Successful treatment with hysteroscopy for infertility due to isthmocele and hydrometra secondary to cesarean section:A case report
2019-04-17LuisPablopezRiveroMiguelJaimesFelipeCamargoEstherpezBayghen
Luis Pablo López Rivero,Miguel Jaimes,Felipe Camargo,Esther López-Bayghen
Abstract
Key words:In vitro fertilization;Isthmocele;Hydrometra;Case report
INTRODUCTION
The routine practice of cesarean sections has increased around the world[1].Mexico has one of the highest cesarean rates,reaching 45% of total births[2].Cesarean sections are associated with many complications,such as placenta accreta,ectopic pregnancy,uterine rupture,and defective scarring in hysteroscopy[3].
Isthmoceles are gap scars that develop in the myometrium due to the healing process associated with cesarean sections.In Mexico,the incidence rate of isthmocele is unknown;however,other countries’ rates range between 19% and 56%[3].Isthmocele is associated with detrimental gynecological symptoms,such as abnormal uterine bleeding,pelvic pain,and infertility[4].With respect to infertility,an accumulation of blood within the isthmocele can cause a retrograde passage of blood to the uterine cavity,instigating inflammation and an adverse environment for embryo implantation[4].This accumulation of blood at the endometrium can be visualized during a transvaginal ultrasound,where an image suggestive of hydrometra can be seen.
In many cases,the diagnosis of an isthmocele is incidental in patients who suffer from intermenstrual “staining”,dyspareunia,dysmenorrhea,or secondary infertility.Current diagnostic methods consist of transvaginal ultrasound,hysterosonography,and video hysteroscopy.With transvaginal ultrasound,the isthmocele appears as an anechoic zone with an isosceles triangle shape.The management of the isthmocele is not standardized,and correction typically involves invasive procedures[5].Here,we present a case of secondary infertility,where the diagnosis of isthmocele was indicated by the recurrent development of hydrometra.After isthmocele correction,the hydrometra was abrogated,and embryo transfer ended in a successful pregnancy.
CASE PRESENTATION
Chief complaints
A 37-year-old woman (Body-Mass Index:23.62 kg/m2) was attending the Ingenes Institute in México City for secondary infertility.
History of present illness
The patient has been trying to get pregnant for 24 mo with negative results,using only natural methods to conceive.We proposed anin vitrofertilization (IVF) protocol,complimented with pre-implantation genetic diagnosis (PGD).
History of past illness
Three years prior to her attending Ingenes,she had one previous pregnancy,which was resolved by a cesarean section after placental detachment.Afterward,she presented abnormal uterine bleeding with bloody or brown vaginal discharge,severe pelvic pain,dyspareunia,and urinary discomfort eight months after the cesarean.
Personal and family history
She had no other medical complications and was not taking any medications.No causes of male infertility were found in her partner.
Physical examination upon admission
The patient underwent a standard course of controlled ovarian stimulation (Depot GnRH agonist,Cetrotide 0.25 mg daily dose,Merck,Darmstadt,Germany).In the immediate days after controlled ovarian stimulation started (9 d),we observed the formation of hydrometra.The patient was given a single dose of Triptorelin (0.2 mg;Gospeptyl daily Ferring Pharmaceuticals,Saint-Prex,Switzerland).Stimulation was prolonged until the diameter of leading follicles was > 18 mm (18-22 mm).Then,recombinant human chorionic gonadotropin (hCG) (Choragon 1000 IU,Ferring Pharmaceuticals,Saint-Prex,Switzerland) was administered,and oocytes were retrieved after 36 h with ultrasound guidance.All 14-18 mm follicles were aspirated,and 20 ova were collected.It was decided to proceed to fertilization and culture.The ova were fertilized by intracytoplasmic sperm injection,and six embryos developed(2AB,2BB,and 2BC Inner cell mass/Trophoblast quality).With the embryos that reached day 5,a trophectoderm biopsy was collected for PGD,and then the embryos were frozen.After PGD,four euploid embryos were considered for implantation;however,it was decided to postpone implantation for one month to allow for endometrial preparation.
The endometrial preparation was carried out with the application of anin situagonist prior to the Luteal phase (Triptorelin 3.75 mg,Gonspeptyl daily,Ferring) and transdermal application of 17-β-estradiol (Evorel 50).On day 10 of endometrial preparation,the formation of hydrometra was again evident,so it was decided to cancel the endometrial preparation cycle and perform an endometrial cavity evaluation-closing of any ostia by hysteroscopy with the suspicion of a possible hydrosalpinx.The proximal closing of tubal ostia was performed without any complications,finding a normal cavity.A second endometrial preparation was performed with the same protocol.On day 10 of the endometrial preparation,the formation of hydrometra was again evident,so we decided to cancel the cycle,and treat the patient with progestin (Utrogestan,100 mg every 12 h,5 d,SEID,Barcelona,Spain).A new endometrial preparation with estradiol valerate (Primogyn,Bayer Health) was tried using 6 mg/d as the maximum dose,and on the 13th d of preparation,the hydrometra formation was again evident.
Laboratory examinations
None.
Imaging examinations
A transvaginal ultrasound was performed,and in addition to hydrometra,the presence of an isthmocele was located at the anterior wall of the uterine isthmus.Its base was 6.6 mm and height was 6.1 mm,indicating the presence of a second-degree isthmocele (Figure 1).Re-evaluation of a previous hysteroscopy video was performed,where a thorough evaluation of the defect area was not performed,probably due to the absence of characteristic symptoms.
FINAL DIAGNOSIS
Presence of a second-degree isthmocele leading to hydrometra.
TREATMENT
The patient agreed to a surgical hysteroscopy,to correct the area containing the isthmocele.The procedure was performed by finding the myocell sac with neovascularization and mucosanguineous content.The procedure consisted of leveling the isthmocele area with a monopolar resectoscope loop (Monopolar Karls Storz 24 Fr.with lens telescope of 2.9 mm and 12),with subsequent application of monopolar ablation energy in the bed of the isthmocele using a roll ball electrode.The procedure was performed with 1.5% glycine as distension medium and guided at times by transabdominal ultrasound (procedure time:25 min,see Figure 2).
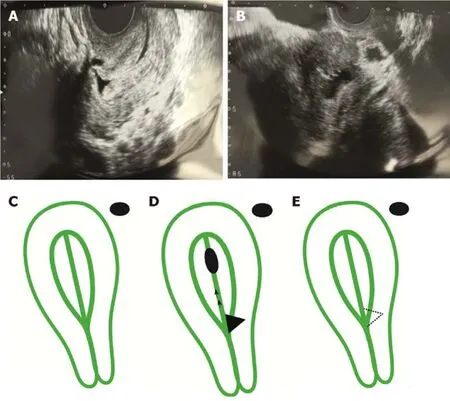
Figure1 Transvaginal ultrasonographic images.
OUTCOME AND FOLLOW-UP
Postoperative monitoring was performed for one week.Afterward,endometrial preparation was resumed with the transdermal application of 17-β-estradiol (Evorel 50,Janssen Pharmaceuticals).This time,there was no hydrometra formation,and embryo transfer could be completed.Two embryos were transferred,and 9 d after the transfer,implantation was confirmed by serum β-hCG (180 mU/mL).We were able to visualize the presence of a gestational sac in the uterine fundus with the presence of an embryo and positive embryocardia at week 6 and normal development parameters at week 12.4 (Figure 3).Pregnancy was followed with regular visits;the patient did not present any complications,and the product was born in good health (3.3 kg).
DISCUSSION
With caesarian sections becoming a more common practice,the rate of isthmocele complications is also augmented.Although isthmocele diagnosis mainly occurs incidentally,identifying factors associated with isthmocele could improve diagnostic rates.Here,we show that recurrent hydrometra during IVF was associated with an unknown isthmocele.The isthmocele was discovered on a secondary,more sensitive ultrasound.
Hydrometra is associated with pregnancy complications and failure[4].Here,we document a case report that demonstrates that hydrometra is an indicator of an isthmocele.Moreover,correction of the isthmocele attenuated the hydrometra,suggesting that the former was the cause of the latter.Hydrometra,the accumulation of mucus or watery fluid in the uterine cavity,would be a predictor as for isthmocele were shown to accumulate blood,leading to a retrograde passage of blood to the uterine cavity[4],promoting a suboptimal state for pregnancy.
Currently,there are only invasive procedures to correct an isthmocele.Using resection and ablation,the isthmocele was corrected,and the persistent hydrometra was attenuated.Afterward,the patient achieved implantation,and the product was delivered with no other complication.This does suggest that,under the conditions presented with this case report,the invasive correction of the isthmocele does not alter the female reproduction potential;however,more studies are required.
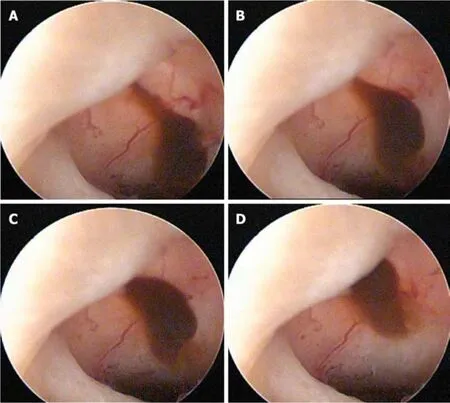
Figure2 lsthmocele at the time of surgery.
Hydrometra could be a prognostic indicator for isthmocele.The patient came to our IVF facility due to secondary infertility and,because of our examination process,the hydrometra was discovered.This presents a problem for women who try to get pregnant,who are not using IVF nor can afford specialized care.Since isthmocele is a possible cause of infertility,examining for isthmocele or hydrometra should be performed if the patient has had a previous caesarian and problems to get a subsequent pregnancy.
CONCLUSION
This is the first report of a successful isthmocele treatment in a patient with secondary infertility,where an isthmocele associated with persistent hydrometra formation and infertility.Physicians,Gynecologists,Human Reproduction Biologists,and Gynecological Endoscopists should keep in mind that isthmocele is a possible cause of secondary infertility,also possibly linked to the formation of persistent hydrometra.
ACKNOWLEDGMENTS
We would like to express our gratitude to the participants of the study,to Adina Neumann and Paola Olvera for their technical assistance in PGT,to the members of the Ingenes-IVF Laboratory,and to Dr.Leonardo M Porchia for his contributions in preparing the manuscript.
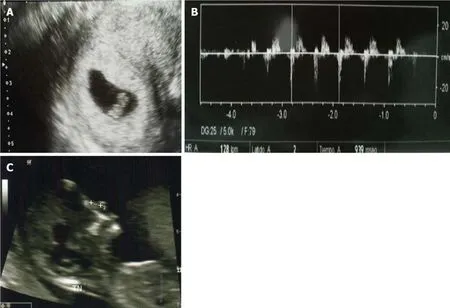
Figure3 Pregnancy ultrasounds.
杂志排行
World Journal of Clinical Cases的其它文章
- Effects of apoptosis on liver aging
- Liver involvement in the drug reaction,eosinophilia,and systemic symptoms syndrome
- Surgical method choice and coincidence rate of pathological diagnoses in transduodenal ampullectomy:A retrospective case series study and review of the literature
- lndividualized minimally invasive treatment for adult testicular hydrocele:A pilot study
- Successful totally transthoracic echocardiography guided transcatheter device closure of atrial septal defect in pregnant women
- Cardiac amyloidosis:A case report and review of literature
