Spontaneous ovarian hyperstimulation syndrome: Report of two cases
2019-04-25JuanGuiJieZhangWangMingXuLeiMing
Juan Gui, Jie Zhang, Wang-Ming Xu, Lei Ming
Juan Gui, Jie Zhang, Wang-Ming Xu, Lei Ming, Reproductive Center, Renmin Hospital of Wuhan University, Wuhan 430060, Hubei Province China
Juan Gui, Wang-Ming Xu, Lei Ming, Assisted Reproduction and Embryogenesis Clinical Research Center of Hubei Province, Wuhan 430060, Hubei Province, China
Corresponding author: Lei Ming, MD, Professor, Reproductive Center, Renmin Hospital of Wuhan University, 238 Jiefang Road, Wuchang District, Wuhan 430060, Hubei Province,China. rmobg2015@163.com
Abstract
Key words: Ovarian hyperstimulation syndrome; Natural pregnancy; Thawed embryo transfer cycle; Case report
INTRODUCTION
Ovarian hyperstimulation syndrome (OHSS) is an iatrogenic potentially lifethreatening disease, usually a complication during ovulation induction in in vitro fertilization embryo transfer. Risk factors for OHSS include young age, low body mass index, history of polycystic ovarian syndrome, history of previous OHSS, high level of anti-muller hormone (AMH), and higher doses of gonadotropins, etc. The pathogenesis of OHSS includes an increase in the permeability of the capillaries,resulting in a fluid shift from the intravascular space to the extravascular compartments, which is responsible for the development of ascites, sometimes pleural and/or pericardial effusion, hemoconcentration, oliguria, and electrolyte imbalance[1].
Spontaneous OHSS (sOHSS) has the similar clinical presentations as OHSS and is a rare event. During the past 30 years, sOHSS has been reported in women with polycystic ovarian syndrome[2], hypothyroidism[3], hydatidiform mole[4], invasive mole[5], gonadotropin-producing pituitary adenoma[6,7], multiple gestation[8], disturbed liver function[9], and follicle-stimulating hormone receptor (FSHR) gene mutation[10-13].Previous studies have postulated that multiple pregnancy, gestational trophoblastic diseases, hypothyroidism, gonadotropin adenoma, and FSHR gene mutations are responsible for sOHSS[10]. In the present paper, we would report one case of sOHSS in a Chinese woman with a singleton gestation who conceived naturally, and another case of sOHSS with a twin pregnancy in the second trimester after a thawed embryo transfer cycle.
CASE PRESENTATION
Chief complaints
Case 1: A 23-year-old Chinese primigravida conceived spontaneously. She presented with complaints of abdominal distension, dyspnea, nausea, and vomiting.
Case 2: A 34-year-old nulligravida woman was admitted to our hospital complaining of abdominal distension, ascites, abruptly enlarged ovaries, and numbness in the right thigh.
History of present illness
Case 1: The patient’s symptoms started 2 d ago with abdominal distension, dyspnea,nausea, and vomiting.
Case 2: The symptoms started one week ago with abdominal distension, ascites,abruptly enlarged ovaries, and numbness in the right thigh.
History of past illness
Case 1: She had regular menstruation before her pregnancy and had no history of ovulation induction. She also denied the history of polycystic ovarian syndrome and hypothyroidism.
Case 2: She had regular menstruation and normal thyroid function. No previous history of illness or operation was noted. The 34-year-old nulligravida woman was referred to assisted reproductive technology due to azoospermatism of her husband.Super-long protocol was used for controlled ovarian hyperstimulation. She was a hyper-reactive patient with a high level of AMH (7.97 ng/mL) and multiple antral follicle counts (more than 20). The starting dose of recombinant follicle-stimulating hormone (FSH) was 125 IU. After 3 d, the dose was reduced to 112.5 IU because of her hyper-response. The total duration of stimulation was 9 d and then 9000 IU human chorionic gonadotropin (HCG) was used to trigger ovulation. Eighteen oocytes were retrieved. All embryos were frozen because of the high risk of OHSS (enlargement of the ovaries, left 9.2 cm × 6.6 cm, right 8.8 cm × 6.5 cm). Three months later, artificial hormone cycle was conducted to prepare frozen embryo transplantation. Oral estradiol valerate (4 mg/d for 7 d, then 6 mg/d) was used to prepare the endometrium. When the woman’s endometrial lining was 10 mm thick and estradiol was 104.5 pg/mL, two embryos were transferred into the uterine cavity 3 d following the start of progesterone administration. There was no follicular growth during this cycle. Twelve days after the embryo transfer, serum β-HCG level was 1389.9 IU/L. A viable twin pregnancy was confirmed by transvaginal ultrasonography 30 d after transplantation. During antenatal care, both ovaries abruptly became enlarged and ascites was observed by pelvic ultrasonography (left 28.46 cm × 12.36 cm, right 15.01 cm × 11.27 cm, ascites 2.95 cm) at the 17 wk of gestation (Figure 1A-C).
Physical examination
Case 1: After admission, her vital signs such as temperature, pulse, respiration, and blood pressure were normal. Abdominal examination revealed a moderately distended, tender, and tense abdomen with positive shifting dullness on percussion.
Case 2: Her vital signs such as temperature, pulse, respiration, and blood pressure were normal. Abdominal examination revealed a severely distended, tender, and tense abdomen.
Laboratory examinations
Case 1: Laboratory studies showed normal levels of haemoglobin (121 g/L) and haematocrit (37.6%), decreased level of albumin (31.20 g/L, normal > 40 g/L), and increased values of β-HCG (>200000 IU/L) and CA125 (447.3 U/mL, normal < 35 U/mL), as well as slightly elevated liver enzymes, ketonuria (2+), and proteinuria(1+). Examinations regarding blood electrolytes, coagulation profile, renal function,thyroid function, blood glucose, carcinoembryonic antigen, and CA153 concentrations were unremarkable.
Case 2: Laboratory studies revealed the following findings: β-HCG 159226 IU/L, FSH 0.43 mIU/mL, luteinizing hormone (LH) 0.09 mIU/mL, human epididymis protein 4(HE4) 74.30 pmol/L, AFP 158.70 ng/mL, and CA125 441.60 U/mL.
Imaging examinations
Case 1: Abdominal ultrasonographic examinations (Figures 2A-C) revealed a viable intrauterine pregnancy of a size consistent with dates, bilateral ovarian enlargement(the size of the left ovary was 14.92 cm × 7.98 cm and the size of the right ovary was 13.33 cm × 8.11 cm), massive ascites and bilateral cystic adnexal masses (left 4.91 cm ×4.33 cm, right 3.86 cm × 2.97 cm), and hepatolithiasis. Ultrasonographic evaluation of the chest disclosed right pleural effusion (4.8 cm).
Case 2: Ultrasonography showed that there was a 4.9 cm pleural effusion in the left thorax and no abnormality was found in the kidneys or adrenal glands.
Further diagnostic work-up
Case 2: Ovarian cyst puncture was performed, the fluid was macroscopically light yellow and clear, and a few mesenchymal cells and lymphocytes were observed under a microscope.
FINAL DIAGNOSIS
Case 1
Due to the patient’s medical history and laboratory findings, a diagnosis of sOHSS was considered.
Case 2
A provisional diagnosis of OHSS was made.
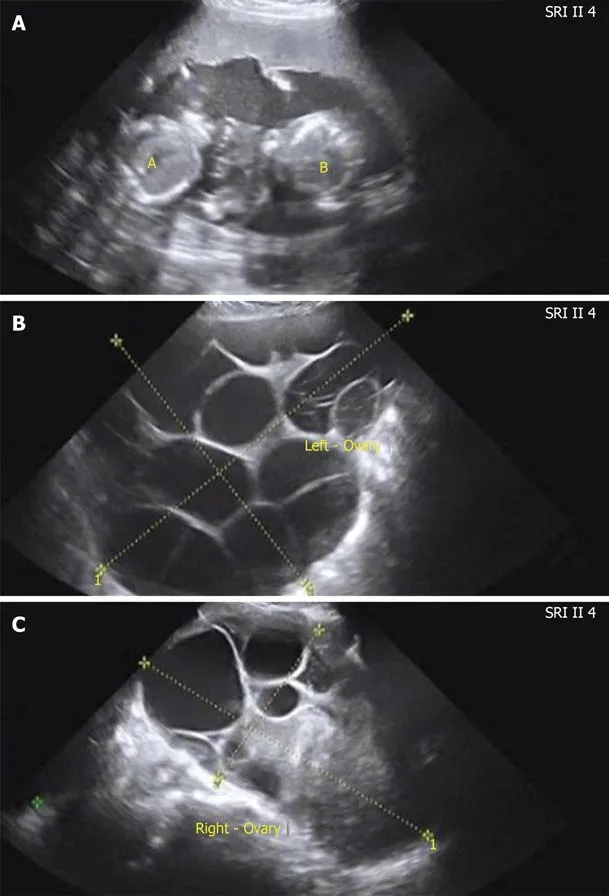
Figure 1 Abdominal ultrasonography of the fetus, ovary, and ascites in case 2. A: Ultrasonography of the fetus showed a twin pregnancy at the 17th gestational week; B: Ultrasonography of the left ovary showed that the size of the left ovary was 28.46 cm × 12.36 cm; C: Ultrasonography of the right ovary showed that the size of the right ovary was right 15.01 cm × 11.27 cm.
TREATMENT
Case 1
Pleural paracentesis was performed to aspirate 400-500 mL/d of pleural effusion to reduce the patient’s discomfort. She was admitted for monitoring and supportive therapy. She received complex conservative therapy at our ward, including intravenous fluid substitution with hydroxyethyl starch 130/0.4 sodium chloride and compound dextran 40 (both 500 mL/d), nutrition support, gastro- and hepatoprotective therapy (rabeprazole sodium 40 mg/d and reduced glutathione 2.4 g/d)every day during hospitalization. She also received intravenous administration of human albumin at 100 mL/d for 3 d.
Case 2
She received intravenous administration of colloid and albumin, and antiinflammatory therapy.

Figure 2 Abdominal ultrasonography of the fetus, ovary, and ascites in case 1. A: Ultrasonography of the fetus showed a single live fetus at 12 + 5 gestational weeks; B: Ultrasonography of the ovaries showed bilateral ovarian enlargement (the size of the left ovary was 14.92 cm × 7.98 cm and the size of the right ovary was 13.33 cm × 8.11 cm) and bilateral cystic adnexal masses (left 4.91 cm × 4.33 cm, right 3.86 cm × 2.97 cm); C: Ultrasonography showed massive ascites.
OUTCOME AND FOLLOW UP
Case 1
The patient responded well to our treatment and was discharged 2 wk after admission. Her abdominal distension was relieved, and she had no dyspnea in the supine position, only with mild cough when she left the hospital. The pregnancy proceeded normally, and the patient had spontaneous onset labor at 40 wk of gestation and underwent an uncomplicated vaginal delivery of a male newborn.
Case 2
After the treatment, her abdominal distension was relieved 10 d later. The volume of the ovaries was gradually reduced during the pregnancy. At 28 wk of gestation, the size of the left ovary was 10.5 cm × 6.3 cm, and the size of the right ovary was 11.5 cm× 7.9 cm. A caesarean section was performed at 35 wk and 1 d of gestation. Bilateral ovarian enlargement was noted (Figure 3). The pregnancy resulted in the live birth of a female baby weighing 2350 g, and a male baby weighing 2150 g.

Figure 3 Enlarged ovary during cesarean section in case 2. Enlarged ovary during operation.
DISCUSSION
sOHSS is extremely rare in the natural pregnancy or after thawed embryo transfer.Previous researchers have divided it into four subtypes according to its clinical manifestations and FSHR mutations[10]. Type I refers to the mutated FSHR cases with normal HCG, thyroid stimulating hormone (TSH), and FSH levels, which can lead to recurrent sOHSS. Several kinds of FSHR gene mutations have been identified, such as Asp567Asn, Thr449Ala, Iso554Thr, Thr449Ile, Asp567Gly, Ser128Tyr, Ala307Thr,Arg634His, and Thr449Asn. Type II corresponds to cases secondary to high HCG levels, such as hydatidiform mole and multiple pregnancies. Type III is related to hypothyroidism with high TSH levels, and levothyroxine could relieve the symptoms to some extent[3]. Type IV is related to gonadotrophin adenomas secreting FSH or LH.In recent years, studies have found that FSHR gene mutations can be activated not only by FSH, but also by the glycoprotein hormones having the same β subunit[14]such as TSH, LH, and HCG, increasing its sensitivity to HCG produced during natural pregnancy and leading to the occurrence of OHSS during natural pregnancy,and this kind of sOHSS has familial disposition and recurrent characteristics[15].Alternative theories might include the presence of variant HCG or variant TSH that exhibits higher biological activity or granulosa cells in an autocrine environment that is more sensitive to FSHR stimulation.
Our first patient presented a singleton natural pregnancy with high HCG values but normal levels of TSH. The second patient showed a twin pregnancy with a history of OHSS and normal TSH values, which fulfills the criteria of type II OHSS. However,we did not detect whether there was a mutation in FSHR, so it was uncertain whether our cases belonged to type I.
Unlike the iatrogenic OHSS, sOHSS usually occurs between 8-14 wk of pregnancy[15]. During the pregnancy, HCG usually peaks between the 8th and 10th gestational week and declines thereafter. The second case is uncommon with regard to three aspects: (1) OHSS occurred in a pregnancy following a thawed embryo transfer cycle; (2) Onset of OHSS was not earlier than 17 wk of gestation; and (3) The syndrome continued until delivery with caesarean section. This case highlights the fact that females with a history of OHSS may have a higher risk for sOHSS and should be closely monitored. A twin pregnancy characterized by elevated HCG levels might be one reason for sOHSS. Single embryo transfer may decrease the risk of the development of severe OHSS in cases in which freezing of all embryos is used to prevent OHSS.
OHSS is a self-limiting disease and could be predicted by high-risk factors during ovulation induction. Effective prevention measures can reduce the risk of moderate to severe iatrogenic OHSS. However, sOHSS cannot be predicted. Therefore, early diagnosis and early management of sOHSS are particularly important. In the diagnostic procedure, the first-line investigation for the diagnosis is pelvic ultrasonography. It is a cost-efficient and non-invasive examination which can reflect the states of the fetus, ovaries, and ascites. Based on the comprehensive understanding of sOHSS, we should also give an eye on ovarian malignant tumors to avoid misdiagnosis and missed diagnosis. The ovaries of woman with sOHSS are mostly bilateral polycystic and the capsule wall is thin. In comparison, an ovarian malignant tumor is characterized by a unilateral solid-cystic cyst with a thick capsule wall. Because CA125 increases during the first trimester, it is not accurate for the diagnosis of ovarian tumor during pregnancy. Currently, conservative treatment is the primary management option, and surgery is only needed for cases of ovarian rupture, ovarian torsion, intra-abdominal hemorrhage, or ectopic pregnancy[13].Pregnancy with sOHSS is a rare disease, and is not easy to detect in the early phase, so obstetricians and gynaecologists should have a comprehensive understanding of sOHSS to make correct diagnosis and treatment, avoiding premature termination of pregnancy and unnecessary surgery damaging the fertility of the patients.
CONCLUSION
sOHSS is extremely rare. It is always confused with ovarian tumors with abdominal distension, ascites, and enlarged ovaries. The first line investigation for the diagnosis is pelvic ultrasonography. Since sOHSS cannot be predicted, patients with a history of OHSS should be closely monitored. Conservative treatment is the primary option of management. Single embryo transfer may decrease the risk of developing severe OHSS in assisted reproductive cases.
ACKNOWLEDGEMENTS
We are grateful to these two patients for providing the information and support, as well as providing the written informed consent allowing us to publish their data. We thank Rui-Hao Wang (from Department of Neurology, University of Erlangen-Nuremberg, Germany) for his assistance in improving the quality of written English.
杂志排行
World Journal of Clinical Cases的其它文章
- Polyunsaturated fatty acids and DNA methylation in colorectal cancer
- lmpact of resection margins on long-term survival after pancreaticoduodenectomy for pancreatic head carcinoma
- Arthroscopy combined with unicondylar knee arthroplasty for treatment of isolated unicompartmental knee arthritis: A long-term comparison
- lntact, pie-crusting and repairing the posterior cruciate ligament in posterior cruciate ligament-retaining total knee arthroplasty: A 5-year follow-up
- Community-acquired pneumonia complicated by rhabdomyolysis: A clinical analysis of 11 cases
- Dissection and ligation of the lateral circumflex femoral artery is not necessary when using the direct anterior approach for total hip arthroplasty
