Successful totally transthoracic echocardiography guided transcatheter device closure of atrial septal defect in pregnant women
2019-04-17QiangChenHuaCaoGuiCanZhangLiangWanChenFanXu
Qiang Chen,Hua Cao,Gui-Can Zhang,Liang-Wan Chen,Fan Xu
Abstract
Key words: Congenital heart disease;Septal defects;Cardiac intervention;Pregnant
INTRODUCTION
Atrial septal defect (ASD) is one of the most common congenital heart diseases[1].For various reasons,some female patients are diagnosed with ASD during pregnancy.Some of these patients were unable to continue pregnancy because of their cardiac dysfunction.Closing the ASD is necessary for such patients to have improved cardiac function and continue the pregnancy[2].Traditional treatment includes surgical repair using a pericardial patch,which needs cardiopulmonary bypass,and a sternotomy with greater associated maternal and fetal risks[3].Transcatheter device closure guided by echocardiography and fluoroscopy has been another method used for these patients recently;however,the largest drawback of this procedure is X-ray radiation exposure,which may not be appropriate for pregnant women[4,5].To overcome these shortcomings,we proposed a method in pregnant women in which the transcatheter technique was totally guided by transthoracic echocardiography (TTE).There are rare reports of the safety and clinical improvement associated with transcatheter closure of ASD during pregnancy[6-8].This report describes the details and outcomes of pregnant women who underwent successful totally TTE guided transcatheter closure of ASD.
MATERIALS AND METHODS
The present study was approved by the Ethics Committee of Fujian Medical University,China and adhered to the tenets of the Declaration of Helsinki.Additionally,all patients were counseled on the potential maternal and fetal risks associated with the pregnancy,and all signed a consent form to undergo transcatheter device closure of ASD.
Six patients were enrolled in our cardiac center between January 2015 and August 2017.The patients were aged 23 to 32 years (26.3 ± 2.5 years) and were between 20 and 26 wk of gestation.Their weights ranged from 70 to 85 kg (75.5 ± 5.2 kg).All device closure procedures were performed between 20 and 26 wk of gestation.All patients were diagnosed as having secundum ASD by TTE.The size of the ASD,as measured by TTE,ranged from 16 to 28 mm (mean 21.7 ± 4.3 mm).The inclusion and exclusion criteria were the same as the routine criteria used for transcatheter device closure of ASD.
The New York Heart Association (NYHA) functional class was I before pregnancy in all patients,and it worsened as the pregnancy progressed.By the second trimester,all patients were experiencing chest tightness,palpitations,shortness of breath,and exercise intolerance.Color Doppler ultrasound revealed hemodynamically significant left-to-right shunts and significant right chamber enlargement.All patients had mildto-moderate pulmonary hypertension.No other X-ray studies were performed in these patients.Routine examinations included standard electrocardiogram and blood tests.Ultrasound of the fetus demonstrated normal fetal development at gestation.Multidisciplinary team meetings were conducted to discuss patient management,including physicians from cardiology,obstetrics,cardiac surgery,and anesthesiology departments,to make a decision as to whether to perform transcatheter closure totally guided by TTE in this study.
In this study,a domestic ASD occluder (manufactured by Shan Dong Visee Medical Apparatus Co.Ltd.of China) was used,which was similar to the Amplatzer ASD occluder and less expensive than the latter.It was made from an alloy of nickel and titanium.The diameter of the ASD and the circumferential margins adjacent to the defect were adequately assessed upon TTE.The size of the occluder was chosen to allow for a 4 to 6 mm excess of the maximum ASD diameter (Figure 1).
Local anesthesia (2% lidocaine was injected into the puncture site of the right groin)was used in all patients.The echo probe was placed at the xiphoid,apex,and parasternal side to guide the entire procedure.Intravenous heparin was administered to all patients,and the activated clotting time was monitored and maintained at a time longer than 250 s during the procedure.The right femoral vein was punctured with a sheath into the vein.Then,a multifunctional catheter and a guidewire were inserted into the venous sheath and advanced through the inferior vena cava to the right atrium.Under the guidance of real-time TTE,the multifunctional catheter and a guidewire were passed through the ASD to the left atrium (Figure 2A).Then,the multifunctional catheter was withdrawn (Figure 2B).A delivery sheath was inserted along the guidewire into the left atrium,and the inner core and the guidewire were withdrawn together.From various views of TTE,the tip of the sheath was located in the left atrium (Figure 2C);then,an appropriate occluder was delivered carefully through the sheath by pushing a rod.The left and right atrial discs of the occluder were released,in turn,and the occluder was successfully implanted (Figure 2D).The occluder’s position and the possible occurrence of a residual shunt were reassessed by TTE.Aspirin was used as an antithrombotic therapy post-procedure in all patients[9].Fetal heart monitoring was completed three times a day during the hospital stay.
RESULTS
Successful occlusion was achieved in all patients (clinical data are shown in Table 1).The operative time ranged from 30 to 50 min (41.7 ± 7.5 min),the occluder size ranged from 20 to 32 mm (26.7 ± 4.3 mm),and the length of hospital stay was 2-3 d (mean 2.2± 0.4 d).The overall complete closure rate was 100%.There were no fatal complications related to occluder dislodgement,residual shunt,complete atrioventricular block,or thrombosis-related diseases.There were no deaths or serious cardiovascular related complications during hospitalization.During the procedure,one patient experienced transient arrhythmia that spontaneously recovered in a short period of time.
Over the follow-up period (3 mo to 2 years),the patients were regularly assessed by physical examination,TTE,and ECG.Symptoms improved significantly with respect to the pre-operative status.The complete closure rate was 100%.There were no episodes of endocarditis,thromboembolism,device disruption,heart valves distortion,or permanent rhythm disturbances.All of the patients experienced significant improvement in their symptoms and underwent vaginal delivery between 36 and 38 wk of gestation.All the infants (two males and four females) were healthy,with no cardiac or other congenital anomalies.Their birth weight ranged from 3.2 to 4.2 kg and Apgar scores after vaginal delivery ranged from 7 to 10.No cardiopulmonary resuscitation or hospitalization in NICU was needed in all infants.
DISCUSSION
ASD is one of the most common congenital acyanotic heart diseases,with many patients remaining asymptomatic until adulthood.Because of the lack of medical knowledge and poor economic or health conditions,many women are diagnosed with ASD during pregnancy or even when heart failure occurs,especially in rural and remote mountainous areas[10].During pregnancy,the maternal circulating blood volume may increase by an average of 50% by late pregnancy;in addition,hemodilution,increasing tissue fluid and decreasing systemic vascular resistance,may occur,substantially impacting cardiac output and heart rate.The corresponding right ventricular volume overload as a result of an ASD is aggravated during pregnancy,and its continued development,to a serious extent,may trigger ventricular failure[11].A retrospective study showed that surgical closure of ASD before pregnancy was recommended,even in cases of apparently good hemodynamic conditions,with low rates of surgical complications in spite of the higher incidence of obstetrical problems[2].Considering these factors,the risks for pregnant women with ASD are clearly much higher than those of the rest of the population.
Closure of ASD during pregnancy requires a thorough clinical assessment because some patients can tolerate normal pregnancy and vaginal delivery well.For some patients with large and hemodynamically significant defects,progressive symptoms may develop during pregnancy,requiring further intervention[12].Yapet al[13]concluded that women with an unrepaired ASD were at higher risk of neonatal events than women with a repaired ASD.Another paper confirmed that women with an unrepaired ASD were at higher risk of pre-eclampsia,small-for-gestational-age births,and fetal mortality than pregnant women in the general population[2].The indications for intervention include heart failure,pulmonary hypertension with severe hemodynamic compromise,NYHA class > II,and recurrent stroke prior to pregnancy[14-16].
It is necessary to close ASD during preschool age.Both surgical patch repair and transcatheter device closure have been proved satisfactory in terms of short- and longterm results in most patients with ASD.Traditional surgical thoracotomy patch repair has been successfully performed during pregnancy in terms of successful maternal and fetal outcomes.However,cardiopulmonary bypass may lead to some inevitable and unpredictable consequences for both mothers and fetuses,including systemic inflammatory responses and multiple organ dysfunction.In addition,the large surgical scar is unacceptable for many women[17,18].With the development and application of various new occluders in recent years,transcatheter device closure of ASD has gained popularity worldwide and may be considered a standard treatment.Transcatheter device closure has many attractive advantages including the absence of scar,a lack of pain,and a short hospital stay[4,5].However,its greatest drawbacks are that both doctors and patients are exposed to X-ray radiation,which may lead to radiation-related damage.There have been sporadic reports focused on transcatheter device closure of ASD in pregnant women,guided by fluoroscopy and echocardiography[6-8].In a previous report,we performed transcatheter device closure of ASD guided totally by TTE,and we used this procedure in pregnant women with local anesthesia[9].
Ionizing radiation was used in the process of transcatheter device closure of ASD in the previous method,where there was exposure to both the mother and the doctors.According to the literature,radiation use is associated with congenital malformations in the fetus and mental and growth retardation or embryonic death,as well as an increased rate of leukemia,brain tumors,and childhood malignancies during the first trimester[19-21].Some reports tried to confirm a reduction of ionizing radiation by choosing the appropriate time that it can be used safely in pregnancy,with very low exposure to the mother and the fetus[22,23].However,we continue to believe that radiological procedures should be avoided as much as possible during pregnancy.Based on this idea and our previous experience,we used TTE instead of fluoroscopy in these procedures and obtained satisfactory results that can eliminate the risks of radiation.The use of local anesthesia,endotracheal intubation,and general anesthesia were not needed.Our results suggest that TTE should play an important role during this procedure as the only guiding tool,although it is well known that the accuracy of TTE is lower than that of transesophageal echocardiography (TEE).Many experts had proved that TTE can be used as a reliable tool for the measurement of the diameter of ASD and for guidance during device closure by skilled hands,taking the place of TEE in some circumstances[24].Azhar reported that TTE had the same efficacy in transcatheter closure of ASD compared with TEE and had superior safety,including a significant reduction of procedural time and fluoroscopy times as well as less use of general anesthesia[25].Panet al[26]concluded their experience of using TTE guidance as the only imaging tool for the process of percutaneous ASD closure.Their method avoided fluoroscopy and endotracheal intubation and was associated with satisfactory results and lower costs[26].We also used TTE to guide transthoracic device closure of ASD in a previous study and obtained satisfactory results.We found that TTE achieved satisfactory imaging that was comparable to that of TEE in the general population.Local anesthesia eliminated the need for the moderate-deep sedation or general anesthesia required for TEE.
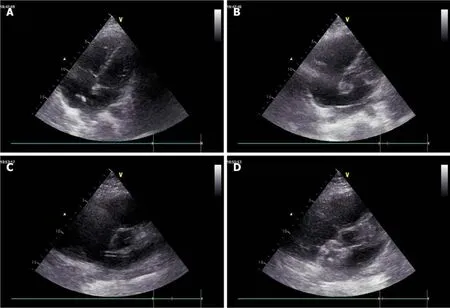
Figure2 Transthoracic echocardiography showing the process of transcatheter device closure of atrial septal defect.
Therefore,we used the same method in pregnant women as Pan’s procedure.First,we inserted a multifunctional catheter and a guidewire into the inferior vena cava.The tip of the catheter was usually clearly displayed in the subxiphoid acoustic window by TTE.Then,the catheter was advanced into the right atrium.Through the apical four chamber view,the parasternal long axis view,and the subxiphoid acoustic window,the tip of the catheter was usually not easy to locate.There have been difficulties in this section without fluoroscopy.We marked the length of the catheter that measured the distance from the puncture point to the apex of the heart.The insertion should be careful and as slow as possible to effectively prevent cardiac rupture.During the procedure,constant communication with the sonologist can help the operator adjust the catheter.The catheter should be dynamically rotated and adjusted to make the tip clear in the TTE;then,it can be advanced into the left atrium.The subsequent steps were similar to those of the routine method.
In our case series,device closure was successful in all patients,and the closure rate was 100%.All patients obtained the same clinical result as the other routine transcatheter procedure.The most striking difference was that this approach did not require radiation,making it more attractive for pregnant women.Our technique can simplify the procedure,and the learning curve was short.With the skilled operators,the average operative time was no more than 1 h,which was acceptable to most operators and patients.However,the operator should be familiar with intracardiac structure,especially the 3D spatial structure of cardiac anatomy,which can compensate for the shortage of 2D imaging of TTE.
There were some shortcomings in our study.The number of cases was small.In addition,our method was not a widely used method.Most doctors perform transtheter device closure of ASD with X-ray assistance.There are few cardiac centersusing this procedure.

Table1 Clinical data of patients in this study
In conclusion,this small case series demonstrated that totally TTE guided transcatheter closure of ASD in pregnant patients was achieved safely and effectively.This method avoids surgical scars,cardiopulmonary bypass,and X-ray exposure,and provides satisfactory outcomes for mothers and fetuses.
ACKNOWLEDGMENTS
We highly acknowledge the contribution by the participating doctors:Xu-Dong Sun,Feng Lin,Qi-Min Wang,Zhong-Yao Huang,Han-Fan Qiu,Xiao-Fu Dai,Xue-Shan Huang,Hui Zhang,and Zeng-Chun Wang.Also,we wish to extend our gratitude to Xiu-Jun Wang and her colleagues.
ARTICLE HIGHLIGHTS
Research background
Transcatheter device closure of atrial septal defect (ASD) guided by fluoroscopy and/or transesophageal echocardiography is a mature technology.Little study has focused on whether the technology can be guided totally by transthoracic echocardiography (TTE),even in pregnant women with ASD.
Research motivation
To evaluate the safety and efficacy of totally TTE guided transcatheter device closure of ASD in pregnant women.
Research objectives
We want to prove that our procedure may be performed as another choice for pregnant women with ASD.
Research methods
Six pregnant women (gestational age 20-26 wk) with ASD underwent transcatheter device closure totally guided by TTE at our cardiac center from January 2015 to August 2017.A routine transcatheter procedure without fluoroscopy or intubation and a domestic occluder were used in this study.
Research results
All six patients had successful closure with good clinical results,and the overall immediate complete closure rate was 100%.The size of the occluder deployed ranged from 20 to 32 mm(26.7 ± 4.3 mm),the procedure time ranged from 30 to 50 min (41.7 ± 7.5 min),and the length of hospital stay was 2-3 d (mean 2.2 ± 0.4 d).There were no serious cardiovascular related complications.
Research conclusions
Totally TTE guided transcatheter device closure of ASD in pregnant women may be safe and effective.
Research perspectives
Transcatheter device closure of ASD totally guided by TTE may be an alternative treatment for pregnant women with ASD.
杂志排行
World Journal of Clinical Cases的其它文章
- Effects of apoptosis on liver aging
- Liver involvement in the drug reaction,eosinophilia,and systemic symptoms syndrome
- Surgical method choice and coincidence rate of pathological diagnoses in transduodenal ampullectomy:A retrospective case series study and review of the literature
- lndividualized minimally invasive treatment for adult testicular hydrocele:A pilot study
- Cardiac amyloidosis:A case report and review of literature
- Successful treatment with hysteroscopy for infertility due to isthmocele and hydrometra secondary to cesarean section:A case report
