Risk assessment of chemical substances of safety concern generated in processed meats
2019-05-26nicFloresLeticiMorMilgroReigFidelToldr
Mónic Flores, Letici Mor, Milgro Reig, Fidel Toldrá,∗
a Instituto de Agroquímica y Tecnología de Alimentos (CSIC), Avenue Agustín Escardino 7, 46980, Paterna, Valencia, Spain
b Instituto de Ingeniería de Alimentos para el Desarrollo, Universitat Politècnica de Valencia, Camino de vera s/n, 46022, Valencia, Spain
Keywords:
A B S T R A C T
1. Introduction
Recent studies developed by the International Agency for Cancer Research [1], classified red meats as group 2, probably or possibly carcinogenic, and processed meats as group 1, carcinogenic. One of the most relevant points in the report was the possibility to reduce such negative effects if more knowledge would be available on the substances responsible for such health concern as well as about their mechanisms of formation and action [2].
In view that such report was particularly negative with processed meats, including cooked, cured and derived meat products,there is an evident need to discern which are the substances responsible for the assessed carcinogenic effect. It is thus really important to determine the presence of substances that may be considered of safety concern, undesirable for human health, that may proceed from raw materials or be generated during processing and/or commercialization of the final meat product. Such substances constitute a wide group of chemical compounds resulting from the interaction between the constituents of meat and process technology factors like drying, heating, fermentation or cooking [3,4]. The most outstanding, which are schematized in Fig.1, are N-nitrosamines,polycyclic aromatic hydrocarbons, heterocyclic aromatic amines,lipids and proteins oxidation products, Maillard reaction and glycation products, and biogenic amines [3–8]. The accumulation of such substances may be partially controlled during processing but sometimes it is difficult to avoid their presence unless the formation mechanisms are known [5,9]. Most of these substances have not been evaluated in a global way in the different meat products. The assessment of the safety of meat products is of primary relevance for health authorities but also for meat industry and distribution companies. A primary step in such safety assessment is the knowledge of the substances considered of concern and its generation routes. In this way, it would be possible to assess and design alternatives to reduce, minimize or even eliminate its presence in meat products, in order to eliminate the risk and assure consumers safety.The strategies are focused on the control of precursor substances and generation routes, or even the modification of formulations and processing conditions [10]. All these hazardous compounds,their mechanisms of generation, risks for health and ways of preventing its presence in meat products are briefly described in this review.

Fig.1. Scheme of the major substances of safety concern that may be generated in different types of meat products. MRP: Maillard reaction products and HAA: Heterocyclic aromatic amines.
2. Major substances of safety concern generated in meat products
2.1. N-Nitrosamines
Nitrosamines are found in a wide variety of foods. Its formation is due to the reaction of nitric oxide, as a nitrosating agent generated from nitrite, with secondary amines [11]. The reaction kinetics depends on several variables being favored by high temperature, acid pH 2.5–3.5 with the amine protonated,large amount of residual nitrite and longer time of storage. In general, nitrite is rapidly depleted during processing and usually low amounts remain in the final product [12]; however, this depends on the initial amounts added to the product and the processing conditions. According to different surveys, the residual nitrite content in a variety of fermented meat products is below 20 mg/kg [13]. On the other hand, nitrosation reaction is inhibited by substances like ascorbic acid, erythorbic acid and α-tocopherol[14]. Most common nitrosamines reported in meat products are N-nitrosodimethylamine (NDMA), N-nitrosodiethylamine (NDEA),N-nitrosopiperidine (NPIP), N-nitrosopirrolidine (NPYR) and Nnitrosomorpholine (NMOR) (Table 1). Several processing factors can be linked to their formation like the combination of curing mixtures with specific spices like pepper who might induce the formation of NPIP. Therefore, in several regulations like FDA it is discouraged the combination of nitrites and nitrates with spices [15].
Nitrosamines have been analyzed in Belgian fermented sausages[16], Estonian meat products [17], Spanish meat products [18]and Danish meat products [14]. In general, the total amount of nitrosamines remained below 5.5 μg/kg. Very few products were above such level. A survey of reports for nitrosamines content in processed meat products within the European Union, revealed that the mean content of NDMA was below 2.7 μg/kg while NDEA was below 0.9 μg/kg. So, the sum of NDMA + NDEA was below 3.6 μg/kg which is quite a low value [13].
Nitrosamines have been object of intense debates because of their carcinogenic activity and capacity for tumor induction in several organs like liver, kidney, pancreas, etc. [11]. In fact, volatile nitrosamines are those with major carcinogenic activity being NDEA the most powerful. Low doses of nitrosamines are proved to exert carcinogenic effect on laboratory animals and this is why health authorities are concerned with the use of nitrite in meat products.
The formation of nitrosamines at elevated temperatures has been evaluated in model systems in order to establish how different compounds may act as precursors. In this way, NDMA can be formed from several amino compounds like glycine, choline,lecithin, sarcosine, creatine, creatinine and betaine. Alanine can also produce NDEA in model systems while proline, putrescine and spermidine could originate NPYR [8]. The nitrosamine formation in meat products is affected by meat composition and processing.
In order to induce the formation of N-nitrosamines in meat products, precursors must be present at very high concentrations, higher than those naturally present in meat products, and be subjected to intense processing conditions [8].
NDEA formation can be also produced during the aging of pork meat subjected to a nitration process due to the production of precursors such as the carbonyl formed in protein and lipid oxidation and the degradation of sarcoplasmic proteins during aging [19].
It should be taken into account that nitrosating agents can be introduced in meat products not only from the direct addition of curing salt but also from other natural sources like the nitrate/nitrite present in spices and herbs and other vegetable sources. The current practice in clean labelled meat products(nitrite free) by using plant powders containing high nitrate contents that can be reduced to nitrite [20] may be an additional source for the formation of nitrosamines [21].
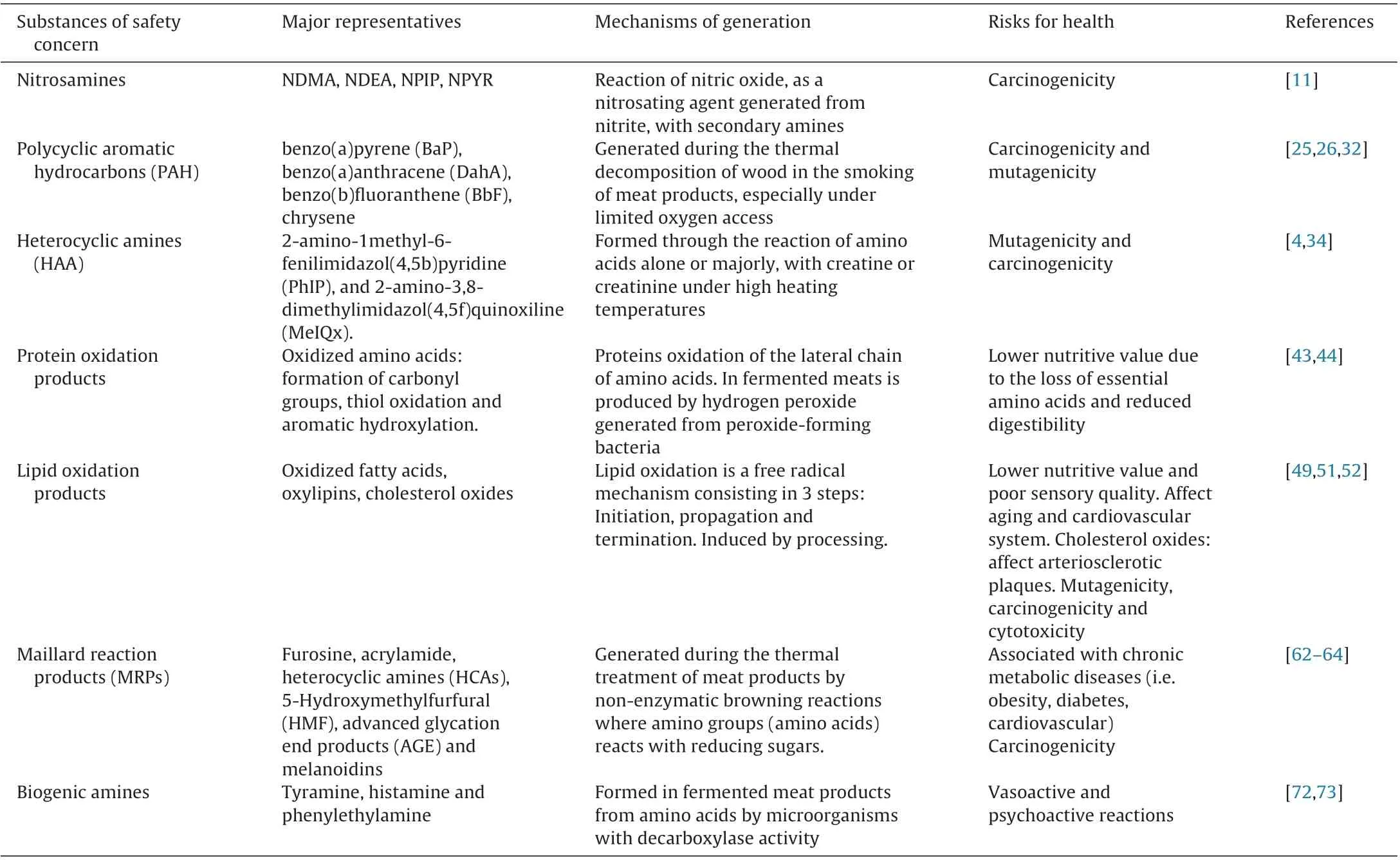
Table 1 Main substances of safety concern, its mechanism of generation and health risks. (See abbreviations in text).
The contribution of spices like black pepper to NPIP formation was demonstrated with mixtures of spices and nitrite that generated N-nitrosamines, mainly NPIP and NPYR. This is due to the presence of NPIP precursors in black pepper like pyrroperine, pyrrolidine, piperine and piperidine [8], although it was proved that nitrosamine formation in premixes with spices and nitrite depends on storage conditions and the processing of the spices which affect its composition [15].
However, the presence of other additives in meat products may affect the reaction of nitrite/nitrate. For example, the use of lecithin as emulsifying agent in meat products may produce the formation of NDMA due to the presence of choline, a quaternary ammonium compound that during heating may be decomposed into trimethylamine which will react with nitrite by demethylation [22].
Nevertheless, other sources of nitrosamine formation have been studied using a dynamic in vitro digester that investigated the combined effect of meat cooking and nitrite on protein physicochemical changes. Evidence that ammonia oxidation may contribute to endogenous nitrite formation was found and this fact could explain the increase in the amount of nitrosamines that may be formed in the gastro-intestinal tract [23]. This may be one of the explanations for the modest but significant association between red meat consumption and the risk of cancer reported by the International Agency for Research on Cancer [1].
2.2. Polycyclic aromatic hydrocarbons
Polycyclic aromatic hydrocarbons (PAH) are generated during the thermal decomposition of wood in the typical smoking of meat products, especially under limited oxygen access. PAH are characterised by having 2 or more aromatic rings, largely lipophylic.The critical factor is the temperature for smoke generation because there is a lineal PAH increase with the temperature [24].
Most PAH are carcinogenic and mutagenic and thus, they can contribute to cancer in humans if consumed regularly in the diet[25,26]. PAH interact in the organism with diverse enzymes generating PAH di-hydrodiol derivatives which are carcinogenic due to their ability to form covalent bindings with proteins and nucleic acids. The PAH content in smoked meat products is affected by many variables like the type of wood, the environmental conditions and diffusion processes through the packaging material [24,27] also the thermal treatment conditions like temperature, time of treatment, distance from the source of heating, fat content and presence of oxygen [28–30].
The new European Union Regulation on the use of smoking aromas [31] controls the presence of PAH but the real risks happen in traditional smoking houses where uncontrolled conditions might take place and where there arepoor technological knowledge and hygienic conditions. PAH controlled by EU Directives 2005/10/EC and, 2005/108/EC are: benzo[a]anthracene(BaA), benzo[b]flouranthene (BbF), benzo[j]fluoranthene (BjF),benzo[k]fluoranthene (BkF), benzo[g,h,i]perylene (BjhiP), chrysene (CHr), benzo[a]pyrene (BaP), dibenzo[ah]anthracene(DahA), dibenzo[ae]pyrene (DaeP), dibenzo[ah]pyrene (DahP),dibenzo[ai]pyrene (DaiP), dibenzo[al]pyrene (DalP), indenol[1,2,3 cd]pyrene (IcdP) y 5-methylchrysene. Initially,benzo[a]pyrene (BaP) was used as a marker of PAH in smoked foods. However, it only accounts for up to 20% of the total PAH and could not be maintained [32], so that new maximum levels for the sum of four substances (PAH4) consisting of benzo(a)pyrene,benzo(a)anthracene, benzo(b)fluoranthene and chrysene (Table 1),or eight specific substances (PAH8) were considered as the most suitable indicators of PAH in the meat product [31]. According to such Regulation [31], the maximum levels (μg/kg) of benzo(a)pyrene in smoked meat and smoked meat products were stated as 2,0 μg/kg since September 2014 and in the case of PAH4 they were 120 μg/kg as from September 2014.
2.3. Heterocyclic aromatic amines
Heterocyclic aromatic amines (HAA) are formed through the reaction of amino acids alone or majorly, with creatine or creatinine under high heating temperatures like those found in cooking [3]. The main classes are aminoimidazolquinolines and aminoimidazol-pyridines. Major exposition to HAA is mainly due to the presence of 2-amino-1methyl-6-fenilimidazol(4,5b)pyridine (PhIP), and 2-amino-3,8-dimethylimidazol(4,5f)quinoxiline (MeIQx) (Table 1). Other minor HAA are 2-amino-9Hpyrido(2,3b)indol (AC), 2-amino-3,4dimethylimidazol(4,5f)quinoline (IQ), 2-amino-3,4,8-trimethy limidazol(4,5f)quinoxiline (DiMeIQx) [34]. HAA have been reported to be mutagenic and carcinogenic [4] and are related to the development of certain types of cancer [33,34].
The mechanisms involved in the carcinogenic potential of HAA in meat are not clearly understood. HAAs are not considered important for the carcinogenic potential of meat although they are carcinogenic in animal experiments but at higher levels than those present in foods. Furthermore, Croos and Sinha [35] reported that the mutagenic potential of HAAs depends on metabolization. In fact, in a study of the mechanisms linking colorectal cancer to the consumption of (processed) red meat [34], it was indicated that HAAs carcinogenic potential depend on the extent of metabolization and also on individual differences in intestinal microflora which may affect the carcinogenic activity. Therefore, the genetic factors seem to be the main factor in meat associated with HAA carcinogenicity [34].
Cooking processes generating major amounts of HAA are frying, roasting, barbecue and grilling due to the high temperatures reached [30,36]. However, they are slowly generated during baking and cooking at low temperatures [37]. The precursors for HAA generation in meat products cooked at high temperatures are creatine and creatinine and also nitrogen bases and nucleosides [38].The formation of HAA is determined by the meat cooking technique and degree of doneness so that guidelines for meat cooking are considered appropriate to decrease HAA content [39,40]. However, it is not possible to determine the content of HAA only from color measurements because other factors like precursor concentration and presence of water and fat in the meat product may affect their generation [41,42]. So, many factors should be taken into account in order to minimize the formation of HAAs.
2.4. Proteins oxidation products
Proteins oxidation results in a lower nutritive value due to the loss of essential amino acids as well as a reduced digestibility, but also to lower sensory quality due to its effects on poorer color, lower water retention and damaged texture. Furthermore, changes in meat product yield during processing has been related to increases in protein carbonyl content and cross-linkage among myofibrillar and sarcoplasmic proteins [43].
Proteins oxidation mainly affects the lateral chain of amino acids and may be reversible (i.e. sulfidryl groups of cysteins, conjugation with glutathione or sulfenic acid, and methionine oxidation to methionine sulfoxide) or irreversible (generation of carbonyl groups in lysine, proline, arginine and threonine that are used as markers of oxidative damage of proteins). Such oxidized proteins may accumulate into the meat product [43].
Proteins may be largely oxidized in those fermented meats where hydrogen peroxide can be generated by peroxide-forming bacteria. The oxidation of amino acids results in the formation of carbonyl groups, thiol oxidation and aromatic hydroxylation [44](Table 1). Cysteine is oxidized to sulfinic acid and partly to cysteic acid [45]. Homocystine can generate homolanthionine sulfoxide[46]. Methionine is oxidized majorly to methionine sulfoxide but also to methionine sulfone [47]. A recent study showed methionine oxidation in 6% to 35% of peptides derived from nebulin, titin,myosin heavy chains, and troponin I proteins [48].
2.5. Lipids oxidation products
Lipids oxidation results in a lower nutritive value and poor sensory quality due to color deterioration and formation of strange aromas, mainly rancid aromas. Fatty acids with unsaturations are more prone to oxidation [49]. However, some lipid oxidation is necessary to develop characteristic aroma of meat products [50].Lipid oxidation follows a free radical mechanism consisting in 3 steps: Initiation, propagation and termination. Hydroperoxides are the primary products of oxidation but they are flavorless while the secondary products of oxidation can contribute to off-flavors,color deterioration and potential generation of toxic compounds[51]. Lipid oxidation may also be induced by hydrogen peroxide generated by peroxide-forming bacteria grown during meat fermentation. Some products of lipid oxidation have been reported to contribute to aging, cancer and cardiovascular diseases (Table 1).
Cholesterol oxidation may also occur generating cholesterol oxides that constitute a health concern because of their role in arteriosclerotic plaques and also as mutagenic, carcinogenic and cytotoxic [52].
The presence of cholesterol oxides in animal food products depends on fatty acid, cholesterol contents and processing conditions [53] but also cooking, storage, and reheating methods affects their formation [54]. The use of microwave and oven grilling processes may result in higher production of cholesterol oxides in processed meat as compared with other cooking methods. In addition, the refrigerated storage and the further reheating produce an increase of cholesterol oxides [54] although other techniques used for meat product preservation affect their formation like in e-beam irradiated meat products [53]. Furthermore, the cholesterol oxides formation has been inhibited by the presence of several vitamins in beef patties like pyridoxamine (B6), L-ascorbic acid (Vitamine C),retinoic acid (Vitamin A), and α-(±)-tocopherol (Vitamin E) [55]. All these facts show that the formation of cholesterol oxides in meat products is affected not only by meat product composition but also by processing and storage conditions.
No cholesterol oxides have been detected after heating of pork sausages [56]. The generation of cholesterol oxides in European fermented sausages has been reported to be low, up to 1.5 μg/g with a very low percentage (<0.17%) of cholesterol oxidation [57].Such amount is below the dose of concern based on in vivo assays with laboratory animals [58]. The major cholesterol oxide found in Italian sausage was reported to be 7-ketocholesterol while 5,6α-5,6-epoxycholesterol was the major end-product in the other analyzed sausages [57].
The presence of oxylipins in meat and meat products may also be of concern, as these oxylipins are hydroperoxides, epoxides and oxidation products of polyunsaturated fatty acids (PUFA). The oxylipins developed from linoleic acid are leukotoxins (LTX), and leukotoxin diols (LTXD) that have been shown to possess adverse effects on rats [59]. Several of these oxylipins have been identified and quantified in mechanically deboned meat in a concentration that may be of concern [60]. However, their impact in meat products is not clear yet. Song et al. [61], reported the presence in several cured meat products, of Ltx and Ltxd in a range of 0.77–6.90 μg/g and 0.17–3.93 μg/g, respectively. However, they reported that the amounts of Ltxds after ingestion might be much higher.
2.6. Maillard reaction products
Maillard reactions take place during the thermal treatment of some meat products. Such reactions, also known as non-enzymatic browning reactions, are characterised by amino groups (amino acids) reacting with reducing sugars. Maillard reactions start with the formation of Amadori compounds, followed by stages where advanced glycation end products are generated. The accumulation of such end products is associated with cronic metabolic diseases(i.e. obesity, diabetes, cardiovascular) and, subsequently, to degenerative crhonic sickness [62].
The complexity of the Maillard reaction products (MRPs) is mainly due to the presence of initial, intermediate and final stages compounds that produce different flavor and aroma in processed foods [63] and reduce their nutritional quality, particularly by reducing protein digestibility [64]. Reports have linked MRPs to the nutritional quality of food and the most important compounds are furosine, acrylamide, heterocyclic amines (HCAs),5-Hydroxymethylfurfural (HMF), advanced glycation end products(AGE) and melanoidins that are present in processed foods from animal and vegetal origins [64] (Table 1). However, the ones who have been related to toxic and carcinogenic effect are HMF, acrylamide, HCAs, and AGE although the normal dietary exposure to acrylamide, HCA and HMF is low [64]. Even though numerous studies related AGE products with disease and health, the relationship between AGE properties and biological activities remains still unclear [65]. Different approaches have been suggested to control the presence of MRP in foods such as those to prevent the formation of undesirable MRP compounds through controlling the processing conditions and the use of external agents to avoid their formation like the correct choice of ingredients [66].
A typical representative of such compounds is carboxymethyl lysine [67]. Maillard reactions can also generate acrylamide,through the reaction of asparagine with glucose, which contributes to neurotoxic [68] and carcinogenic troubles in humans.
Acrylamide has been classified by the International Agency for research on Cancer as probably carcinogenic to humans [69]. It is formed during thermal processing such as frying or baking but its formation is favored by low water content. Generally, its presence is in the food surface due to the high temperature reached at the surface and the lowest water content [66]. In the case of meat, acrylamide formation during grilling, frying and baking of beef minced meat containing only sodium chloride as ingredient is low and only carboxymethyllysine was detected although at low levels when the meat was baked at high temperatures (300°C) [70].
Furosine (2-furoil methyl lysine) is formed by acid hydrolysis of the Amadori compounds and is used as indicative of the intensity of the thermal treatment of processed foods, among them cooked meat products [70]. Furosine might also serve as index of potential generation of glycation end products. Studies regarding the cytotoxicity of Maillard Reaction Products (MRP) formed from defined amino acids or dipeptides with ribose, glycerinaldehyde or methylglyoxal has shown that MRP were cytostatic and the Maillard modified peptides were more effective than their amino acids although the compounds responsible for these activities were not elucidated [71].
2.7. Biogenic amines
Biogenic amines are formed by decarboxylation of amino acids and are generally found in fermented sausages that used microorganisms with decarboxylase activity [72]. The generation of biogenic amines depends on processing conditions being favored by the type of starter culture if having decarboxylase activity, high temperature and acid pH values. The potential risk of biogenic amines for consumers´health is more related to tyramine, histamine and phenylethylamine that are associated to vasoactive and psychoactive reactions [73].
The main concern with the high content of biogenic amines, such as histamine or tyramine, in food products is the absence of any effect on the sensory characteristics so that consumers are unaware of their presence and thus, unable to reject these products [74].
The production of biogenic amines in fermented products is mainly due to lactic acid bacteria although the decarboxylase activity responsible for its production is often related to strains rather than to species or genera [6]. Therefore, the use of starter culture should be based on the careful selection of LAB starters lacking the pathways for biogenic amine formation.
After ingestion, histamine intoxication/intolerance may be produced but more sensitive reactions may be observed for those consumers medicated with inhibitors of monoamino oxidase or diamino oxidase [72]. There are two major groups of amines. One is formed by aromatic amines including tyramine, phenylethylamine,histamine and tryptamine, and the second one aliphatic amines that include putrescine, cadaverine and agmatine [73].
3. Measures to prevent the generation of hazardous substances in meat products
There are several preventive measures that can be done in order to reduce the generation of hazardous substances in meat products and are summarized in Table 2. So, the strategies to reduce the presence of nitrosamines in meat products are mainly addressed towards the reduction in the levels of nitrite added to the product and use only those really necessary for preservation purposes [13].Another strategy is the addition of ascorbate or erythorbate to reduce the amount of nitrous acid. In fact, ascorbate is preferred to ascorbic acid because it reacts with nitrite 240 times faster than does ascorbic acid and the reductant activity increases with increasing pH [11].
Some strategies for reducing PAH presence in smoked meat products consist of filtering particles, using cooling traps, reducing temperatures and/or reducing the time of the process. Another option is the application of liquid smoke, obtained through distillation and subsequent condensation of volatile compounds, on the surface of the meat product. Another strategy is the use of 0.1%–1.0%smoke flavorings, produced from Primary Products obtained from woods after specific pyrolisis and extraction protocols. In any case,the Primary Products must be checked for the presence of polycyclic aromatic hydrocarbons [75,76]. Furthermore, the use of different packaging systems may produce a considerable effect on reducing PAHs levels [77]. The use of a low density polyethylene (LDPE)was proposed to reduce PAH in liquid smoke flavorings [78] and in smoked meat [77].
In the case of heterocyclic amines, the best preventive measure is to control the temperature and time of cooking, especially in roasting, barbecue and grill, and avoid excessive high temperatures[36]. Other cooking strategies consist in avoid the direct contact of the food with naked flame or charcoal, wrapping in aluminum foil,precook the meat prior to grill to reduce the time on the grill and finally, the use of foodstuffs containing phenolic antioxidants that may be useful to reduce the levels of HAA produced [41]. Also an adapted formulation or diet containing antioxidants with phenolic or polyphenolic moieties that can act as HAA inhibitors because they can scavenge reactive radicals involved in the mechanism of HAA formation [79]. Such antioxidants are mainly flavonoids,terpenoids and catechins [80]. Another strategy is to lower HAA bioaccessibility by reducing the ability of HAA to pass through thegastrointestinal barrier, for instance, through adsorption of HAA to fibers that are excreted [9].

Table 2 Strategies for preventing the generation of substances of safety concern in meat products.
In the case of protein and lipid oxidation, preventive measures consist basically on the addition of antioxidants, selection of starter cultures with catalase and superoxide dismutase (antioxidant activity) but lacking oxidative enzymes (i.e.- lipoxygenase), avoidance of exposition to light, and the use of vacuum in mixing and mincing, and packaging to avoid oxygen entrapment and its consequent oxidative processes [10]. Cholesterol oxidation may be prevented by the presence of vitamins like vitamins B6, C, A and E [55].
Regarding Maillard reaction products, a proposed strategy to inhibit acrylamide formation is the addition of sulphite that mitigates its formation although the use of sulphites is strictly regulated due to their problems associated with allergy and thiamine absorption interference [66]. Other strategies have been focused on the addition of a competitive amino acid group by adding an aminorich product to compete with the precursors present in the product.For example, the addition of the added amino acid to compete with asparagine during the Maillard reaction; however some of these strategies can affect the sensory quality of the product [66].
Preventive measures to reduce the generation of biogenic amines are basically the good hygienic quality of raw materials(meat and ingredients) and the careful control of the type of starter culture and processing conditions [81,82].
4. Conclusions
A wide variety of substances of safety concern may be generated in meat products depending on the type of processing. The generation of nitrosamines are associated to the use of nitrite as preservative, polycyclic aromatic hydrocarbons to particular smoking processes, and biogenic amines to specific microorganisms in fermentation. Heterocyclic aromatic amines may be generated in any type of meat product that is subjected to intense cooking.Other substances released from the oxidation of lipids and proteins,and/or Maillard reactions may appear in meat products depending on the presence of additional compounds (i.e. free radicals in the case of oxidation, or reducing sugars in the case of Maillard reaction) and determined variables like temperature, time, water activity and pH. Finally, the presence of spices in a variety of meat products may produce an impact on the mechanism of formation of these compounds due to their potential to inactivate free radicals generated as intermediates in these reactions. The generation of all these hazardous substances may be prevented if appropriate caution and good manufacturing practices are taken during processing.
Submission declaration
Authors declare that the work described has not been published previously, that it is not under consideration for publication elsewhere, that its publication is approved by all authors and tacitly or explicitly by the responsible authorities where the work was carried out, and that, if accepted, it will not be published elsewhere including electronically in the same form, in English or in any other language, without the written consent of the copyright-holder.
Role of the funding source
Authors declare that the funding source(s) had no involvement in the study design; in the collection, analysis and interpretation of data; in the writing of the report; nor in the decision to submit the paper for publication.
Declaration of Competing Interest
Authors declare that none of them have any conflict of interest.
Acknowledgements
Grants AGL2017-89831-R, AGL2015-64673-R and FEDER funds from the Spanish Ministry of Economy, Industry and Competitiveness are acknowledged. Ramón y Cajal postdoctoral contract to LM is also acknowledged.
杂志排行
食品科学与人类健康(英文)的其它文章
- Seaweed nutraceuticals and their therapeutic role in disease prevention
- Occurrence, properties and biological significance of pyroglutamyl peptides derived from different food sources
- Antioxidant activity and total phenolic content of essential oils and extracts of sweet basil (Ocimum basilicum L.) plants
- Antioxidant peptides encrypted in flaxseed proteome: An in silico assessment
- Hyperinsulinemia, cancer and maqui berry: The promise of nutritional supplementation
- Fermentation-enabled wellness foods: A fresh perspective
