浴上研磨成匀浆, 在4℃、9000 r min-1下离心25 min, 取上清液(即粗提液)保存于4℃中备用, 采用硫代巴比妥酸(TBA)法[31]测定丙二醛(MDA)含量。
1.3.3 叶片光合特性参数 在R1期和R5期选择晴朗无风的天气, 于上午8:30–11:00, 随机选取每个品种每个处理长势一致的4盆植株, 用CI-340光合仪(CID, USA)测定每盆中5株绿豆主茎顶部倒数第3片复叶中间小叶的净光合速率(n)、蒸腾速率(r)、气孔导度(s)和胞间CO2浓度(i), 取其平均值。测定时温度为28~34℃, 大气CO2浓度为430~530 μmol L-1, 相对湿度为70%~80%, 光合有效辐射为800~1600 μmol m-2s-1。
1.3.4 产量及产量构成因素的测定 于收获期对每品种每处理的4盆绿豆植株进行室内考种, 统计每盆中5株植株中各株的单株荚数、单荚粒数、百粒重和单株产量, 取其平均值。
1.4 数据处理
采用Microsoft Excel 2016进行数据的录入、整理及绘图, 用SPSS 23.0对数据进行方差分析。
2 结果与分析
2.1 S3307对不同时期淹水绿豆生理特性参数的影响
2.1.1 叶片SPAD值 由图1可知, 在R1期和R5期淹水胁迫后, 绿豆叶片SPAD值整体呈下降趋势。与CK相比, 绿丰2号和绿丰5号均表现为R1期淹水胁迫(W)处理的SPAD降低幅度高于R5期, 并且两时期淹水胁迫下绿丰5号W处理的SPAD降低幅度高于绿丰2号。喷施S3307后均提高了淹水后及胁迫解除后绿豆叶片SPAD值。2个时期淹水后(R1-10和R5-10), 绿豆W处理叶片SPAD较CK均降低, W+S处理在R1期较W处理分别增加了3.49%和28.27%, R5期分别增加了11.25%和9.64%。胁迫解除后(R1-15和R5-15), 绿豆W处理的叶片SPAD较各自CK均显著降低, 绿丰2号和绿丰5号W+S处理在R1期较W处理分别增加了10.06%和35.22%, R5期分别增加了5.45%和32.59%。
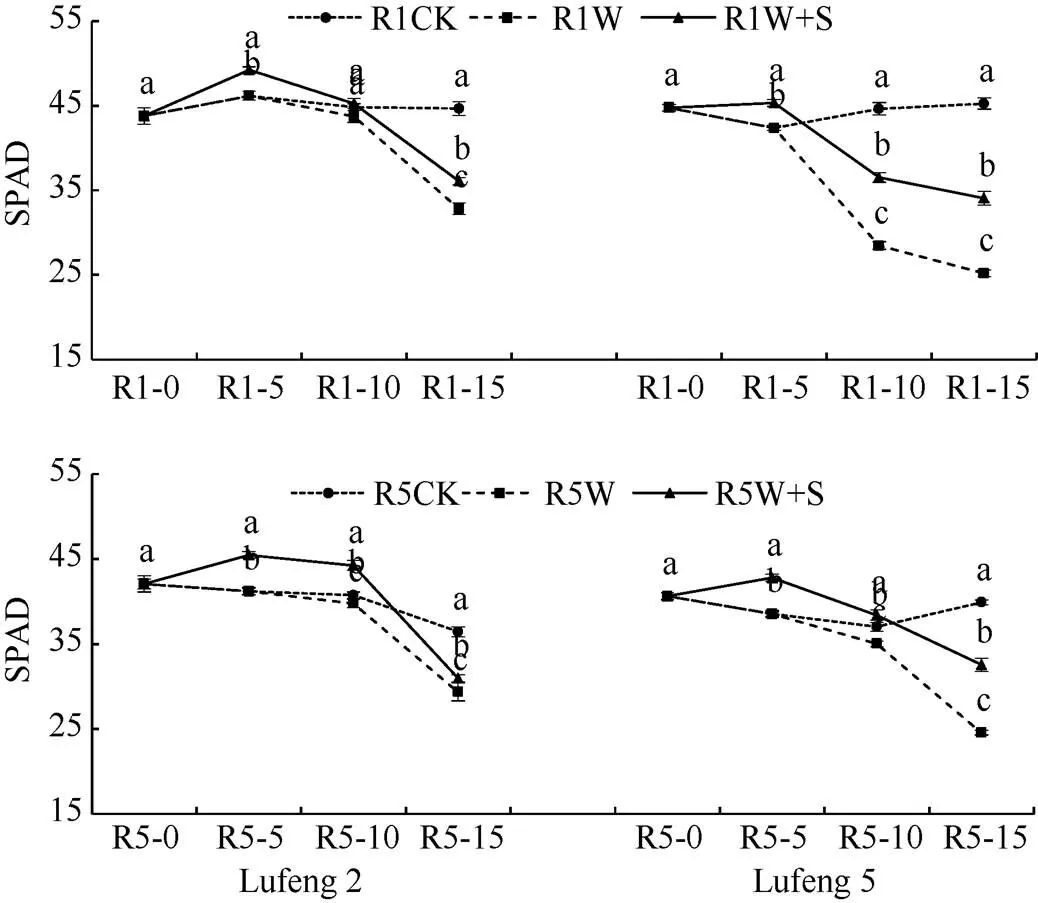
图1 S3307对R1期和R5期淹水胁迫下绿豆叶片SPAD的影响
R1CK: 始花期对照; R1W: 始花期喷施蒸馏水后淹水胁迫处理; R1W+S: 始花期喷施S3307后淹水胁迫处理; R5CK: 始粒期对照; R5W: 始粒期喷施蒸馏水后淹水胁迫处理; R5W+S: 始粒期喷施S3307后淹水胁迫处理。R1-0: 始花期喷施蒸馏水或S3307; R1-5: 始花期后5 d (即喷药5 d后); R1-10: 始花期后10 d (即淹水5 d后); R1-15: 始花期后15 d (即胁迫解除5 d后), R5-0: 始粒期喷施蒸馏水或S3307; R5-5: 始粒期后5 d (即喷药5 d后); R5-10: 始粒期后10 d (即淹水5 d后); R5-15: 始粒期后15 d (即胁迫解除5 d后)。同天内标以不同字母的值在= 0.05水平上差异显著。
R1CK: the beginning bloom control; R1W: exposed to waterlogging stress after spraying water at the beginning bloom; R1W+S: exposed to waterlogging stress after spraying S3307 at the beginning bloom; R5CK: the beginning seed control; R5W: exposed to waterlogging stress after spraying water at the beginning seed; R5W+S: exposed to waterlogging stress after spraying S3307 at the beginning seed.R1-0: spayed water or S3307 at the beginning bloom; R1-5: 5 d after the beginning bloom (5 d after spayed water or S3307); R1-10: 10 d after the beginning bloom (5 d after waterlogging stress); R1-15: 15 d after the beginning bloom (recovered 5 d), R5-0: spayed water or S3307 at the beginning seed; R5-5: 5 d after the beginning seed (5 d after spayed water or S3307); R5-10: 10 d after the beginning seed (5 d after waterlogging stress); R5-15: 15 d after the beginning seed (recovered 5 d). Values of SPAD within the same day with different letters are significantly different at the 0.05 probalility level.
2.1.2 叶片丙二醛(MDA)含量 由图2可知, 除绿丰2号在R1期淹水后W处理MDA含量较CK显著降低了21.29%外, 其余淹水时期绿豆的W处理MDA含量较CK显著增加。R1期淹水后(R1-10), 绿丰2号W+S处理较W处理增加了9.44%, 绿丰5号降低了8.81%; 胁迫解除后(R1-15), 绿丰2号和绿丰5号W+S处理较W处理分别显著降低了7.49%和33.43%。R5期淹水后(R5-10), 绿丰2号和绿丰5号W+S处理较W处理分别显著降低了9.72%和10.33%, 胁迫解除后(R5-15)分别显著降低了13.17%和12.68%。
2.2 S3307对不同时期淹水绿豆叶片光合特性参数的影响
2.2.1 对叶片净光合速率(n) 由图3可知, 绿豆在淹水及缓解后其净光合速率整体变化趋势为CK>W+S处理>W处理。绿丰2号W处理的净光合速率淹水后(R1-10和R5-10)在R1期和R5期较CK分别显著降低52.21%和43.06%, W+S处理净光合速率较W处理显著增加74.43%和42.83%; 绿丰2号W处理净光合速率缓解后(R1-15和R5-15)在R1期和R5期较CK分别降低18.17%和7.53%, W+S处理净光合速率在R1期较W处理显著增加26.09%, R5期降低0.29%且差异不显著。绿丰5号在不同时期受淹及缓解后的净光合速率变化趋势与绿丰2号基本一致, 但其下降幅度大于绿丰2号。
2.2.2 对叶片蒸腾速率(r) 由图4可知, 在R1期和R5期绿丰2号W处理在淹水后(R1-10和R5-10)叶片蒸腾速率均显著低于CK, W+S处理叶片蒸腾速率均显著高于W处理; 而在缓解后(R1-15和R5-15)各个处理变化趋势不同, W处理均高于CK, 而W+S处理在R1期高于W处理, 在R5期低于W处理, 但均高于各自CK。绿丰5号在淹水及缓解后, 除R5期的W+S处理在淹水后(R5-10)略高于CK外, 其他处理变化趋势均为CK>W+S处理>W处理。
2.2.3 对叶片气孔导度(s) 由图5可知, 绿丰2号在R1和R5期喷施S3307 5 d后(R1-5和R5-5)叶片气孔导度较CK显著增加; 绿丰2号W处理在淹水后(R1-10和R5-10)叶片气孔导度均显著低于CK, W+S处理叶片气孔导度均显著高于W处理; 在缓解后(R1-15和R5-15) W处理在R1期高于CK, 在R5期低于CK, 差异均不显著, W+S处理叶片气孔导度在两时期均高于CK和W处理。绿丰5号除在R5期缓解后(R5-15)W+S处理叶片气孔导度大于CK外, 在R1期和R5期淹水及缓解后的变化趋势均为CK>W+S处理>W处理。
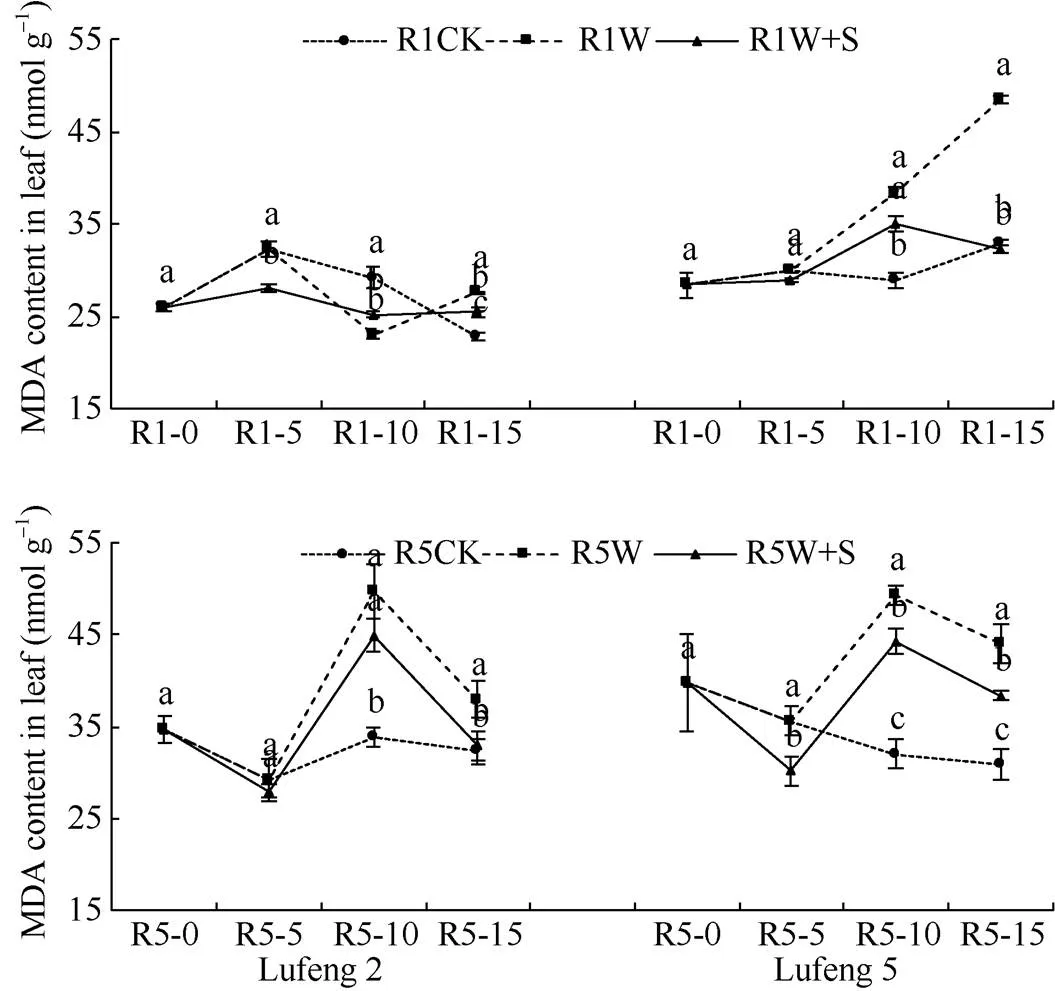
图2 S3307对R1期和R5期淹水胁迫下绿豆叶片MDA含量的影响
缩写同图1。同天内标以不同字母的值在= 0.05水平上差异显著。
Abbreviations are the same as those given in Fig. 1. Values of MDA content within the same day with different letters are significantly different at the 0.05 probalility level.

图3 S3307对R1期和R5期淹水胁迫下绿豆叶片净光合速率的影响
缩写同图1。同天内标以不同字母的值在= 0.05水平上差异显著。
Abbreviations are the same as those given in Fig. 1. Values of the photosynthetic rate within the same day with different letters are significantly different at the 0.05 probalility level.
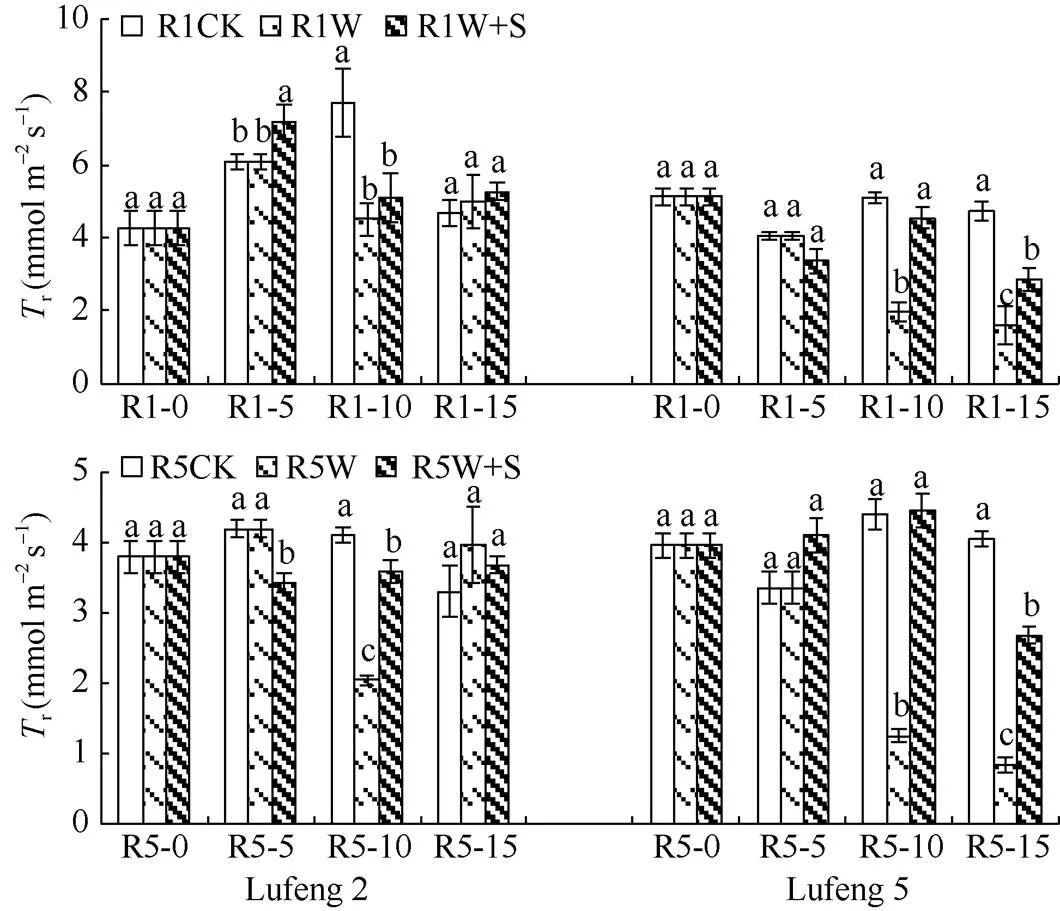
图4 S3307对R1期和R5期淹水胁迫下绿豆叶片蒸腾速率的影响
缩写同图1。同天内标以不同字母的值在= 0.05水平上差异显著。
Abbreviations are the same as those given in Fig. 1. Values of the transpiration rate within the same day with different letters are significantly different at the 0.05 probalility level.

图5 S3307对R1期和R5期淹水胁迫下绿豆叶片气孔导度的影响
缩写同图1。同天内标以不同字母的值在= 0.05水平上差异显著。
Abbreviations are the same as those given in Fig. 1. Values of the stomatal conductance within the same day with different letters are significantly different at the 0.05 probalility level.
2.2.4 对叶片胞间CO2浓度(i) 由图6可知, 绿丰2号和绿丰5号在R1和R5期喷施S3307 5 d后(R1-5和R5-5)W+S处理叶片胞间CO2浓度较CK均增加; 绿丰2号W处理的叶片胞间CO2浓度在淹水后(R1-10和R5-10)均显著低于CK, W+S处理叶片胞间CO2浓度均高于W处理, 但差异不显著; 绿丰2号在R1期缓解后(R1-15和R5-15)的变化趋势为W+S处理>CK>W处理, R5期的变化趋势为CK>W+S处理>W处理。绿丰5号在两时期淹水后的变化趋势均为W+S处理>CK>W处理;在两时期缓解后的变化趋势均为CK>W+S处理>W处理。
2.3 S3307对不同时期淹水绿豆籽粒产量的影响
由表1可知, R1期绿丰2号和绿丰5号R1W单株产量较R1CK分别显著降低了24.70%和29.54%; R1W+S较R1W分别显著增加了32.40%和28.91%。两品种绿豆单株荚数表现为R1W+S>R1CK>R1W, 单荚粒数表现为R1CK>R1W+S>R1W。绿丰2号百粒重在处理之间差异不显著, 绿丰5号百粒重表现为 R1W
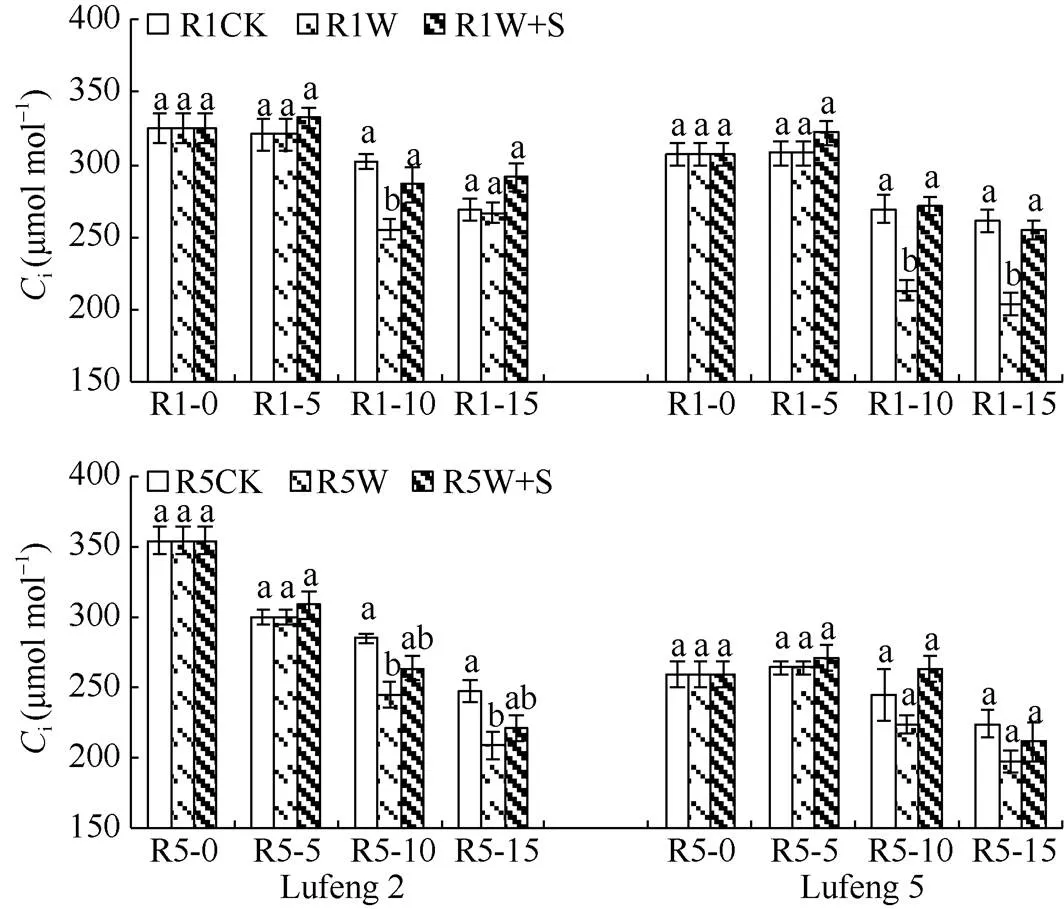
图6 S3307对R1期和R5期淹水胁迫下绿豆叶片胞间CO2浓度的影响
缩写同图1。同天内标以不同字母的值在= 0.05水平上差异显著。
Abbreviations are the same as those given in Fig. 1. Values of the intercellular CO2concentration within the same day with different letters are significantly different at the 0.05 probalility level.
由表2可知, R1期绿丰2号和绿丰5号淹水处理的单株产量较对照分别降低27.16%和33.63%, R5期分别降低18.07%和28.87%, R1期S3307处理较淹水处理分别增加34.08%和52.34%, R5期分别增加13.77%和21.18%。由表1~表3可知, 在淹水胁迫和S3307处理下, 2017—2018年绿豆产量及产量构成因素的变化趋势基本一致, 均表现为2个绿豆品种在R1期减产幅度高于R5期, 同时期下绿丰2号的减产幅度低于绿丰5号。R1期淹水胁迫影响了绿豆的单株荚数、单荚粒数和百粒重, R5期影响了绿豆单荚粒数和百粒重。喷施 S3307后使淹水胁迫下绿豆单株产量显著增加。
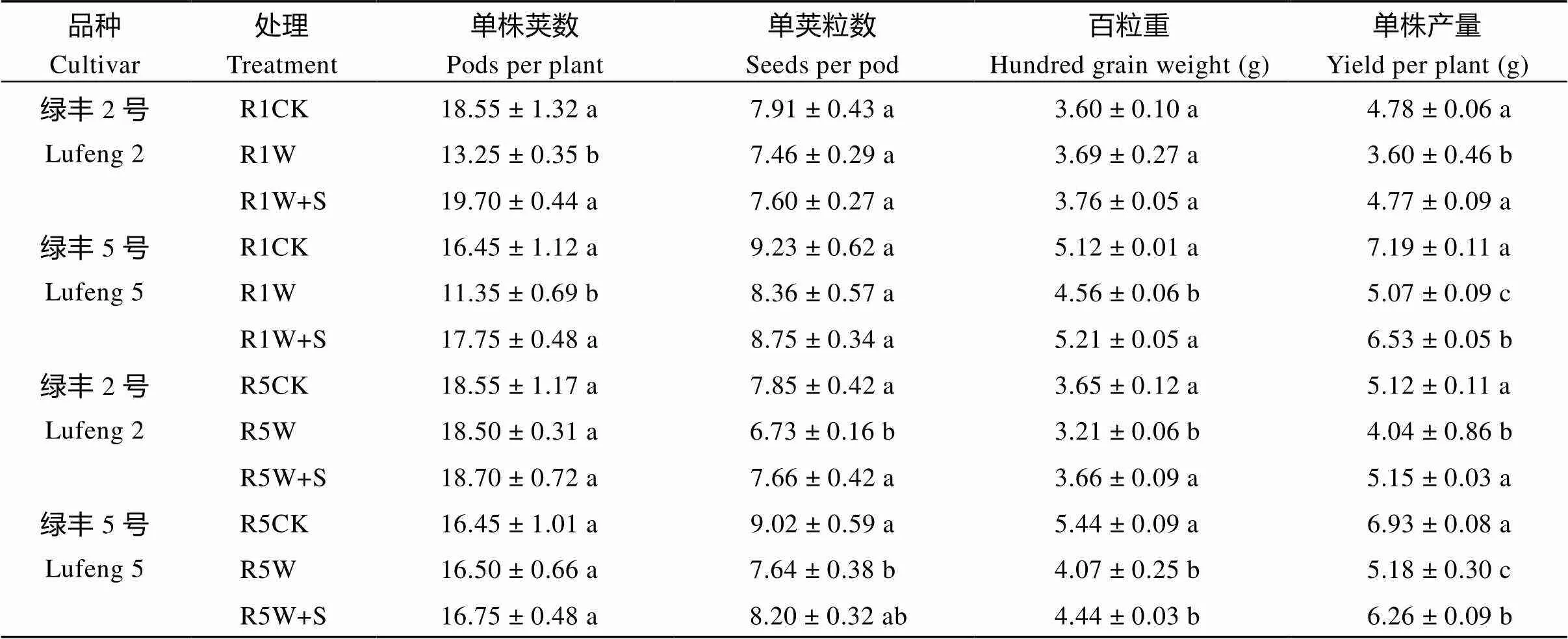
表1 S3307对R1期和R5期淹水胁迫下绿豆产量及产量构成因素的影响(2017年)
缩写同图1。同栏内标以不同字母的值在= 0.05水平上差异显著。
Abbreviations are the same as those given in Fig. 1. Values of yield and yield components within the same column with different letters are significantly different at the 0.05 probalility level.
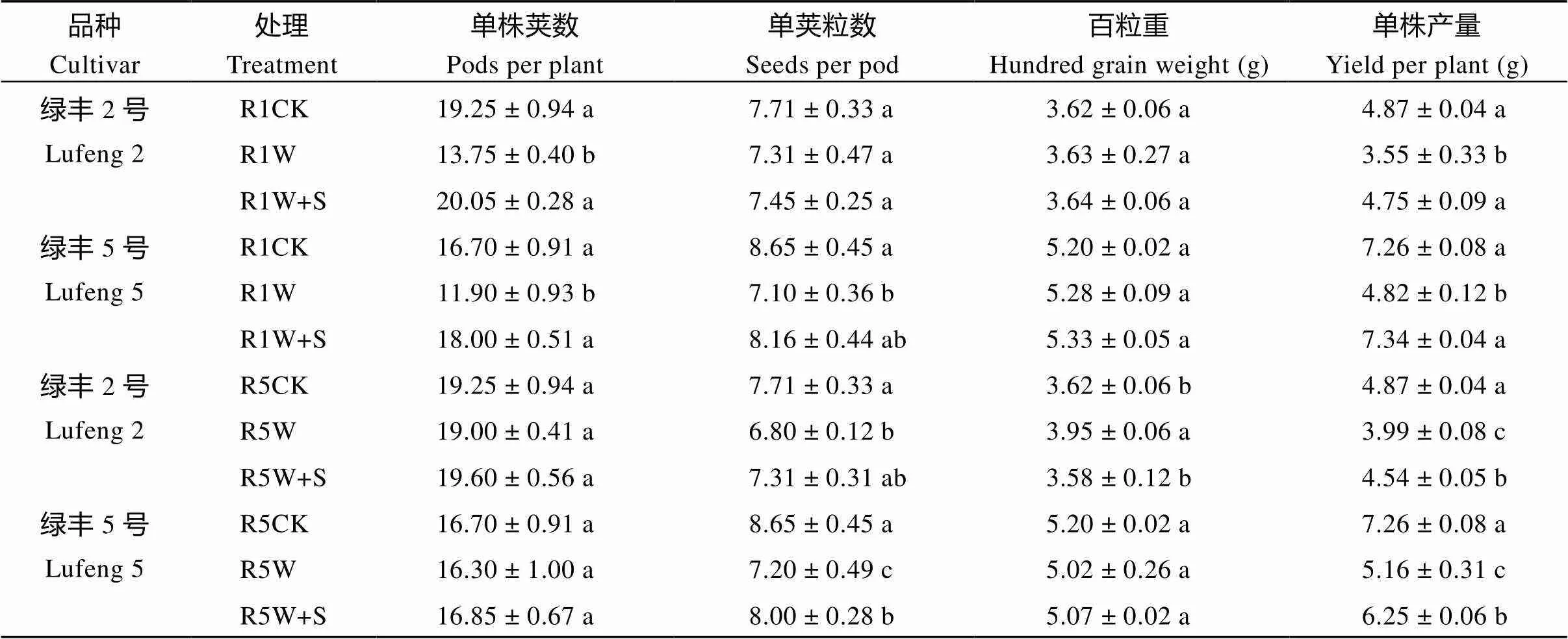
表2 S3307对R1期和R5期淹水绿豆产量及产量构成因素的影响(2018年)
缩写同图1。同栏内标以不同字母的值在= 0.05水平上差异显著。
Abbreviations are the same as those given in Fig. 1. Values of yield and yield components within the same column with different letters are significantly different at the 0.05 probalility level.

表3 2017–2018年R1期和R5期淹水胁迫下绿豆单株产量的减产率及缓解率
R1: 始花期; R5: 始粒期; 减产率 = (W – CK)/CK×100%; 缓解率 = (W+ S3307 – W)/W×100%, 以每个品种每个处理单株产量计算。
R1: the beginning bloom; R5: the beginning seed; Yield reduction rate = (waterlogging treatment – control)/control×100%; Remission rate = (waterlogging+S3307 treatment – waterlogging treatment)/waterlogging treatment×100%. The calculation in laced on yield per plant per treatment of each variety.
3 讨论
淹水胁迫会对植物的生长及生理代谢造成严重影响, 随着胁迫时间延长或程度加深, 植物受害越严重。叶绿素的含量变化是植物对水分胁迫响应较为敏感的生理指标之一, 反映了植物叶片的光合性能及衰老程度[14]。丙二醛(MDA)作为膜质过氧化指标, 其含量的高低代表了植物在逆境条件下受害程度的深浅[32]。绿豆在开花期和鼓粒期维持较高的叶绿素含量及光合速率对籽粒产量形成具有重要意义[33]。本试验结果表明, R1和R5期淹水胁迫会影响绿豆的正常生长, 降低叶绿素含量, 使细胞膜质过氧化程度加深, 丙二醛(MDA)含量增加。但不同绿豆品种对淹水胁迫的响应有所不同。绿丰2号在R1期受淹及缓解后, 淹水处理的MDA含量较对照相比出现先降低后增加的变化趋势, 绿丰5号淹水处理较对照则呈现显著上升趋势。说明在一定淹水胁迫时间内, 绿丰2号对淹水胁迫具有一定的适应性, 这与曹旖旎等[34]研究结果相似。在逆境胁迫条件下应用S3307后, 会缓解胁迫对植物造成的危害。有研究发现[35-36], 叶面喷施S3307后能够提高盐胁迫下大豆的叶绿素含量, 在R1期喷施S3307后可降低大豆叶片MDA含量, 缓解胁迫对大豆叶片细胞膜的伤害。本试验发现, 淹水胁迫条件下, 喷施S3307增加了受淹绿豆叶片的叶绿素含量并降低MDA含量, 减轻淹水胁迫下绿豆叶片的细胞膜质过氧化作用, 保证绿豆幼苗在淹水逆境下的生长及生理代谢, 这也是S3307增强绿豆植株耐淹性的原因之一。
光合作用是作物生长发育过程中重要的能量转化代谢系统, 光合能力的提高为籽粒干物质的积累奠定了基础, 是作物产量形成的物质基础。耐淹植物通常可以保持较高的光合特性及光合速率, 这些光合气体交换参数在一定程度上可以作为判定植物耐淹性的指标[37]。研究表明, 喷施S3307后可提高芸豆[38]和大豆[39]鼓粒后期不同冠层叶片的净光合速率、蒸腾速率和气孔导度。在干旱胁迫条件下, 经S3307处理后百日草幼苗的净光合速率、气孔导度和蒸腾速率有所提高, 说明S3307能够提高胁迫下百日草幼苗的光合能力[40]。本研究发现, 2个绿豆品种在淹水胁迫后光合气体交换参数均有不同程度的降低, 喷施S3307后能显著提高淹水胁迫下绿豆的净光合速率、蒸腾速率和气孔导度。但不同品种对淹水胁迫及S3307的调控响应有所不同, 在胁迫解除后, 绿丰2号淹水处理和S3307处理的净光合速率、蒸腾速率和气孔导度均有所缓解, 并且蒸腾速率和气孔导度均高于对照, 然而绿丰5号淹水处理在胁迫解除后净光合速率仍低于对照。说明绿丰2号在胁迫解除后具有较强的恢复能力, 耐淹性高于绿丰5号。这与Chaves等[41]和Antonio等[42]关于不同耐淹性品种对淹水胁迫响应不同的研究结果基本一致。此外, 在R1期淹水胁迫下绿豆淹水处理的叶绿素含量、净光合速率和气孔导度的降低幅度高于R5期淹水。说明绿豆在R1期受淹水胁迫危害程度高于R5期。
绿豆受淹后根系处于低氧环境, 使地上部光合参数值下降, 影响作物正常的光合作用, 进而影响绿豆的干物质积累, 对绿豆单株荚数、单荚粒数和百粒重等产量构成因素造成严重影响, 最终降低绿豆的产量。本试验结果表明, R1期淹水胁迫使绿豆单株荚数和单荚粒数显著降低, 产量显著降低24.70%~33.63%; R5期淹水胁迫使绿豆单荚粒数显著降低, 产量显著降低18.07%~28.87%。这与前人研究结果[17-19]相似。有研究发现[43], 在大豆始花期和盛花期喷施S3307后会增加大豆的单株荚数和单株粒数并提高产量。本试验在喷施S3307后均能显著提高R1和 R5期淹水胁迫下绿豆的单株产量, 同时S3307对不同时期淹水胁迫下绿豆的产量构成因素调控不同, R1期喷施S3307后能提高胁迫下绿豆的单株荚数、单荚粒数和百粒重; R5期喷施S3307后能显著提高胁迫下绿豆的单株荚数和单荚粒数。2年试验结果均显示, R1期淹水胁迫下绿豆的减产率高于R5期, 再次说明绿豆在R1期受淹水胁迫的危害程度大于R5期, 同时 S3307 对 R1 期淹水胁迫的缓解率高于R5期, 调控效应较好。这与Linkemer等[44]研究结果基本一致。有关喷施S3307对淹水胁迫下绿豆叶片及根系抗氧化酶系统、碳氮代谢、活性氧代谢系统及相关耐淹性基因表达调控等影响有待进一步研究。
4 结论
喷施烯效唑(S3307)减轻了淹水胁迫下绿豆叶片细胞膜质过氧化作用, 提高了始花期(R1期)和始粒期(R5期)淹水胁迫下绿豆的叶绿素含量及光合性能, 进而提高了单株产量。绿豆在R1期受淹水胁迫危害程度高于R5期, 绿丰2号的耐淹性强于绿丰5号。S3307对不同时期淹水胁迫下不同绿豆品种的调控效应有所不同, 但可用于绿豆抗涝栽培, 缓解淹水胁迫产生的危害。
[1] Chen L R, Ko C Y, Folk W R, Lin T Y. Chilling susceptibility in mungbean varieties is associated with their differentially expressed genes., 2017, 58, doi: 10.1186/s40529-017- 0161-2.
[2] Noble T, Douglas C, Williams R, Williams B, Mundree S. Development of the mungbean nested association mapping (NAM) resource. In: InterDrought V, 21−25 February 2017, Hyderabad, India. pp 21−25. https://eprints.qut.edu.au/104759/.
[3] Kumawat N, Kumar R, Sharma O P. Nutrient uptake and yield of mungbean(L.) Wilczek as influenced by organic manures, PSB and phosphorus fertilization., 2009, 27: 2002–2005.
[4] Bhanu A N, Singh M N, Srivastava K. Screening mungbean [(L.) Wilczek] genotypes for mungbean yellow mosaic virus resistance under natural condition., 2017, 7: 00276.
[5] Ren B, Dong S, Zhao B, Liu P, Zhang J. Responses of nitrogen metabolism, uptake and translocation of maize to waterlogging at different growth stages., 2017, 8, doi: 10.3389/fpls.2017.01216.
[6] Xu Q T, Yang L, Zhou Z Q, Mei F Z, Qu L H, Zhou G S. Process of aerenchyma formation and reactive oxygen species induced by waterlogging in wheat seminal roots., 2013, 238: 969– 982.
[7] ShabalaS. Physiological and cellular aspects of phytotoxicity tolerance in plants: the role of membrane transporters and implications for crop breeding for waterlogging tolerance.. 2011, 190: 289–298.
[8] Voesenek L A, Sasidharan R. Ethylene- and oxygen signaling- drive plant survival during flooding., 2013, 15: 426– 435.
[9] Shabala S, Shabala L, Barcelo J, Poschenrieder C. Membrane transporters mediating root signalling and adaptive responses to oxygen deprivation and soil flooding., 2015, 37: 2216–2233.
[10] Zhang H J, Li N H, Cheng X Z, Katinka W. The impact of mungbean research in ChinaWorld Vegetable Center, Taiwan (AVRDC), China, 2003, https://www.eldis.org/document/A20817.
[11] 张娇, 王东勇, 朱佳宁, 郑媛媛, 姚叶青. 淮河流域持续性强降水的重要前期信号. 气象, 2011, 37: 1329–1335. Zhang J, Wang D Y, Zhu J N, Zheng Y Y, Yao Y Q. The precursor signals of persistent and strong precipitation along the Huaihe River Valley., 2011, 37: 1329–1335 (in Chinese with English abstract).
[12] Normile D. Reinventing rice to feed the world., 2008, 321: 330–333.
[13] 周珺, 魏虹, 吕茜,李昌晓, 王振夏, 高伟, 陈伟. 土壤水分对湿地松幼苗光合特征的影响. 生态学杂志, 2012, 31: 30–37. Zhou J, Wei H, Lyu Q, Li C X, Wang Z X, Gao W, Chen W. Effects of soil water regime on leaf photosynthetic characteristics of slash pine (Engelm.) seedlings., 2012, 31: 30–37 (in Chinese with English abstract).
[14] 孙小艳, 陈铭, 李彦强, 吴照祥, 钟永达, 余发新. 淹水胁迫下北美鹅掌楸无性系生理生化响应差异. 植物生理学报, 2018, 54: 473–482. Sun X Y, Chen M, Li Y Q, Wu Z X, Zhong Y D, Yu F X. Variations in physiological and biochemical responses in clones ofunder flooding stress.2018, 54: 473–482 (in Chinese with English abstract).
[15] 曹昀, 郑祥, 杨阳, 陈冰祥, 国志昌, 吴海英. 淹水对灰化苔草幼苗生长及抗氧化物酶活性的影响. 生态学杂志, 2016, 35: 3273–3278. Cao Y, Zheng X, Yang Y, Chen B X, Guo Z C, Wu H Y. Effects of waterlogging on the growth and antioxidant enzyme activity ofseedlings., 2016, 35: 3273–3278 (in Chinese with English abstract).
[16] 僧珊珊, 王群, 张永恩, 李潮海, 刘天学, 赵龙飞, 刘怀攀. 外源亚精胺对淹水胁迫玉米的生理调控效应. 作物学报, 2012, 38: 1042–1050. Seng S S, Wang Q, Zhang Y E, Li C H, Liu T X, Zhao L F, Liu H P. Effects of exogenous spermidine on physiological regulatory of maize after waterlogging stress., 2012, 38: 1042–1050 (in Chinese with English abstract).
[17] 余卫东, 冯利平, 胡程达, 彭记永. 苗期涝渍对黄淮地区夏玉米生长和产量的影响. 生态学杂志, 2015, 34: 2161–2166. Yu W D, Feng L P, Hu C D, Peng J Y. Effects of waterlogging during seedling stage on the growth and yield of summer maize in Huang-Huai region., 2015, 34: 2161–2166 (in Chinese with English abstract).
[18] 李彩霞, 周新国, 王和州, 郭冬冬, 郭树龙, 陈金平, 姜新. 小麦花后淹水胁迫对根区土温及籽粒灌浆的影响. 麦类作物学报, 2013, 33: 1232–1236. Li C X, Zhou X G, Wang H Z, Guo D D, Guo S L, Chen J P, Jiang X. Root zone soil temperature and grain filling progress of winter wheat under water flooding at grain filling stage., 2013, 33: 1232–1236 (in Chinese with English abstract).
[19] Duhan S, Kumari A, Bala S, Sharma N, Sheokand S. Effects of waterlogging, salinity and their combination on stress indices and yield attributes in pigeonpea (L. Millsp.) genotypes., 2018, 23: 1–12.
[20] 李金航, 郭丽丽, 孔祥生, 张淑玲, 闫臻. 6-BA和GA3对牡丹叶片衰老过程中生理特性的影响. 植物生理学报, 2014, 50: 1243–1247. Li J H, Guo L L, Kong X S, Zhang S L, Yan Z. Effects of 6-BA and GA3on physiological characteristics during leaf senescence of,, 2014, 50: 1243–1247 (in Chinese with English abstract).
[21] Noguchli H. New plant growth regulators and S-3307D., 1987, 51: 15–22.
[22] Zhen H L, Yan Z H, Feng L J. Effects of chlormequat chloride on the growth and endogenous hormones contents ofand their correlation analysis., 2012, 29: 76–82.
[23] 张洪鹏, 张盼盼, 李冰, 李东, 刘文彬, 冯乃杰, 郑殿峰. 烯效唑对淹水胁迫下大豆叶片光合特性及产量的影响. 中国油料作物学报, 2016, 38: 611–618. Zhang H P, Zhang P P, Li B, Li D, Liu W B, Feng N J, Zheng D F. Effects of uniconazole on leaf photosynthetic characteristics and yield of soybean under waterlogging stress., 2016, 38: 611–618 (in Chinese with English abstract).
[24] Spent J E, Hume D J, Kumudini S V. Soybean yield potential: a genetic and physiological perspective., 1999, 39: 1560–1570.
[25] 刘洋, 郑殿峰, 冯乃杰, 张盼盼, 陈文浩, 张红梅. 鼓粒期叶施烯效唑对绿豆各器官糖分积累及籽粒产量的影响. 中国农学通报, 2015, 31(30): 143–148. Liu Y, Zheng D F, Feng N J, Zhang P P, Chen W H, Zhang H M. Effect of foliar spraying uniconazole in seed filling period on sugar accumulation in various organs and grain yield of mung bean., 2015, 31(30): 143–148 (in Chinese with English abstract).
[26] Wan Y, Luo Q M, Yan Y H, Yang W Y, Cao X N. Response of morphological characters of soybean to application of growth retardant (uniconazole) at third trifoliate stage., 2013, 14: 792–797.
[27] Yan Y H, Gong W Z, Yang W Y, Wan Y, Chen X L, Chen Z Q, Wang L Y. Seed treatment with uniconazole powder improve soybean seedling growth under shading by corn in relay strip intercropping system., 2010, 13: 367–374.
[28] 曾红, 王小春, 陈国鹏, 陈诚, 蒲甜, 彭霄, 刘婷, 宋靖, 阳苏书, 杨文钰. 喷施烯效唑对玉米–大豆套作群体株型及产量的影响. 核农学报, 2016, 30: 1420–1426. Zeng H, Wang X C, Chen G P, Chen C, Pu T, Peng X, Liu T, Song J, Yang S S, Yang W Y. Effects of spraying uniconazole on morphological and yield of groups in maize-soybean strip intercropping system., 2016, 30: 1420–1426 (in Chinese with English abstract).
[29] 杨文钰, 于振文, 余松烈, 樊高琼, 韩惠芳, 董兆勇, 梁雪莲. 烯效唑干拌种对小麦的增产作用. 作物学报, 2004, 30: 502–506. Yang W Y, Yu Z W, Yu S L, Fan G Q, Han H F, Dong Z Y, Liang X L. Effect of uniconazole waterless-dressing seed on yield of wheat., 2004, 30: 502–506 (in Chinese with English abstract).
[30] Gawad M H, Batal M A. Response of maize productivity to the growth retardant “Uniconazole” under high nitrogen fertilization and plant density., 1996, 10: 553–556.
[31] Kumar G N M, Knowles N R. Changes in lipid peroxidation and lipolytic and free-radical scavenging enzyme activities during aging and sprouting of potato () seed-tubers., 1993, 102: 115–124.
[32] Liu X Z, Huang B R.Heat stress injury in relation to membrane lipid peroxidation in creeping bentgrass., 2000, 40: 503–510.
[33] 高小丽, 孙健敏, 高金锋, 冯佰利, 柴岩, 贾志宽. 不同基因型绿豆叶片光合性能研究. 作物学报, 2007, 33: 1154–1161. Gao X L, Sun J M, Gao J F, Feng B L, Chai Y, Jia Z K. Photosynthetic performance in the leaves of different mung bean genotypes., 2007, 33: 1154–1161 (in Chinese with English abstract).
[34] 曹旖旎, 蔡泽宇, 李晓刚, 张建锋, 陈光才. 土壤淹水和铜污染对杞柳形态及生理生化特性的影响. 生态学杂志, 2018, doi: 10.13292/j.1000-4890.201902.017. Cao Y N, Cai Z Y, Li X G, Zhang J F, Chen G C. Effects of flooding and copper stress on phenotypic and physiological characteristics ofseedlings., 2018, doi: 10.13292/j.1000-4890.201902.017 (in Chinese with English abstract).
[35] 孟娜, 徐航, 魏明, 魏胜华. 叶面喷施烯效唑对盐胁迫下大豆幼苗生理及解剖结构的影响. 西北植物学报, 2017, 37: 1988–1995. Meng N, Xu H, Wei M, Wei S H. Effect of foliar uniconazole spaying under salt stress on physiological and anatomical characteristics in.-, 2017, 37: 106–113 (in Chinese with English abstract).
[36] 孙福东, 冯乃杰, 郑殿峰, 崔洪秋, 刘春娟, 何天明, 赵晶晶. 植物生长调节剂S3307和DTA-6对大豆荚的生理代谢及的影响. 中国农业科学,2016, 49: 1267–1276.Sun F D, Feng N J, Zheng D F, Cui H Q, Liu C J, He T M, Zhao J J. Effects of plant growth regulators S3307 and DTA-6 on physiological metabolism andgene expression in soybean., 2016, 49: 1267–1276 (in Chinese with English abstract).
[37] Chen H J, Qualls R G, Blank R R. Effect of soil flooding on photosynthesis, carbohydrate partitioning and nutrient uptake in the invasive exotic., 2005, 82: 260–268.
[38] 王畅, 赵海东, 冯乃杰, 郑殿峰, 梁晓艳, 齐德强, 李建英, 韩毅强, 黄文婷. S3307和DTA-6对芸豆生殖生长阶段光合特性和产量的影响. 草业学报, 2018, 27(11): 162–170. Wang C, Zhao H D, Feng N J, Zheng D F, Liang X Y, Qi D Q, Li J Y, Han Y Q, Huang W T. Effects of S3307and DTA-6 on the photosynthetic characteristics and yield of kidney bean plants in the reproductive stage., 2018, 27(11): 162–170 (in Chinese with English abstract).
[39] 宮相伟, 刘春娟, 冯乃杰, 郑殿峰, 王畅. S3307和DTA-6对大豆不同冠层叶片光合特性及产量的影响. 植物生理学报, 2017, 53: 1867–1876. Gong X W, Liu C J, Feng N J, Zheng D F, Wang C. Effects of plant growth regulators S3307and DTA-6 on photosynthetic characteristics and yield in soybean canopy., 2017, 53: 1867–1876 (in Chinese with English abstract).
[40] 李宁毅, 时彦平, 王吉振. 水分胁迫下烯效唑对百日草幼苗光合特性及叶解剖结构的影响. 西北植物学报, 2012, 32: 1626–1631. Li N Y, Shi Y P, Wang J Z. Effect of Uniconazole on photosynthetic characters and leaf anatomical structure of zinnia seedings under water stress., 2012, 32: 1626–1631 (in Chinese with English abstract).
[41] Chaves M M, Pereira J S, Maroco J, Rodrigues M L, Ricardo C P P, Osorio M L, Carvalho I, Faria T, Pinheiro C. How plants cope with water stress in the field. Photosynthesis and growth., 2002, 89: 907–916.
[42] Antonio O V, Francisco G S, Silvia S G, Inmaculada S, Vicente L, Manuel N, Juan J M N. Physiological responses of three pomegranate cultivars under flooded conditions., 2017, 224: 171–179.
[43] 汪惠芳, 陈润兴. S3307对秋大豆株型和产量的影响. 植物生理学通讯, 1997, 33: 181–183. Wang H F, Chen R X. The effect of S3307 on plant-form and yield of autumn soybean., 1997, 33: 181–183 (in Chinese with English abstract).
[44] Linkemer G, Board J E, Musgrave M E. Waterlogging effects on growth and yield components in late-planted soybean., 1998, 38: 1576–1584.
Effects of S3307 on the photosynthesis and yield of mung bean at R1 and R5 stages under waterlogging stress
YU Qi1, FENG Nai-Jie1, WANG Shi-Ya1, ZUO Guan-Qiang1, and ZHENG Dian-Feng1,2,*
1College of Agronomy, Heilongjiang Bayi Agricultural University, Daqing 163319, Heilongjiang, China;2National Coarse Cereals Engineering Research Center, Heilongjiang Bayi Agricultural University, Daqing 163319, Heilongjiang, China
Waterlogging stress is one of the main abiotic stresses during the growth and development of crops. It is of great significance to explore the mechanisms for improving the flood resistance and waterlogging resistance cultivation of mung bean under waterlogging stress. In this experiment, the effects of uniconazole (S3307) on physiology, photosynthesis and yield of mung bean leaves under waterlogging stress were investigated in pot culture with different flood resistance mung bean varieties Lufeng 2 and Lufeng 5 from 2017 to 2018. Under the stress of waterlogging at different growth stages, the chlorophyll content (SPAD) and photosynthetic characteristic parameters of mung beans leaves were significantly decreased, malondialdehyde (MDA) content was significantly increased. The yield reduction rate of mung beans under the stress of waterlogging was 24.70%–33.63% at the beginning bloom (R1 stage), and 18.07%–28.87% at the beginning seed (R5 stage). Both mung bean varieties showed that the effect of waterlogging stress at R1 stage was greater than that at R5 stage, and the flood resistance of Lufeng 2 was stronger than that of Lufeng 5. After S3307 sprayed, SPAD, net photosynthetic rate (n), transpiration rate (r), and stomatal conductance (s) in the leaves of mung beans could be significantly increased and MDA content could be significantly decreased. The remission rate of mung beans under waterlogging stress was 28.91%–52.34% at R1 stage, and 13.77%–27.36% at R5 stage. The results showed that S3307 sprayed on the leaf surface could effectively alleviate the physiological function and photosynthetic capacity of mung bean leaves under the stress of waterlogging, thus reduce the yield reduction, but there were differences in the regulatory response of mung bean varieties to S3307 in different waterlogging periods.
mung bean; uniconazole (S3307); waterlogging stress; photosynthesis; yield
2018-11-26;
2019-01-19;
2019-03-08.
10.3724/SP.J.1006.2019.84160
郑殿峰, E-mail: byndzdf@126.com
E-mail: yrkiyrki@163.com
本研究由国家自然科学基金项目(31871576)和国家“十二五”科技支撑计划项目(2014BAD07B05)资助。
This study was supported by the National Natural Science Foundation of China (31871576) and the National Key Technology Support Program of China for the 12th Five-Year Plan (2014BAD07B05).
URL: http://kns.cnki.net/kcms/detail/11.1809.S.20190307.1438.002.html
