A New Species of the Genus Trimeresurus from Southwest China(Squamata: Viperidae)
2019-03-27ZeningCHENLiangZHANGJingsongSHIYezhongTANGYuhongGUOZhaobinSONGandLiDING
Zening CHEN, Liang ZHANG, Jingsong SHI, Yezhong TANG, Yuhong GUO, Zhaobin SONG and Li DING*
1 Chengdu Institute of Biology, Chinese Academy of Sciences, Chengdu 610041, Sichuan, China
2 Key Laboratory of Bio-Resource and Eco-Environment of Ministry of Education, College of Life Sciences, Sichuan University, Chengdu 610065, Sichuan, China
3 Guangdong Key Laboratory of Animal Conservation and Resource Utilization, Guangdong Public Laboratory of Wild Animal Conservation and Utilization, Guangdong Institute of Applied Biological Resources, Guangzhou 510260,Guangdong, China
4 Key Laboratory of Vertebrate Evolution and Human Origins of Chinese Academy of Sciences, Institute of Vertebrate Paleontology and Paleoanthropology, Chinese Academy of Sciences, Beijing 100044, China
5 School of Chemistry and Life sciences, Guizhou Education University, Guiyang 550018, Guizhou, China
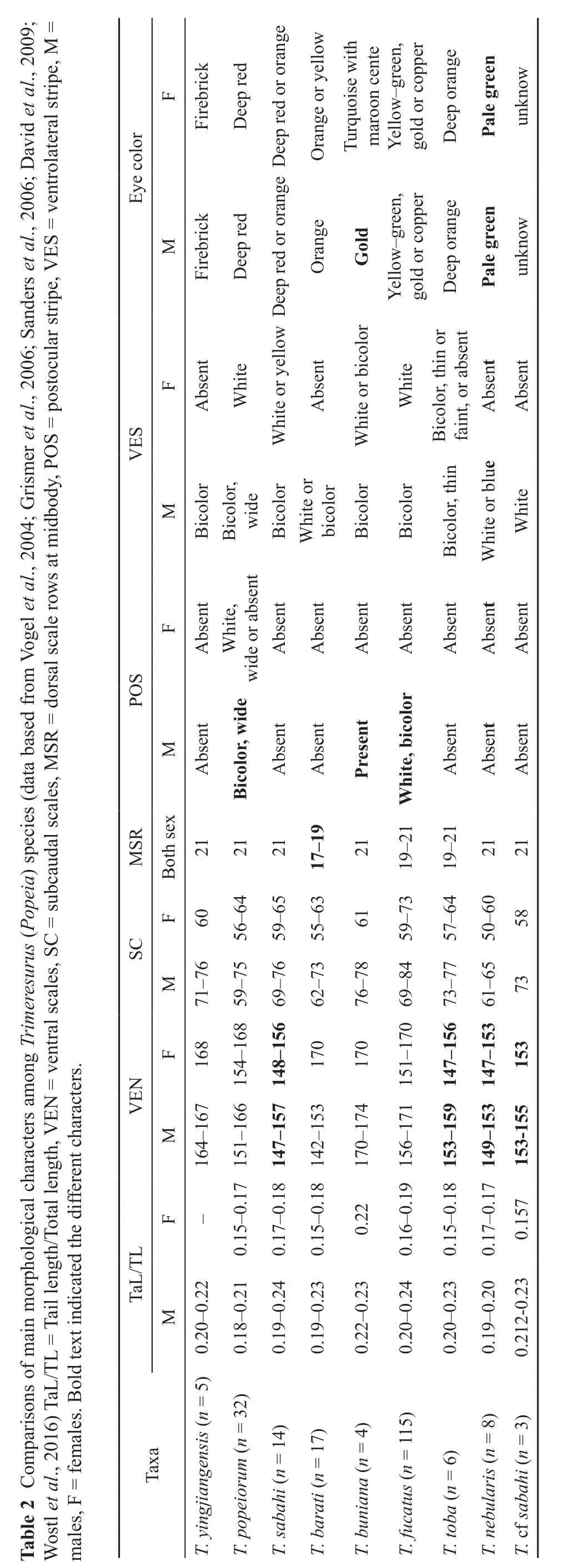
Abstract Species from the Trimeresurus popeiorum complex (Subgenus: Popeia) is a very complex group. T.popeiorum is the only Popeia species known from China. During the past two years, five adult Popeia specimens(4 males, 1 female) were collected from Yingjiang County, Southern Yunnan, China. Molecular, morphological and ecological data show distinct differences from known species, herein we describe these specimens as a new species Trimeresurus yingjiangensis sp. nov Chen, Ding, Shi and Zhang, 2018. Morphologically, the new species distinct from other Popeia species by a combination of following characters: (1) dorsal body olive drab,without cross bands on the scales; (2) a conspicuous bicolor ventrolateral stripe present on each side of males, fi rst row of dorsal scales fi rebrick with a white ellipse dot on posterior upper part in male, these strips absent in females; (3) eyes fi rebrick in both gender;(4) suboculars separated from 3rd upper labial by one scale on each side; (5) ventrals 164-168 (n = 5); (6) MSR 21.
Keywords Popeia, morphology, phylogenetics, geographical isolation, Trimeresurus yingjiangensis sp. nov.
1. Introduction
Asian green pitvipers (Trimeresurus) is a very complex group, generally with a green throughout body, tail red on back, and most with lateral lines and postocular stripes in males, while in females these lateral lines and postocular stripes are often absent (Creer et al., 2004; Gumprecht et al., 2004; Malhotra and Thorpe, 2004; Vogel et al.,2004; Guo et al., 2015). There are 51 species in the genus Trimeresurus recorded to date in Asia, and the diversity of this genus may still be underestimated (Uetz et al., 2018;
Wostl et al., 2016). Due to their similar body pattern and scales among species and widespread distribution of few species, the species in this genus is difficult to be identified. Based on morphological and molecular characters, genus Popeia was split from Trimeresurus by Malhotra and Thorpe (2004). Subsequently David et al.(2009) and Sumontha et al. (2011) relegated Popeia as the status of a subgenus, respectively. However, broad scale phylogenetic analysis places them in a single monophyletic clade and are considered as Trimeresurus(Figueroa et al., 2016; Pyron et al., 2013). For the simplicity purpose, we will use Trimeresurus instead of Trimeresurus (Popeia) when referring the species of subgenus Popeia in following text. As well as for the subspecies of T. sabahi, we will omit “sabahi” at the middle.
Vogel et al. (2004) made a new comb of the T.popeiorum complex based on morphological characters,and described two new species: T. fucatus and T.nebularis. Grismer et al. (2006) reported T. buniana as a new species referred to this subgenus. Then David et al.(2009) described T. toba from Indonesia. Thus, subgenus Popeia was consisted of six species: T. popeiorum, T.fucatus, T. nebularis, T. sabahi, T. barati and T. toba.
A recent research argued that T. fucatus, T. nebularis,T. sabahi, T. barati and T. toba might be barely a single species, T. sabahi (Wostl et al., 2016), because differences in the morphological characters between species could be eliminated when adding new specimens with medium measures. In addition, their molecular characters could not be discriminated. However, considering the morphological distinctiveness, genetic diversity and ecologically distinct, Mulcahy et al. (2017) believed that T. popeiorum, T. nebularis, T. sabahi should be consider as distinct species, as well as the specimens from Phangt-nga, Thailand (B467) should be recognize as T.phuketensis and the specimens from Phetburi, Thailand(B52 and B34) and Tanintharyi Div.; Kawthaung Dist.Myanmar should be recognized as a new species T. sp(Mulcahy et al., 2017). Additionally, those remaining controversial and closely related populations of T. sabahi(T. sabahi fucatus, T. sabahi barati, T. sabahi buniana and T. sabahi toba) should be recognized as subspecies of T. sabahi. Indeed, the concept of subspecies is very helpful in solving the current chaotic situation, but this seems to be a compromise for the controversial situation.Therefore, the taxonomy within this genus remains controversial.
So far, although Trimeresurus is widespread in many places of Asia (Davie et al, 2009; Malhotra and Thorpe,2004; Mulcahy et al., 2017; Vogel et al., 2004; Wostl et al., 2016), T. popeiorum is the only Popeia species distributed in China (Guo et al., 2015). However,during our animal surveys in Yunnan Province in 2017,we collected five specimens of subgenus Popeia, they are similar in morphology to T. popeiorum, but can be distinguished from T. popeiorum by the absence of postocular streaks, differences in scales and body patterns.Herein, based on the molecular and morphological data by including all available specimens available in GenBank,we proposed it as a new species.
2. Materials and Methods
2.1. Morphological analysis We examined three male specimens preserved in the museum of Chengdu Institute of Biology (CIB), Chinese Academy of Sciences, one male specimen preserved in the museum of Institute of Vertebrate Paleontology and Paleoanthropology (IVPP),Chinese Academy of Sciences and one female specimen preserved in the museum of Guangdong Institute of Applied Biological Resources (GIABR).
Body and tail length were measured with a tape ruler to the nearest 1 mm: total length (TL) from the tip of snout to the tip of tail; snout-vent length (SVL) from the tip of snout to anterior margin of cloaca; tail length(TaL) from posterior margin of cloaca to the tip of tail.Other measurements were conducted with a digital caliper to the nearest 0.1 mm: head length (HL) from the snout tip to the posterior margin of the mandible; head width (HW) at the maximal widest part of the head; head height (HH) at the maximal highest part of the head;the eye horizontal diameter (ED); Ratio of tail length to total length (TaL/TL). The same observer to avoid inter-observer bias collected all data. The following comparative scales and maxillary teeth were counted:the dorsal scale rows (DSR); the ventrals (VEN); the subcaudals (SC); the supralabials (SL); the infralabials(INF) and Cephalic scales (scales on a line between the middle of supraoculars, Cep); the eye vertical diameter(VED); distance lower eye margin-edge of the lip(DEL). Morphological measurements are listed in Table 1. Information on some morphological characters were also obtained from written accounts from Pope and Pope(1933), Taylor et al (1958), Vogel et al. (2004), Grismer et al. (2006), Sanders et al. (2006), David et al. (2009),Wostl et al. (2016). Color description reference wiki color-coding.
2.2. Molecular Phylogenetic analysis Genomic DNA was extracted from the collected tissue samples at the CIB. Four specimens from Yingjiang County, Yunnan Province were used for DNA extraction. Four fragments of the mitochondrial were specifically amplified,including cytochrome b and one nuclear gene fragment(cyt b, L14910/H16064, 1100bp, Burbrink, et al. 2000),NADH dehydrogenase subunit 4 (ND4, ND4/Leu, 670bp,Parkinson et al., 2000; Shi et al., 2017), 12S ribosomal RNA (12S, 12SFPh/RVal, 659 bp, Knight and Mindell,1993) and 16S ribosomal RNA (16S, 16sF-L/16sR-H,500bp, Parkinson et al., 2000), Oocyte maturation factor Mos gene (C-mos, S77/S78, 587bp, Pyron et al., 2009).

DE 4.31-9.21 2.31 2.41 HH 7.11-5.9 8.9 7.11 WH 2.22-1.81 7.91 8.02 LH 5.13-8.92 4.13 6.33/LaTLT 402.0 361.0 991.0 902.0 912.0 LT 468 948 247 358 688 LaT 671 931 841 871 491 LVS 886 017 495 576 296 peC 1111111121 FNI 21/2121/21 21/21 21/21 21/21 LS 01/01 01/01 11/01 01/01 01/9”-“CS 3706672717 htiw d NEV 461 861 461 761 661 etoned era atad g ROD 51-12-12 51-12-12 51-12-12 51-12-12 51-12-12 nissi xeS M F M M M M .von .ps sisnegnaijgniy suytilacoL nannuY ,gnaijgniY nannuY ,gnaijgniY nannuY ,gnaijgniY nannuY ,gnaijgniY nannuY ,gnaijgniY ruseremirT fo )mm 1.0( stneevreserP rehcuoV BIC 1010707102LD RBAIG 108102glgnypstLZ BIC 201070102LD BIC 301070102LD PPVI 1762VO merusaeM 1 elbaT axaT suruseremirT sisnegnaijgniy sisnegnaijgniy.T sisnegnaijgniy.T sisnegnaijgniy.T sisnegnaijgniy.T
PCR ampli fi cations were performed in 25 μl reactions by using the following cycling conditions: initial denaturation for 2min at 95 °C, followed by 35 cycles:denaturation at 94 °C for 40 s, annealing at different temperatures (48.5 °C for cyt b, 56 °C for ND4 and C-mos, 52 °C for 12S and 16S) for 25 s, elongation at 72 °C for 15 s, and then fi nalized with elongation step of 2min at 72 °C, with the PTC-100 thermal cycler (BioRad,USA). The products were purified by using the DNA Agarose Gel Extraction Kit (Omega, USA) according to the manufacturer’s instructions. Purified PCR products were sequenced using the same PCR primers. Sequencing was completed by Beijing Qingke New Industry Biotechnology Co., Ltd. Sequence data were uploaded to GenBank, available accession numbers showed in Table S1.
All sequences were aligned with other retrieved sequences on the same gene loci, respectively, by using software MEGA 7 (Kumar et al., 2016), after converting the file format with the software BioEdit (Alzohairy,2011). Our dataset totally contained 38 specimens, each with 2 567 base pairs. Partition finder 2.1.1 under BIC(Lanfear et al., 2012) identified the optimal models of sequence evolution for each partition. Therefore, dataset was combined into three partitions. The evolution models of each partition combination were as follows: partition 1:16S 1, 12S 3, 12S 1, 16S 3, 16S 2, 12S 2, cyt b 1, ND4, 3,HKY+I+G, 1424 bp; partition 2: ND4 1, cyt b 2, HKY+I,572bp; partition 3: cyt b 3, ND4 2, GTR+G, 571bp.
General time reversible (GTR+I+G) model, the most probable substitution model for the uncorrected cyt b p-distance matrix, was applied on MEGA 7 (Kumar et al., 2016). Phylogenetic relationships derived from the combined gene fragments were performed based on Bayesian Inference (BI), which was conducted by using MrBayes 3.2 (Ronquist et al., 2012), and all searches consist of three heated chains and a single cold chain.Three independent iterations with each comprising two runs of 20 000 000 generations are applied, sampling every 1 000 generations. The parameter estimates were plotted against the generation. The first 25 percent of the samples were discarded as burning. ML trees were constructed by using the program RaxML v7.2.6(Stamatakis, 2006) in GTRGAMMA model with 1 000 fast bootstrap repeats. Outgroups were chosen based on close phylogenetic relationship between taxa (Mulcahy et al., 2017).
3. Result
Taxonomic position
Class: Reptilia, Order: Squamata, Suborder: Serpentes,Family: Viperidae
Trimeresurus yingjiangensis sp. nov. Chen, Ding, Shi and Zhang, 2018.
ZooBank accession:BD3E2F08-1C1E-40E7-AEBAE37380268F06
Etymology. The specific name yingjiangensis refers to the location of type specimens, Yingjiang Country,Yunnan Province, China. The common name is suggested as “Yingjiang green pitviper” in English and “Yíng jiāng zhú yè qīng (盈江竹叶青 )” in Chinese.
Holotype. DL2017070101 (CIB), adult male, collected from the Heihe Village, Kachang Town, Yingjiang County, Yunnan Province (24.782° N, 97.878° E, 1 112 m a.s.l) by Li DING on 19 July 2017 (Figure 1).
Paratypes (four specimens). ZL-tspynglg-2018-01(allotype, GIABR) adult female, collected from Heping Village, Tongbiguan Town, Yingjiang County, Yunnan Province (24.584° N, 97.738° E, 1 200 m) by Jian XU(Figure 1 and Figure 2). DL201070102 (CIB), adult male,and DL201070103 (CIB), adult male, collected by Li DING at the same time as Holotype. OV2671 (IVPP),collected from the Yingjiang County (24.734° N, 97.843°E, 1 074 m) by Jingsong SHI on 6 September 2017(Figure 2).
Diagnosis. Trimeresurus yingjiangensis sp. nov. is assigned to Popeia group by hemipenes morphology(Malhotra and Thorpe, 2004), differ with its congeners by a combination of following characters: (1) dorsal body olive drab,without cross bands on the scales; (2)a conspicuous bicolor ventrolateral stripe present on each side of males, first row of dorsal scales firebrick with a white ellipse dot on posterior upper part in male,these strips absent in females; (3) the eyes firebrick in both gender; tail red, mottled with green laterally, and the ventrolateral stripes discontinuous on the tail; (4)hemipenes long, reaching 23rdto 25thSC, forked opposite 5-6th SC (n = 4), bifurcated near the base and the sulcus spermaticus split from the apex to basal without spines;(5) 21 DSR at middle body, moderately keeled; VEN =164-168 (n = 5), SC = 60-76 (n = 5); Sexual dimorphism,the female has more ventrals and fewer subcaudals than males; (6) tail long, with ratios of TaL/TL between 0.199 and 0.219 in male.

Figure 1 Trimeresurus yingjiangensis sp. nov in life: A and B, holotype, male: DL2017070101, photo by Li DING; C allotype, female: ZL-tspynglg-2018-01, photo by Liang ZHANG; D, paratype, male: DL201070102, has a weak and short post ocular streak, photographed by Li DING.
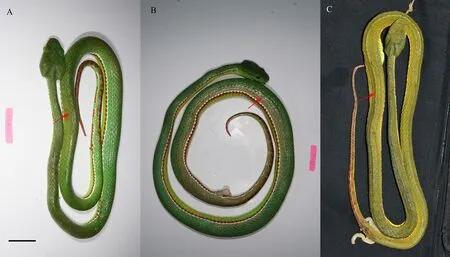
Figure 2 Three of the paratypes of T. yingjiangensis sp. nov.: DL201070102 (A), picture took after the specimen euthanized by freezing,body color start to change from olive drab to green yellow (photographed by Shengchao SHI); DL201070103 (B), picture took after the specimen injecting alcohol, body color start to change from olive drab to olive (photographed by Shengchao SHI); OV2671 (C), picture took after the specimen conserved in 80% ethanol after seven months, body color becomes olive (photographed by Jingsong SHI). The red arrow indicates the color change. Scale: 25 mm
Comparison with other species. T. yingjiangensis sp.nov. is distinct from T. popeiorum (Lectotype, Pope and Pope, 1933) by the following characters: (1) First row of dorsal scales on each side firebrick with a white ellipse dot on posterior upper part in males vs. “brown with yellow tip” in males; (2) lowest quarter of second row white in males vs. “yellow below keel”; (3) suboculars separated from 3rdupper labial by one scale on each side vs. “two scales on each side”; (4) upper part of second upper labial separated from nasal by two small scales smaller than nostril on either side vs. “separated from nasal by two large scales on either side”.
T. yingjiangensis sp. nov. is distinct from T. nebularis by the following characters: (1) body olive drab, upper lips green with white tip, vs. body bright green with blue tones and blue upper lips; (2) eyes firebrick vs.pale green; (3) ventrolateral stripes bicolor in males vs.uniformly white or blue; (4) in males: ventrals 164-167(n = 4), vs. 149-153 (n = 8), subcaudals 71-76 (n = 4) vs.61-65 (n = 8).
T. yingjiangensis sp. nov. is different from T. sabahi, T.cf sabahi (from Sumatra), T. fucatus, T. barati and T. toba by following characters: (1) MSR 21 (vs. MSR 19 in T.barati); (2) dorsal color was olive drab with conspicuous bicolor ventrolateral stripes and no crossbars (vs. dorsal color was green with irregular rusty or reddish-brown dorsal crossbands and white dots on the vertebral scales in T. fucatus); (3) the ventrolateral stripe bicolor in males(vs. white in males of T. cf sabahi from Sumatra, and white in males of T. toba); (4) the temporal scales smaller than those in T. toba; (5) no ventrolateral stripe present on females (vs. white or yellow ventrolateral stripe present on females of T. sabahi); (6) the eyes fi rebrick (vs. yellow in T. sabahi, and orange in T. barati); (7) VEN 164-168,n = 5 (vs. VEN 142-158, n = 17 in T. barati, 147-157,n = 14, in T. sabahi, and 153-155, n = 3, in Sumatra T. cf sabahi). The comparisons of main morphological characters are summarized in Table 2.
Description of the holotype. Body elongated; head triangular, wide at base, length 1.4 times as long as width,clearly distinct from neck; snout moderate, accounting for 23.4% of HL, 1.7 times as long as ED, eyes large, VED/DEL ratio 0.8, pupil vertical in life.
SVL: 688 mm; TaL: 176 mm; TL: 864 mm; TaL/TL:0.204.
VEN: 164; SC: 73, paired, plus one terminal scale; anal shield entire.DSR: 21-21-15 scales, moderately keeled, first row smooth.
Hemipenes long, reaching to 23-24 SC, forked opposite 5-6th plate, no spines (Figure 3F)
Rostral overall trapezoidal, lower margin of rostral nearly 3 timess wider than upper margin, height two third of width at base, obliquely truncated when seen from lateral side; nasal large, undivided, dorsal margin forming part of canthus rostralis; internasals distinct,elongate about 2.3 times as long as wide, separated by a scale of one-half size of each internasal; 4/4 canthal scales bordering the canthus rostralis between the internasal and corresponding supraocular, slightly larger than adjacent snout scales; 1 elongated loreal between upper preocular and nasal; 2 upper preoculars above the loreal pit; elongated and in contact with the loreal; 2/1 postoculars; 1 entire, long and elongated supraocular on left side, about 3.9 times as long as wide, about 1.2 times as wide as the internasals, supraocular on right side broken into 2 scales, the former scale twice larger than the rear scale; supraocular indented on their inner margin by the upper head scales; scales on upper snout surface smooth, juxtaposed, irregular in shape, enlarged, with 6 snout scales on a line between the scale separating the internasals and a line connecting the anterior margins of eyes; cephalic scales small, irregular, smooth, 11 Cep in a line between supraoculars; occipital scales fl at and feebly keeled; temporals small, subequal, in 4/4 rows, smooth;1 thin, elongated, subocular crescent-like, surrounded by 12/11 scales; 10 supralabials on both sides; the 1stsupralabial triangular; the 2nd, high, 1.8 times as high as wide, upper part concave, forming the anterior border of loreal pit, separated from nasal by two small scales on either side of the size about or smaller than nostrils, the upper of the two scales inverted trapezoidal while the lower scale long rectangular and smaller; the 3rdwidest,1.2 times as wide as high, separated from subocular by one small scale on either side; the 4thirregularly quadrangular/pentagonal, smaller than the 3rd, right below the pupil and separated from subocular at least by one scale on both sides. the 5thsmaller than the 4th, separated from subocular by at least 2/1 scales, others in contact with the first row of temporals; 12 infralabials on both sides, the first pair of infralabials in contact with each other and the first three pairs in contact with the chin shields.
?
In life, the dorsal color was olive drab with conspicuous bicolor ventrolateral stripes and no crossbars; first row of DSR on each side firebrick with an white ellipse dots on posterior upper part; lowest quarter of second row white, from neck to over half of tail; gradually disconnected on tail; upper half of 2ndrow to 11throw of DSR edged with sky blue and mottled with irregular sky blue dots. Ventral surface was yellow green,ventrals start from 101thto anal scale edged with fi rebrick on both sides. Dorsal tail heavily mottled with dark red blotches, the dark red blotches contiguous posteriorly; tip of tail Indian red on dorsal while dark salmon on ventral.Head olive drab, without postocular streak; eyes fi rebrick,with a narrow golden edge around pupil; upper lips green yellow, supralabials and infralabials green yellow edged with white and molted with light cyan.
Intraspecific morphological variation. All five specimens were collected from the same location with similar body coloration and body shape with only one sample (DL201070102, Figure 1) with a short postocular streak posterior to eyes; 10 supralabials in general but one sample (OV2671) with only 9 and 11 supralabials on two sides, respectively; ventrals 164-167 in males, while 168 in females; subcaudals 71-76 in males, while 60 in females.
Molecular phylogeny. Considering that there are very little nuclear gene data about genus Trimeresurus in NCBI, and the sequence is rarely from the same specimen,we only listed the C-mos gene accession numbers for future research, but did not use this gene fragments when building the gene tree. Result showed that the two consistent phylogenetic trees were achieved by using Bayesian Inference (BI) and Maximum likelihood(ML) methods (Figure 4), respectively. The topological structure of the phylogenetic trees are approximately identical with those of earlier studies (Guo et al., 2015;Kate et al., 2004; Malhotra et al., 2000; Malhotra et al.,2004; Mulcahy et al., 2017). All subgune species should be consider as six clades including T. sabahi complex,T. yingjiangensis sp. nov., T. popeiorum, T. nebularis,T. phuketensis and T. sp. Additionally, all of the four specimens of T. yingjiangensis sp. nov. performs as a strongly supported monophyletic group and then shares a common ancestor with T. sabahi and other Popeia species. T. yingjiangensis sp. nov. was placed sibling to T.sabahi complex with high support (100%).

Figure 3 Trimeresurus yingjiangensis sp. nov. holotype: DL2017070101. Head of view: A, dorsal; B, ventral; C and D, latera (left and right);E, lateral view of the body (left side); F, hemipenes (photographed by Shengchao SHI). Scale: 5 mm
We support the newest classification by Mulcahy et al. (2017), the species from west Thai (AB52, B34; clade F) as well as T. phuketensis and T. nebularis should be considered as distinct species, but the specimens of T. sp(clade F) performs as a weak supported (27%, indicated by red in the phylogenetic trees). This is different from the previous research by Mulcahy et al. (2017), in their study, the popularity is 60%. This may be due to the different data we use. Additionally, in the clade A, the support rates between the subspecies are generally low(indicated by red in the phylogenetic trees), especially the T. barati and T. fucatus do not performs as a monophyletic group, the phylogenetic relation between T. barati and T. buniana could not be discriminated. Same as this the p-distances in the clade A are low too, ranging from 0.011 to 0.031 (Table 3). The greatest disparity is between a specimen of T. fucata (A203) and a specimen of T.barati (UTA-R61639). In comparison, the uncorrected p-distances within a samples of T. yingjiangensis sp. nov.and a samples of T. sp from Myanmar (CAS247754)are greater than those between other congeneric species(0.059 for cyt b, Table 3). Therefore, our results con fi rm clearly that the individuals from the Yingjiang Country,Yunnan Province as a distinct species.
Distribution and habitat. Trimeresurus yingjiangensis sp. nov. was found in Yingjiang Country, Yunnan Province. At its typical locality, this species prefers to inhabit in streams about 1 000 meters elevation, and perch on branches waiting for prey (Figure 5).
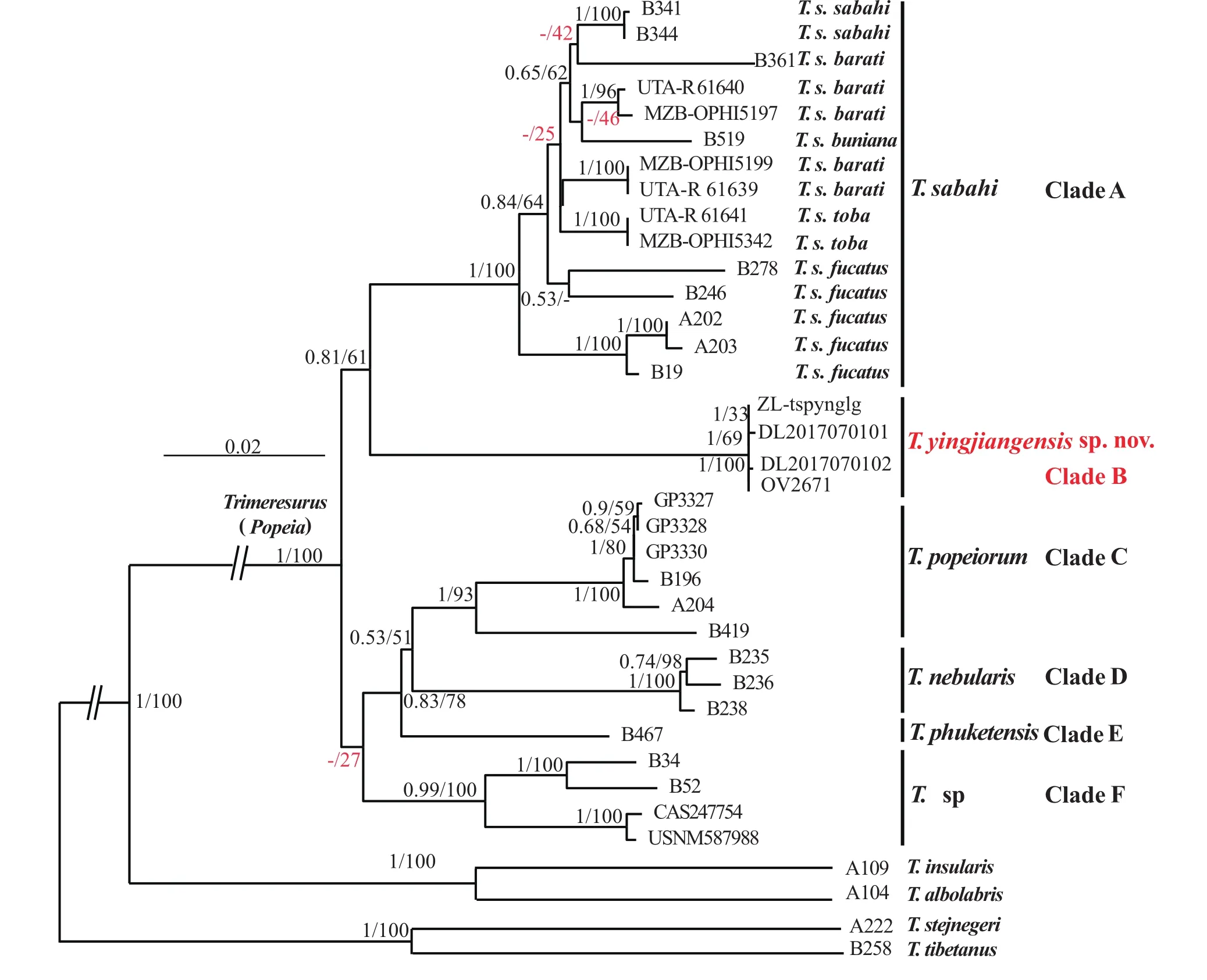
Figure 4 Bayesian phylogenetic tree of the genus Trimeresurus based on cytb, ND4, 12S and 16S. The major cladogenetic events ML/BI bootstrap and posterior probabilities values were presented (the ones lower than 50% are displayed as “-”). The clade of new species was marked in red. T. tibetanus and T. stejnegeri were used as outgroup.
4. Discussion
Being in very complex genus, many species of Asian green pitvipers (Trimeresurus) shared similar morphological characteristics (Gumprecht et al., 2004;Guo et al., 2015; Malhotra and Thorpe, 2004; Mulcahy et al., 2017; Vogel et al., 2004), so that some validated species were often diagnosed as one species. For example,T. gumprechti and T. popeiorum distributed in Yunnan,China were recognized as T. stejnegeri before Guo (2010,2015). Therefore, combined morphological and molecular characters and even ecological traits could be helpful to solve the classification problem of Trimeresurus(Figueroa et al., 2016; Malhotra, 2004; Mulcahy et al.,2017; Sanders et al., 2006; Pyron et al., 2013; Wostl et al., 2016).

Table 3 Uncorrected p-distances between Trimeresurus (Popeia) sp. based on 731 base pairs from the mitochondrial genes cyt b. Specimens T. yingjiangensis sp. nov. are in bold font.

Figure 5 Habitat of Trimeresurus yingjiangensis sp. nov., Heping Village, Tongbiguan Town, Yingjiang County, Yunnan Province (24.584° N,97.738° E, 1 200 m a.s.l.), photographed by Jian XU.
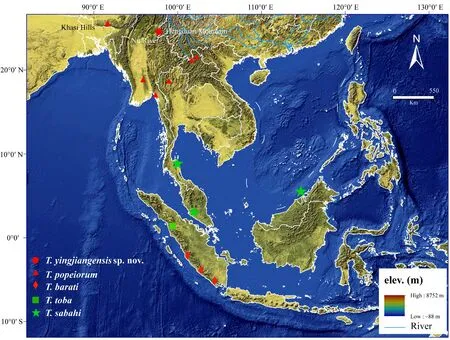
Figure 6 Distribution map of the T. yingjiangensis sp. nov., T. popeiorum, T. barati, T. toba and T. sabahi. The samples which are included in this study are labeled with the codes (constructed by ArcMap 10.3)
T. yingjiangensis sp. nov. inhabits in Yingjiang County,Southern Yunnan Province which locates geographically in the southeastern-most corner of the Tibetan Plateau and the southern-most tip of the Hengduan Mountains. There are numerous mountains and rivers in this area, which restrict gene flows, and lead to regional divergences of species and generate biodiversity (Huang et al., 2009;Peng et al., 2015). Although 31 species of snakes have been recorded in this region to date, the local biodiversity of reptiles may be largely underestimated (Vogel and Luo, 2011; Peng et al., 2015). T. yingjiangensis sp. nov is the only species of Popeia was found in this area, and,nearby, T. popeiorum is distributed in Mengla County,the vicinity of Yingjiang County. Due to the geographical isolations between two closed counties (e.g. Lancang River, Nu River and Gaoligong Mountain, Figure 6),there are evident differences in both morphological characters and mtDNA sequences between two species.The same situation also can be found when comparing T. yingjiangensis sp. nov with the other species. Thus,we believed that T. yingjiangensis sp. nov should be considered as a distinct species.
Acknowledgements This research was financially supported by grants from the National Natural Science Foundation of China (NSFC31460558). We thank Jian XU for providing samples and taking photos and thank Shengchao SHI for taking photos.
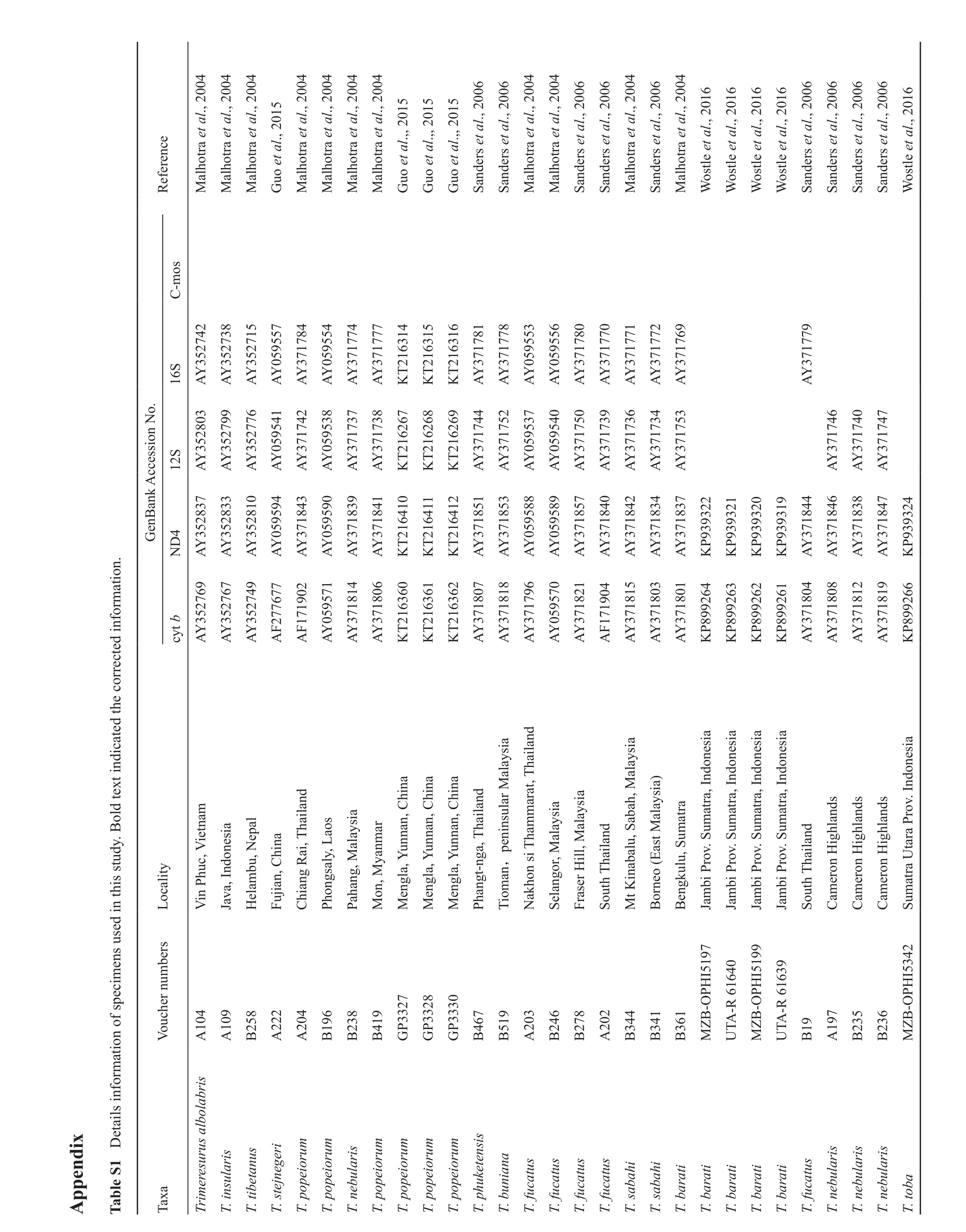
i x p e n d A p i c a t e d t h e c o r r e c t e d i n f o r m a t i o n.t i n d o l d t e x e t a i l s i n f o r m a t i o n o f s p e c i m e n s u s e d i n t h i s s t u d y. B 1 D T a b l e S 6 M a l h o t r a e t a l., 2 0 0 4 M a l h o t r a e t a l., 2 0 0 4 M a l h o t r a e t a l., 2 0 0 4 6 6 6 6 6 6 6 6 5 0 1 5 0 1 5 0 1 5 0 1 6 l., 2 0 1 6 l., 2 0 1 6 l., 2 0 1 6 l., 2 0 1 6 l., 2 G u o e t a l., 2 0 1 M a l h o t r a e t a l., 2 0 0 4 M a l h o t r a e t a l., 2 0 0 4 M a l h o t r a e t a l., 2 0 0 4 M a l h o t r a e t a l., 2 0 0 4 G u o e t a l.,, 2 G u o e t a l.,, 2 G u o e t a l.,, 2 S a n d e r s e t a l., 2 0 0 S a n d e r s e t a l., 2 0 0 M a l h o t r a e t a l., 2 0 0 4 M a l h o t r a e t a l., 2 0 0 4 S a n d e r s e t a l., 2 0 0 S a n d e r s e t a l., 2 0 0 M a l h o t r a e t a l., 2 0 0 4 S a n d e r s e t a l., 2 0 0 M a l h o t r a e t a l., 2 0 0 4 W o s t l e e t a W o s t l e e t a W o s t l e e t a W o s t l e e t a S a n d e r s e t a l., 2 0 0 S a n d e r s e t a l., 2 0 0 S a n d e r s e t a l., 2 0 0 S a n d e r s e t a l., 2 0 0 W o s t l e e t a C-m o s 1 4 o.R e f e r e n c e 1 5 1 6 S 3 5 2 7 4 2 3 5 2 7 3 8 3 5 2 7 1 5 0 5 9 5 5 7 3 7 1 7 8 4 0 5 9 5 5 4 3 7 1 7 7 4 3 7 1 7 7 7 2 1 6 3 2 1 6 3 2 1 6 3 3 7 1 7 8 1 3 7 1 7 7 8 0 5 9 5 5 3 0 5 9 5 5 6 3 7 1 7 8 0 3 7 1 7 7 0 3 7 1 7 7 1 3 7 1 7 7 2 3 7 1 7 6 9 3 7 1 7 7 9 1 6 A Y A Y A Y A Y A Y A Y A Y A Y K T K T K T A Y A Y A Y A Y A Y A Y A Y A Y A Y A Y n N 6 7 6 8 6 9 a n k A c c e s s i o S 3 5 2 8 0 3 3 5 2 7 9 9 3 5 2 7 7 6 0 5 9 5 4 1 3 7 1 7 4 2 0 5 9 5 3 8 3 7 1 7 3 7 3 7 1 7 3 8 2 1 6 2 2 1 6 2 2 1 6 2 3 7 1 7 4 4 3 7 1 7 5 2 0 5 9 5 3 7 0 5 9 5 4 0 3 7 1 7 5 0 3 7 1 7 3 9 3 7 1 7 3 6 3 7 1 7 3 4 3 7 1 7 5 3 3 7 1 7 4 6 3 7 1 7 4 0 3 7 1 7 4 7 1 2 A Y A Y A Y A Y A Y A Y A Y A Y K T K T K T A Y A Y A Y A Y A Y A Y A Y A Y A Y A Y A Y A Y G e n B 1 0 1 1 1 2 2 2 2 1 2 0 1 9 2 4 4 3 5 2 8 3 7 3 5 2 8 3 3 3 5 2 8 1 0 0 5 9 5 9 4 3 7 1 8 4 3 0 5 9 5 9 0 3 7 1 8 3 9 3 7 1 8 4 1 2 1 6 4 2 1 6 4 2 1 6 4 3 7 1 8 5 1 3 7 1 8 5 3 0 5 9 5 8 8 0 5 9 5 8 9 3 7 1 8 5 7 3 7 1 8 4 0 3 7 1 8 4 2 3 7 1 8 3 4 3 7 1 8 3 7 9 3 9 3 9 3 9 3 9 3 9 3 9 3 9 3 3 7 1 8 4 4 3 7 1 8 4 6 3 7 1 8 3 8 3 7 1 8 4 7 9 3 9 3 N D A Y A Y A Y A Y A Y A Y A Y A Y K T K T K T A Y A Y A Y A Y A Y A Y A Y A Y A Y K P K P K P K P A Y A Y A Y A Y K P 3 5 2 7 6 9 7 7 6 2 0 4 3 5 2 7 6 7 0 2 3 7 1 8 1 4 2 7 7 6 6 4 6 3 3 7 1 8 0 6 6 2 6 1 2 1 6 3 6 6 3 5 2 7 4 9 6 1 A F c y t b A Y 6 0 A Y 1 7 1 9 2 1 6 3 A F A Y 2 1 6 3 0 5 9 5 7 1 3 7 1 8 0 7 3 7 1 8 1 8 3 7 1 7 9 6 0 5 9 5 7 0 3 7 1 8 2 1 1 7 1 9 3 7 1 8 1 5 3 7 1 8 0 3 3 7 1 8 0 1 8 9 9 2 8 9 9 2 8 9 9 2 8 9 9 2 3 7 1 8 0 4 3 7 1 8 0 8 3 7 1 8 1 2 3 7 1 8 1 9 8 9 9 2 A Y A Y A Y K T K T K T A Y A Y A Y A Y A Y A F A Y A Y A Y K P K P K P K P A Y A Y A Y A Y K P d s i a u n n a n, C h a i l a n a m u n n a n, C a n,p e n i n s u l a r M h i n a h i n a a l a y a r a t, T s i a a l a y s i a v. I n d o n e s i a a h, M a t r a, I n d o n e s i a h i n a i e t n a l a y s i a a b s a r a t r a, I n d o n e s i a e p a l h a i l a n d a l a y u n n a n, C a l a y s i a)a t r a h a m m a t r a, I n d o n e s i a a l a y s i a u m h a i l a n d a t r a, I n d o n e s i a r o u m u m o n g s a l y, L v. S h i n a u m u m o n e s i a v. S c, V i l l, M v. S a o h a i l a n d v. S i g h l a n d s i g h l a n d s i g h l a n d s t a r a P c a l i t y h u a b a l u, S H e l a m b u, N o n s i T i n , C P a h a n g (E R a i, T r o r o M e n g l a, Y r o y a n m a n g t-n g a, T r o M e n g l a, Y h a i l a n d L o M e n g l a, Y , M S e l a n g o r, M a s t M P h M o V i n P F u j i a n u t h T C h i a n g P h u t h T n, M m a t r a U T i o m J a v a, I n d N a k h F r a s e r H S o M t K B o r n e o B e n g k u l u, S J a m b i P J a m b i P J a m b i P J a m b i P S o C a m e r o n H C a m e r o n H C a m e r o n H S u b e r s H I 5 1 9 7 V o u c h e r n u m H I 5 1 9 9 H I 5 3 4 2 0 4 2 7 5 8 0 9 2 2 0 4 9 6 3 8 1 9 3 3 2 8 3 3 3 0 3 3 6 7 1 9 0 3 4 6 7 8 0 2 4 4 4 1 6 1 B-O P A-R 6 1 6 4 0 B-O P A-R 6 1 6 3 9 9 9 7 3 5 3 6 B-O P A 1 A 1 B 2 A 2 A 2 B 1 B 2 B 4 G P G P G P B 4 B 5 A 2 B 2 B 2 A 2 B 3 B 3 B 3 M Z U T M Z U T B 1 A 1 B 2 B 2 M Z r i s e g e r i e i o r u m e i o r u m e i o r u m e i o r u m e i o r u m e i o r u m k e t e n s i s n a i a T a x a e r e s u r u s a l b o l a b u l a r i s u l a r i s u l a r i s u l a r i s T r i m T. i n s u l a r i s e t a n u s T. t i b T. s t e j n T. p o p T. p o p T. n e b T. p o p T. p o p T. p o p T. p o p T. p h u T. b u n T. f u c a t u s T. f u c a t u s T. f u c a t u s T. f u c a t u s T. s a b a h i T. s a b a h i T. b a r a t i T. b a r a t i T. b a r a t i T. b a r a t i T. b a r a t i T. f u c a t u s T. n e b T. n e b T. n e b T. t o b a

?
猜你喜欢
杂志排行
Asian Herpetological Research的其它文章
- Resurrection of the Genus Leptomantis, with Description of a New Genus to the Family Rhacophoridae (Amphibia: Anura)
- Female-biased Dispersal of the Emei Moustache Toad(Leptobrachium boringii) under Local Resource Competition
- Macroecological Patterns of Climatic Niche Breadth Variation in Lacertid Lizards
- Microhabitat Segregation of Parapatric Frogs in the Qinling Mountains
- Embryonic Growth and Yolk Depletion during Incubation in the Chinese Skink, Plestiodon chinensis
- Investigating the Effectiveness of Road-related Mitigation Measures under Semi-controlled Conditions: A Case Study on Asian Amphibians
