有双重固硫作用的PEDOT包覆MnO2纳米管阴极用于高性能的锂硫电池
2019-05-07潘沛锋刘瑞卿朱红丽冯晓苗沈清明黄镇东马延文
葛 优 潘沛锋 彭 霞 刘瑞卿 朱红丽 冯晓苗 沈清明 黄镇东 马延文
(南京邮电大学有机电子与信息显示重点实验室暨先进材料研究所(IAM),南京 210023)
0 Introduction
At present,among the rechargeable batteries,lithium-sulfur batteries are expected to satisfy various energy storage and conversion demands because of high theoretical specific capacity of sulfur element(1 675 mAh·g-1)and specific energy density of Li-S batteries (2 600 Wh·kg-1),abundant resources in natural,low cost,and environmentally friendly[1-7].However,the practical application of lithium-sulphur batteries is still hindered by some drawbacks.For instance,(a)large volumetric change of sulfur cathode during charge and discharge process leading poor cycling performance[8];(b)the excessive dissolution of polysulfides and subsequent “shuttle effect” resulting in specific capacity decline rapidly[9-15];(c)insulative sulfur and lithium polysulfide with low electronic conductivity reducing the rate capacities of Li-S batteries[16-17].
In order to solve the above problems,a number of strategies have been developed to optimize the composition and the structure of the sulfur cathode.Most host materials for sulfur are carbonous materials,such as micro-/mesoporous carbon[18],carbon spheres[19],carbon nanotubes/nanofibers[20],and graphene[21].These hostmaterials notonly improve the electronic conductivity ofsulfur-based electrodes,butalso capture the polysulfides to retard the shuttling of soluble polysulfides.On the other hand,conducting polymers,such as poly(3,4-ethylenedioxythiophene)(PEDOT)[22-23],polyaniline (PANI)[24]and poly(acrylic acid)(PAA)[25],have also been used as host materials forsulfurattributing to theirhigh conductivity,flexibility and thermal stability.However,the intermolecular interactions between lithium polysulfides(LiPSs) and carbonous materials or conducting polymers are weak because of physically confining LiPSs due to their non-polar characteristic,the LiPSs diffuse out of the cathode and eventually migrate to the anode easily.
Recently,sulfur host materials exhibiting strong chemical interactions with LiPSs have been studied and appeared to be an effective approach to stabilizing the capacity,including modified carbonaceous materials[26],functional polymeric materials[27].Introduction of electronegative N atoms into the carbon lattice,such as mesoporous N-doped carbon,induces asymmetric charge distribution.This affects the net polarity,creating sites for binding LiPSs[28-29].Moreover,metal oxides such as MnO2[30],TiO2[31],TiO[32],V2O5[33]and Al2O3[34],and metal sulfides including TiS2[35],WS2[36]and CoS2[37]have been proposed as effective lithium polysulfides trappers by utilizing the strong chemical adsorption.As a polar oxide,MnO2has high binding energy between MnO2and LiPSs because of the presence of stronger polar chemical bond.Extensive efforts have been devoted to the development of nanostructures for trapping LiPSs,such as MnO2nanowires[38],MnO2nanosheets[39]and MnO2nanoparticles[40].However,these solid structures restrict the content of sulfur loading and cannot solve the large volumetric change ofsulfurcathode.Meantime,conductivities of metal oxides are low,leading to low sulfur utilization and poor rate performance.Therefore,the combination ofpolarhollow materials and conducting matrix with rationally designed structures is a desired strategy to enhance the electrochemical properties of sulfur cathode.So far,the research of hollow MnO2nanotubes for sulfur host is comparatively rare.Only the PPy-MnO2coaxial nanotubes have been synthesized to solve the above problems due to the high conductivity of PPy,the strong chemical adsorption and hollow structure of MnO2nanotubes[41].However,in the preparation procedure,the MnO2nanowires were used as template and the hollow MnO2nanotubes were prepared by acid etching.The complex preparation process significantly affected the manufacturability of the sulfur cathode.Therefore,there is still a challenge to design innovative method and nanostructures for efficaciously encapsulation of sulfur.
Herein,the hollow α-MnO2-PEDOT nanotubes were prepared through a facile template-free hydrothermalself-assembly in situ polymerization to immobilize sulfur.In the composite structure,the MnO2hollow nanotubes not only enhance the sulfur loading and accommodate volumetric change of sulfur,but also provide combined role of chemical and physicaladsorption forlithium polysulfides.The chemical adsorption attribute to Mn-S bond which is conducive to limit lithium polysulfides.The physical adsorption is produced by hollow nanotube morphology.Besides,S@MnO2combiningwithPEDOT could enhance the conductive of S@α-MnO2-PEDOT and reduce the excessive dissolution of polysulfides.After combining with the conductive polymer PEDOT,the S@MnO2-PEDOT nanocomposites electrode revealed the excellent discharge specific capacity of 774.8 mAh·g-1at 1.0C after 200 cycles,which effectively perfects the electrochemical properties of Li-S batteries.
1 Experimental
1.1 Synthesis of urchin-like α-MnO2nanotubes
All the reactants and solvents were analytical grade and used without further purification.Urchinlike α-MnO2nanotubes were produced by hydrothermal method.In a typical process,0.17 g of KMnO4was dispersed in 18 mL of deionized water(DI)to form a clear solution under magnetic stirring for 30 min.Subsequently,2 mL of HCl(2 mol·L-1)was added to the clear solution dropwise under magnetic stirring.It was then transferred into an autoclave and heated to 120℃for 12 h.The hydrothermal product was collected by centrifugation and rinsed several times with DI and ethanol.
1.2 Synthesis of S@α-MnO2composites
The mixtures of prepared urchin-like α-MnO2nanotubes and sulfur were sealed and heated to 155℃for 10 hours.Then,the mixtures were heated to 250℃under argon flow for 30 min in tube furnace to eliminate the sulfur on the outside surface of the α-MnO2nanotubes by evaporation.The resulting S@α-MnO2composites with the sulfur loading of 71.09%(w/w)were obtained,according to the thermogravimetric analysis(TGA).
1.3 Synthesis of S@α-MnO2-PEDOT
A simple in situ polymerization process was used to coat the PEDOTs on the surface of S@α-MnO2composite.Typically,50 mg S@α-MnO2composites were dispersed into the different quantity(4,2,1 mg)of 3,4-ethylenedioxythiophene(EDOT)solution at room temperature.Then different quantity(12.50,6.25,3.12 mg)of oxidant FeCl3solution was added dropwise sequencingly.After stirring for 6 hours,the final product was collected by centrifugation,washed by distilled water several times,and then dried at 60℃overnight.
1.4 Characterization
XRD measurements were carried out on a Philip XRD X′PERT PRO X-ray diffractometer operating at 40 kV and 40 mA,and using Cu Kα radiation(λ=0.154 18 nm).The diffraction patterns were performed in the 2θ range of 10°~80°.The structure and morphology were characterized by SEM (Hitachi S-4800 at 10 kV)and TEM (Hitachi 7700 at 100 kV).High-resolution TEM(HRTEM)images were recorded on FEITalosF200X field-emission transmission electron microscope operated at 200 kV.Raman spectroscopy was carried out using a Renishaw inVia Raman microscope with a 532 nm laser with exposure time of 10 s,the laser power was reduced to 1%to minimize the sublimation of sulfur due to the laser heating.Chemical bonding nature was analyzed by X-ray photoelectron spectroscopy(PHI 5000 Versa Probe).Thermogravimetric analysis was used to determine the sulfur content of the material on a TGA instrument(NETZSCH STA-449 C)employing a heating rate of 10℃·min-1from room temperature to 700℃ under a nitrogen flow.
CR2032-type coin cells were assembled in a glovebox filled with argon.The working electrodes were prepared by mixing 70%(w/w)active materials,20%(w/w)acetylene black and 10%(w/w)polyvinylidene fluoride (PVDF)binder in N-methyl pyrrolidinone(NMP).The slurries were homogeneously coated on to aluminum foil current collectors.The electrodes were dried at 60℃for 12 h under vacuum.Subsequently,the electrodes were cut into disks with a diameter of 13 mm.A piece of lithium foil was used for the combined counter and reference electrodes.1.0 mol·L-1lithium bis(trifluoromethanesulfonyl)imide(LiTFSI)in 1,3-dioxolane and 1,2-dimethoxyethane(volume ratio,1∶1)with 1%(w/w)LiNO3as an additive was used as the electrolyte.The LiNO3was added to help passivate the surface of the lithium anode and reduce the “shuttle effect”.Celgard 2400 was used as a separatorfilm.The cycle performances,rate capability and galvanostatic charge/discharge tests were carried out on LAND CT2001A in a potential range of 1.5~2.8 V (vs Li/Li+).The specific capacity was calculated based on the weight of sulfur.Cyclic voltammetry (scan rate:0.2 mV·s-1,cut-off voltage:1.5~2.8 V)and electrochemical impedance spectra(frequency range from 100 kHz to 10 mHz)were measured with an electrochemical workstation VMP3.
2 Results and discussion
2.1 Structure characterization
The formation mechanism of hollow urchin-like morphology of manganese dioxide can be explained by the process ofOstwald maturation.In general,permanganic acidradical is unstable on thermodynamics and it is easy to be reduced to manganese dioxide.Therefore,the reaction occurred rapidly in an acidic environment and at a high temperature under hydrothermal condition.Atthebeginningofthe reaction,a large number of MnO2crystal nuclei condensed into MnO2microspheres and MnO2nanorods grew outwards along the surface of the microsphere,the formation of the hollow urchin-like structure was attributed to the gradual disappearance of the core.The formation of MnO2nanotubes was mainly due to the etching of MnO2nanorods by hydrochloric acid.In the hydrothermal condition,hollow urchin-like morphology of MnO2can be reflected in the intensification of the etching.The redox reaction can be described by Equation(1)as follows.
The scanning electron microscopy (SEM)images of α-MnO2(Fig.1(a~c),Fig.S1,Supporting Information)exhibit the 3D structure of urchin-like morphology at different magnifications.The urchin-like microspheres assembled by a large number of short-sized nanotubes of manganese dioxide dispersed from the center to the outside can be observed from Fig.1(a,b).From Fig.1c,the manganese dioxide nanotubes with hollow structure can be seen clearly as green ellipses shown.TEM images(Fig.1(d~e))also revealed that the obvious hollow structure ofα-MnO2nanotubes and the nanotubes had a diameter of approximately 120 nm.The lattice fringe with an interplanar spacing of about 0.31 nm that corresponded to the (310)plane of α-MnO2was further identified from the inset of Fig.1e.The high-angle annular dark field scanning transmission electron microscopy (HAADF-STEM)image and elemental mappings of MnO2nanotubes exhibit the element distribution of Mn and O along the length of nanotube in Fig.2f.
After the melt-diffusion stage,the S@α-MnO2nanotubes morphology (Fig.2a)was maintained well except for the rough surface compared with the pristine MnO2nanotubes (Fig.1c).To give further insight into the morphology and structure of the S@α-MnO2,TEM analysis has been carried out.Fig.2b reveals that the solid rods,indicating the sulfur was successfully encapsulated in hollow tubes of MnO2.After the solid rods of S@α-MnO2were coated by PEDOT,there was obviously cladding on the outer surface as shown in Fig.2c,and the enlarged image(Fig.S2)shows the wrapped nanotube of S@α-MnO2-PEDOT with a diameter of approximately 140 nm.Fig.2d further reveals the presence of PEDOT and the thickness was approximately 10 nm under the mass ratio of S@α-MnO2composites and EDOT with 50∶2.For comparison,other different mass ratios(50∶1,50∶4)were also carried out,their thicknesses were 5 and 20 nm,respectively (Fig.S3).Moreover,the HAADFSTEM and TEM elemental mappings confirmed the existence and homogeneous distribution of Mn,O,S and C elements in S@α-MnO2(Fig.2e)and S@α-MnO2-PEDOT (Fig.2f),respectively.The high voltage and volatilization of some sulfur during TEM testing resulted in the hollow structure to some extent.
The X-ray diffraction (XRD)pattern indicates tetragonal crystal system of hollow urchin-like α-MnO2.The α-MnO2had tunnel structures which was one of five known mineral polymorphs of manganese oxide.The crystal structure of α-MnO2was tunnel structures of[1×1]and [2×2]which being composed by singleordouble chain according to[MnO6]octahedral along public edges[42].As shown in the Fig.3a,the main diffraction peaks of α-MnO2at around 12.9°,17.9°,28.6°,37.5°,42.0°,49.8°,56.3°,60.1°,65.4°,69.4°and 73.2°,which was consistent with the diffraction peaks of the tetragonal crystal system of α-MnO2(PDF No.44-0141)reported in the literature[43].The product had good crystallization because all diffraction peaks were sharp in Fig.3a.Also,the XRD patterns of S@α-MnO2and S@α-MnO2-PEDOT were almost the same to that of elemental sulfur with preponderant peaks at 23.02°,26.26°,27.65°,and 28.61°,indicating sublimed sulfur was successfully infiltrated into α-MnO2nanotubes and α-MnO2-PEDOT composites,and the diffraction peaks were in good agreement with the standard cards of sulfur(PDF No.08-0247)and manganese dioxide.Because of the existence of PEDOT,the diffraction peak intensity was weak compared with pure MnO2.The sulfur contents of S@α-MnO2-PEDOT composites with different mass ratio of S@α-MnO2composites and EDOT were demonstrated by thermogravimetric analysis(TGA)in Fig.S4.In the TGA,the sulfur volatilized when temperature reached~160℃ showing steep weight loss and the slight weight loss around 500℃could be attributed to the pyrolysis of PEDOT.In the control sample,the residualmoisture content was about 3%(w/w).The TGA reveals that the sulfur contents(mass percentage)of S@α-MnO2-PEDOT nanocomposites are 69.75%(50∶1),70.40%(50∶2)and 71.91%(50∶4).The Raman spectrum of S@α-MnO2-PEDOT composites was as provided to investigate the structural information(Fig.3b).The intensive peak at 1 443 cm-1was chiefly due to the C-C stretching vibration of the thiophene ring,rooting in the neutral parts existing between the localized elementary excitationssuch aspositive polarons or bipolarons generated upon doping[44-46].The peaks consisted in 1 502 and 1 565 cm-1were put down to the C=C asymmetric stretching vibrations of the thiophene rings in the middle and at the end of the chains,respectively[47].The peak at 635 cm-1was attributed to MnO2.Moreover,There were obvious Raman spectra of pure sulfur at 152.9,218.4 and 471.6 cm-1,which corresponded to the vibration of S-S bond in S@α-MnO2-PEDOT composites[48].To prove the interaction of polysulfides with α-MnO2nanotubes,the XPS analysis of pristine α-MnO2,the polysulfide and α-MnO2nanotubes were carried out.The XPS spectra of the whole spectrum of S@α-MnO2-PEDOT and Li2S6@α-MnO2-PEDOT are shown in Fig.S5.In Fig.3c,the Mn2p3/2spectrum showed two deconvoluted peaks at 641 and 642.3 eV,corresponding to Mn3+and Mn4+of pristine α-MnO2,respectively[49].As evident from Fig.3d,the oxidation of Li2S6resulted in the partial reduction of Mn4+to Mn3+and Mn2+.The peak intensity of Mn3+significantly enhanced,and the new peak located at 640~641 eV,which could be assigned to the Mn2+oxidation states.The results can be clearly seen further confirming the strong interaction of polysulfides with MnO2nanotubes,which can load more sulfur species around the Mn ion and trap more soluble polysulfides[50].
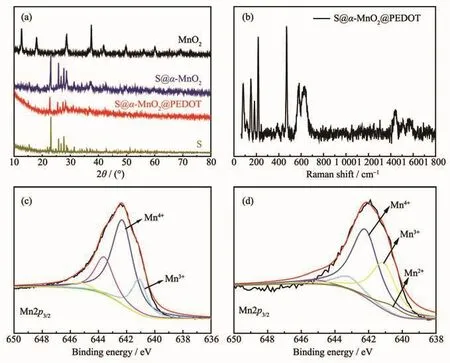
Fig.3 (a)X-ray diffraction patterns of pristine sulfur,α-MnO2,S@α-MnO2andS@α-MnO2-PEDOT;(b)Raman spectra of S@α-MnO2-PEDOT;XPS spectra of(c)S@α-MnO2-PEDOT and(d)Li2S6@α-MnO2-PEDOT for Mn2p3/2
2.2 Electrochemical performance
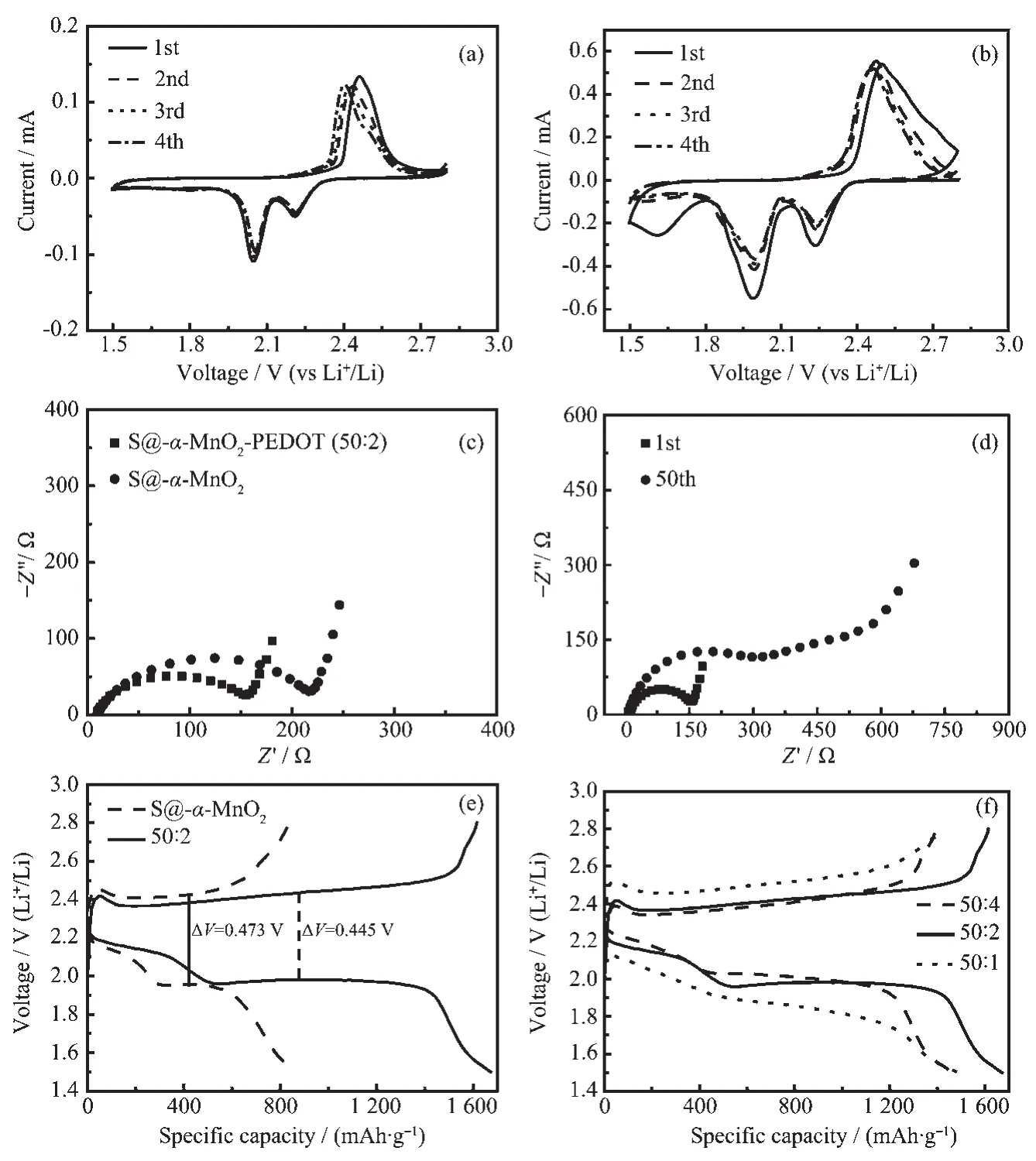
Fig.4 CV curves of(a)S@α-MnO2electrode and(b)S@α-MnO2-PEDOT electrode for the first four cycles at a scan rate of 0.2 mV·s-1;(c)Nyquist plots of S@α-MnO2electrode and S@α-MnO2-PEDOT electrode before cycling;(d)Nyquist plots of S@α-MnO2-PEDOT electrode after cycling;(e)Initial discharge/charge voltage profiles of S@α-MnO2and S@α-MnO2-PEDOT electrodes(50∶2)at 1.0C;(f)Initial discharge/charge voltage profiles of S@α-MnO2-PEDOT electrodes with different PEDOT contents at 1.0C
The electrochemical performances of the S@α-MnO2and S@α-MnO2-PEDOT composites were illustrated in the following research.The first four cycles of the CV curves of S@α-MnO2and S@α-MnO2-PEDOT nanocomposites are presented in Fig.4(a,b)in the potential range of 1.5~2.8 V at a scan rate of 0.2 mV·s-1.Unlike the CV curves of S@α-MnO2,the CV curves of S@α-MnO2-PEDOT had higher current,suggesting the betterconductivity ofS@α-MnO2-PEDOT by introduction of PEDOT.However,both of CV curves showed representative reduction and oxidation characteristic peaks for the lithium-sulfur batteries,and the peaks were at almost the same positions.Two typical reduction peaks at around 2.0 and 2.24 V could be attributed to the multistep reduction reaction of sublimed sulfur[51].The former was attributed to the reduction of S8to S82-,and the latter was related to further transformation of soluble long-chain polysulfide (Li2Sn,n=4~6)to produce insoluble short-chain polysulfides (Li2S or Li2S2)[52].In the oxidation process,the main peak at around 2.45 V corresponded to the reverse reactions of polysulfides back to S8.There was a significant reduction peak of CV in the first circle between 1.5 and 1.8 V in Fig.4b,which may be attributed to the formation of SEI film.Obviously,as the cycle proceeds,the cathodic peaks were shifted to higher potentials and the anodic peaks were shifted to lower potentials,indicating an improvement of reversibility of S@α-MnO2.To further investigate the dynamics of S@α-MnO2and S@α-MnO2-PEDOT nanocomposite.As shown in the Fig.4c,the electrochemical impedance spectroscopy(EIS)measurement was emerged.It can be seen that the EIS spectra included a depressed semicircle in the high frequency region and a sloping line(Warburg impedance)in the low frequency region.The semicircle is attributed to the charge-transfer process,which corresponds to the resistance over Li+diffusion through the contacting interface between the electrolyte and active material electrode.The sloping line reflects a semi-infinite Warburg diffusion process[53].The semicircle in the high frequency region is due to the interfacial charge transfer resistance(Rct).It is obviously shown that S@α-MnO2-PEDOT exhibited a lower Rct(ca.160 Ω)than that of S@α-MnO2(ca.220 Ω),revealing thatthe S@α-MnO2-PEDOT composites electrode has excellent electrical conductivity during the charge and discharge process and the presence of PEDOT in favor of electrochemical reaction kinetics.Fig.4d shows the EIS spectra of S@α-MnO2-PEDOT electrode after cycling.After the 50th cycle,the resistance of S@α-MnO2-PEDOT electrode was more than 300 Ω,which was larger than that after the 1st cycle,indicating a lowerelectronic conductivity because of the generation of LiPSs during cycling process.
Fig.4e showsinitialdischarge/charge voltage profiles of S@α-MnO2and S@α-MnO2-PEDOT electrodes(50∶2).It is observed that there were two plateaus at typical discharge process.The lower voltage plateau at ca.2.0 V reflected the further reduction of highorder polysulfides to low-order polysulfides(Li2Sn,n<4)and finally to insoluble lithium sulfides(Li2S2/Li2S).In addition,the upper voltage plateau conformed with the conversion from S8to long chain polysulfides(Li2S8,Li2S6,or Li2S4).However,the charge plateau is related to the transformation from Li2S2/Li2S to Li2S8/S8,which agreed well with the CV analysis.The S@α-MnO2-PEDOT composites electrode(50∶2)showed the higher initial specific discharge capacity(1 672.2 mAh·g-1)than that of S@α-MnO2electrode(868.1 mAh·g-1).Importantly,the smaller voltage platform difference(0.445 V)of S@α-MnO2-PEDOT electrode compared with the voltage platform difference(0.473 V)of S@α-MnO2electrode suggests that the electrochemical reaction reversibility of the S@α-MnO2-PEDOT is higher relative to S@α-MnO2because of the presence of PEDOT.Fig.4f shows initial discharge/charge voltage profiles of S@α-MnO2-PEDOT electrodes with different PEDOT contents.These S@α-MnO2-PEDOT composites electrodes with different PEDOT contents show the initial specific discharge capacity values of 1 487.7(50∶1),1 672.2(50∶2)and 1 465.7 mAh·g-1(50∶4).
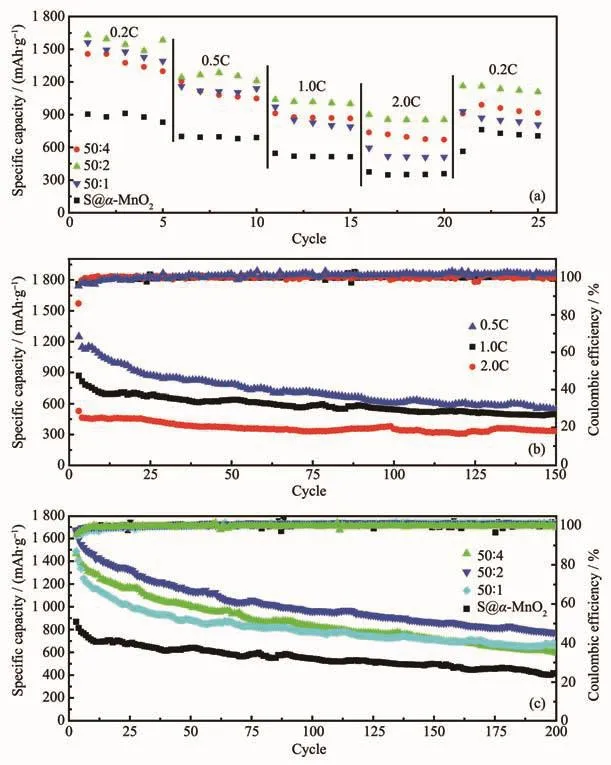
Fig.5 (a)Rate capabilities of S@α-MnO2-PEDOT electrodes with different PEDOT contents;(b)Cycling performances of S@α-MnO2electrode at different current densities;(c)Cycling performances of S@α-MnO2-PEDOT electrodes with different PEDOT contents at the current density of 1.0C
As shown in Fig.5a,the rate capabilities of S@α-MnO2-PEDOT composite electrodeswith different PEDOT contents under different current densities.It was tested by varying current densities from 335(0.2C)to 3 350 mA·g-1(2.0C).The rate capabilities of S@α-MnO2-PEDOT composites electrodes are much higher than that of S@α-MnO2electrode.It was clearly visible that the composite with mass ratio of 50∶2 obtained the best rate performance.The discharge capacities were 1 582.8,1 209.4,1 000.1 and 854.1 mAh·g-1at the current densities of 0.2C,0.5C,1.0C and 2.0C,respectively.When the current density was reduced back to 0.2C,the capacity was recovered to 1 108.6 mAh·g-1,indicating that relatively good stability at different current densities.The cycling performance and coulombic efficiency of S@α-MnO2electrode at the different current densities(0.5C,1C,2C;1C=1 675 mA·g-1)are shown in Fig.5b.The initial discharge capacities were 1 248.7(0.5C),868.1(1.0C)and 529.2(2.0C)mAh·g-1,respectively.And after 150 cycles,the capacities decayed to 549.2,496.8 and 332.9 mAh·g-1at 0.5C,1.0C and 2.0C,respectively.The capacity retentions respectively are 44.0%,57.2%and 62.9%.The coulombic efficiencies were basically above 97%.Apparently,it was important that α-MnO2nanotubes structure could effectively physically restrain the produced polysulfides and also had valid chemicalbond ofMn-S to adsorb polysulfides efficiently,which promoting the utilization of the active material and the reaction kinetics.Fig.5c presents the cycle performances and coulombic efficiencies of S@α-MnO2-PEDOT composite electrodes with different contents of PEDOT at the current densities of 1.0C.Among them,the initial specific discharge capacity values were 1 465.7(50∶4),1 672.2(50∶2),1 487.7(50∶1)and 868.1(50∶0)mAh·g-1,respectively.Obviously,the S@α-MnO2-PEDOT composite(50∶2)has the most remarkable cycle performance.After testing 200 cycles,the specific capacity could still achieve 774.4 mAh·g-1,and the coulombic effciency curve was relatively stable.The excellent electrochemical properties of S@α-MnO2-PEDOT composites were attributed to dual function of MnO2nanotubes and conductive PEDOT buffer layer.In the hybrid composites,MnO2nanotubes and PEDOT layer not only could physically restrict the sulfur/polysulfides inside the inner hollow structure to prevent polysulfides from dissolving into the electrolyte and succedent shuttle effect,but also relieve the volume expansion of S during discharge/charge process and provided a convenient transport channel for ions[54].Moreover,the bonding interaction between polysulfides and MnO2inhibited the chronic seeping of polysulfides and maintained the relative integrity of the electrode.In addition,highly conducting PEDOT layer provided efficient pathways for electron transport.The thicker PEDOT coating,the stronger the conductivity of the composite and more effective at binding sulfur,which resulted in the better electrochemical performance.However,the cycle performances of the S@α-MnO2-PEDOT composite(50∶4)and(50∶1)were similar after 100 cycles.This may be ascribed to the fact that the thicker PEDOT coating has a slight effect on the migration of ions.Therefore,the most superior electrochemical properties of S@α-MnO2-PEDOT composite(50∶2)were presented.The presence of conductive PEDOT buffer layer,effective MnO2nanotubes matrix,bonding interaction between polysulfides and MnO2,and synergistic effect among them yield a robust architecture for enhancing the electrochemical performances of lithium sulfur batteries.
3 Conclusions
In summary,a sulfur cathode has been prepared using hollow α-MnO2nanotubes and exterior PEDOT coating as host materials to immobilize sulfur and capture polysulfides.The hollow α-MnO2nanotube structure not only can accommodate volumetric change ofsulfur,butalso can provide conveniention channels and physical constraint for sulfur/polysulfides.It also can effectively adsorb the polysulfides produced during charge-discharge process by the formation of chemical bond of Mn-S.Moreover,a buffer layer of PEDOT was covered on the surface of MnO2to further enhance the conductivity of the composite cathodes,accelerate electronic transmission,and boost stability of the composites.The S@α-MnO2-PEDOT composites exhibited excellent electrochemical performance.It could reach a capacity of 774.4 mAh·g-1at a current density of 1 675 mA·g-1(1C)after 200 cycles and 854.1 mAh·g-1at a current density of 3 350 mA·g-1(2C).The research provides an effective approach for the preparation of highperformance sulfur cathodes based on tubular transition metal oxides with functionalizing conducting polymer,manifesting a promising pattern to achieve perfect stability,excellent reversibility,fast kinetics,high energy density and long cycle life for Li-S batteries.
Supporting information is available at http://www.wjhxxb.cn
