A New Species of Trimeresurus Lacépède,1804 (Squamata:Viperidae) from Southwestern China,Vietnam,Thailand and Myanmar
2021-06-30ZeningCHENShengchaoSHIJunGAOGernotVOGELZhaobinSONGLiDINGandRongDAI
Zening CHEN,Shengchao SHI,Jun GAO,Gernot VOGEL,Zhaobin SONG,Li DING and Rong DAI*
1 Key Laboratory of Bio-Resource and Eco-Environment of Ministry of Education,College of Life Sciences,Sichuan University,Chengdu 610065,Sichuan,China
2 Chengdu Institute of Biology,Chinese Academy of Sciences,Chengdu 610041,Sichuan,China
3 Nanjing Institute of Environmental Sciences,Ministry of Ecology and Environment of the People's Republic of China,Nanjing 210042,Jiangsu,China
4 State Environmental Protection Key Laboratory of Biosafety,Ministry of Ecology and Environment of China,Nanjing 210042,Jiangsu,China
5 Society for Southeast Asian Herpetology,Im Sand 3,D-69115 Heidelberg,Germany
Abstract The pit vipers of the genus Trimeresurus Lacépède,1804 is one of the largest groups of Asian snakes,distributed from India to China and Indonesia.Recent surveys in Jiangcheng and Simao,Yunnan Province,China resulted in a new species previously allocated to T.albolabris.Combining morphological and molecular data,we describe it as Trimeresurus guoi sp.nov.The new species morphologically differs from T.albolabris in the yellow green ventral color;an indistinct ventrolateral line;the absence of a postocular stripe;the firebrickred iris;a dark red stripe on dorsal tail;hemipenes with relatively weak sparse papillae,reaching 23rd subcaudal when unextruded.Molecularly,the new species forms a clearly divergent lineage (BPP 1.00/ UFB 100).Uncorrected pairwise distances of mitochondrial gene Cyt b between the new species and other known species of the subgenus Trimeresurus range from 0.052 (T.albolabris) to 0.071 (T.insularis).
Keywords morphology,phylogenetics,taxonomy,Trimeresurus
1.Introduction
The Asian green pit vipers (Trimeresurus
Lacépède,1804) are a complex and species-rich group of venomous snakes (Gumprechtet al.
,2004;Malhotra and Thorpe 2004;Vogelet al.
,2004;Guoet al.
,2015).There are 50 known species,but the diversity of the genusTrimeresurus
is still underestimated (Wostlet al.
,2016;Mulcahyet al.
,2017;Chenet al.
2019;Chenet al.
2020;Uetzet al.
,2020;Mirzaet al.
,2020).Trimeresurus albolabris
(Gray,1842) is widely recorded in Southern China,and adjacent Southeastern Asia (Zhu,et al.
,2016).Two subspecies of this species have been raised to full speciesT.insularis
andT
.septentrionalis
based on molecular evidence (Giannasiet al.
,2001).Furthermore,Chenet al.
(2020) describedT.caudornatus
based on populations previously identified asT.albolabris
.Mirzaet al.
(2020) describedT
.salazar
,which is similar toT.septentrionalis
andT.albolabris
.Besides,Zhuet al.
(2016) indicate thatT.albolabris
from Southern China and Southeastern Asia forms a clade containing five lineages with significant divergence.These indicate thatT.albolabris
is a species complex.During several field surveys in Yunnan Province in 2019,a series of specimens resemblingT.albolabris
were collected from Jiangcheng and Simao,Yunnan Provinces,China.However,subsequent morphological and molecular analysis revealed these specimens represent a species different fromT.albolabris
and all other recognized congeners.Herein,it is described as a new species.2.Materials and Methods
2.1.Sampling
Ten individuals of the new species were collected in the field,including six road kills and two individuals kept alive.Specimens were fixed and stored in 80% ethanol and deposited at the Chengdu Institute of Biology (CIB),Chengdu,China.Living individuals are kept in the laboratory of CIB,and their saliva was collected for molecular analysis.2.2.Morphologic analysis
The description is based on morphological characters regarded as taxonomically significant by Vogelet al.
(2004).Body and tail length were measured with a tape ruler to the nearest 1 mm.Other measurements were conducted with a digital caliper to the nearest 0.1 mm.Body length (SVL) and tail length (TaL) were taken with a string,which was then measured using a scale.Ventral scales were counted according to Dowling (1951).The number of dorsal scale rows are given at one head length behind head,at midbody,and at one head length before vent,respectively.Cephalic scales (Cep) were counted on a straight line between the middle of the supraoculars.Abbreviations used in the description:HL:head length (from the tip of the snout to corner of mouth);SVL:snout-vent length;TaL:tail length;TL:total length.ED:the horizontal eye diameter.DSR:dorsal scale row;IL:infralabial scale;SC:subcaudal scale;SL:supralabial scale;VEN:ventral scale.Color description was made according to wiki colorcoding.Sex is determined by examination of the hemipenes.The abbreviations for institutions and comparative material examined are presented in Appendix I.
2.3.Molecular analysis
Total genomic DNA was isolated from liver specimens or saliva stored in 95% ethanol using the EasyPure® Genomic DNA kit (TransGen Biotech,Beijing China).Genomic DNA was visualized using 1.0% agarose gel electrophoresis and stored in -20°C.We amplified fragments of mitochondrial cytochrome b (Cytb
),NADH dehydrogenase subunit 4 (ND4),12S ribosomal RNA (12S) and 16S ribosomal RNA (16S) genes using the primer pairs L14910/H16064 (Burbrinket al.
,2000),ND4/Leu (Parkinsonet al.
,2000),L1091/H1557 (Knight and Mindell,1993),16Sar-L/16Sbr-H (Parkinson,et al.
,2000),respectively.PCR amplifications were performed in 25 μL reactions:1.0 μL genomic DNA,1 μL light strand primer (10 μmol/L),1.0 μL heavy strand primer (10 μmol/L),12.0 μL I-5-2 × High-Fidelity Master Mix (Beijing Qingke New Industry Biotechnology Co.,Ltd),10.0 μL ddHO.Polymerase Chain Reaction (PCR) under the following conditions:initial denaturation for 2 min at 95 °C,followed by 35 cycles:denaturation at 94 °C for 40 s,annealing temperature 48 °C for Cytb
,56 °C for ND4,52 °C for 12S and 54 °C for 16S for 25 s,elongation at 72 °C for 15 s,and then finalized with elongation step of 2 min at 72 °C,with a PTC-100 thermal cycler (BioRad,USA).All PCR products were visualized using 1.0% agarose gel electrophoresis.Successful targeted PCR products were then outsourced to Beijing Qingke New Industry Biotechnology Co.,Ltd.for PCR purification,cycle sequencing,and sequencing.Details of sequences and GenBank accession numbers are presented in supporting files (Table S1).Taxa for molecular phylogenetics were selected based on the tree topologies recovered by Zhuet al.
(2016) and Chenet al.
(2020).GenBank accession numbers are presented in supporting files (Table S1).The sequences were aligned by Mega 7 (Kumaret al.
,2016),checked by eyes,and adjusted if necessary.The optimal models of sequence evolution for both genes and codons were identified by Partition finder 2.1.1 under Bayesian Information Criterion (BIC) (Lanfearet al.
,2012).Phylogenetic trees were constructed based on a concatenated dataset using maximum likelihood (ML) and Bayesian inference (BI).The ML analysis was conducted by using IQ-TREE (Nguyenet al.
,2015) with following models:GTR+ I+Γ was the bestfit for the 12S,16S,and the first codon position of Cytb
and ND4,HKY+I for the second codon position of Cytb
and ND4,and GTR+G for the third codon position of Cytb
and ND4.Ultrafast Bootstrap Approximation (UFB;Hoanget al.
,2017) using 5000 bootstrap replicates to assess node support.Nodes with UFB values of 95 and above were considered significantly supported (Hulsenbecket al.
,2001;Wilcoxet al.
,2002).The BI analysis was conducted by using MrBayes 3.2 (Ronquistet al.
,2012) with the same models of ML analysis.Two simultaneous runs were performed with four chains,three hot and one cold.The simulation ran for 10 million generations,was sampled every 1000 generations using the Markov Chain Monte Carlo (MCMC).The first 1000 trees were discarded as burn-in,and posterior probabilities were determined from the remaining trees.Nodes with Bayesian posterior probabilities (BPP) of 0.95 and above were considered well supported (Hulsenbecket al.
,2001;Wilcoxet al.
,2002).Azemiops feae
andProtobothrops elegans
were used as outgroup.After removing outgroup taxa,MEGA7 (Kumaret al.
,2016) was used to calculate uncorrected pairwise sequence divergence between the species.3.Results
Taxonomy
sp.nov.Chen,Shi,Vogel,and Ding
Figures 1-3
ZooBank No.:103364A6-75A4-435E-B1A4-7A899F3FEA93
Holotype.
YNJC0012,adult male,a roadkill,collected from Jiangcheng County,Yunnan Province (22.5738°N,101.6095° E) on 9 September 2019 (Figure 1).Paratype.
One adult female DL2019111703 (alive),two juvenile females JCR2019062401,DL2019090630;three adult males DL2019111701,DL2019090530 and DL2019090830;and three juvenile males YNJC0010,JCR2019061901,DL2019111702.Detailed information is presented in Table 1.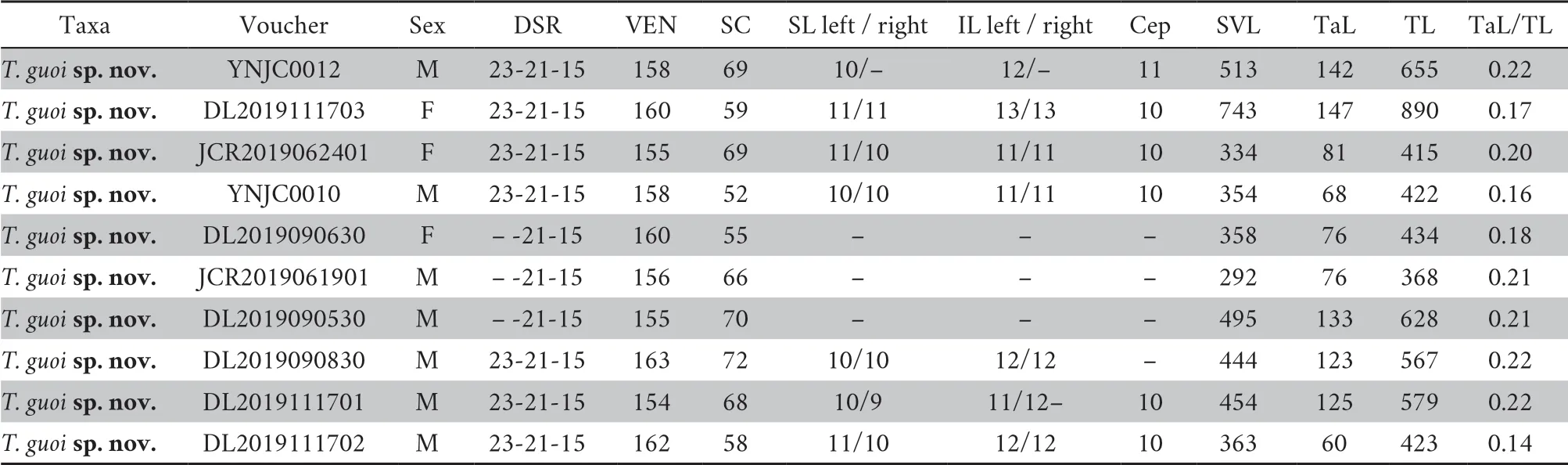
Table 1 Main morphological characters of Trimeresurus guoi sp.nov.Abbreviations are listed in the Materials and Methods.“-” indicated the data missing.
Diagnosis.
(1) Dorsal body jungle-green with faint transverse dark bands on skin,ventral body yellow green.(2) Lateral head jungle-green above lower margin of eyes,and green yellow below,without postocular stripes.(3) Ventrolateral line of male yellow-green,narrow,only present on outermost row of dorsal scales.(4) Iris firebrick-red in both sexes.(5) First supralabial fused with nasal.(6) Head scales feebly keeled;dorsal scale row 23-21-15 (N=
10),feebly keeled except the outermost rows;ventral scale 154-163 in males (N=
6),158-160 in females (N=
3);subcaudal scale 58-72 in males (N=
7),52-59 in females (N=
3).(7) Hemipenes long,reaching 23/32subcaudals when unextruded/extruded,papillae relatively weak and sparse.Description of holotype (Figure 1).
Body elongated;head triangular,slightly damaged,distinct from neck;head length 34.6 mm,4.8% of snout-vent length;eyes large,the horizontal eye diameter 13.4% of head length,pupil vertical.
Figure 1 Holotype (adult male YNJC0012) of Trimeresurus guoi sp.nov.in preservation.Dorsal (A) and ventral (B) view of the specimen;dorsal (C),lateral (D) and ventral (E) view of head;lateral view of middle body (F).Photographed by Shengchao SHI after two months preservation in 80% ethanol.

Figure 2 Comparison of coloration between Trimeresurus guoi sp.nov (Left) and T.albolabris (Right).A and B:Different body pattern (yellow green vs.multicolor) in female;C and D:Different ventrolateral line (absent vs.present) and postocular stripe (absent vs.present) in male;E and F:Different ventral (yellow green vs.yellow).Specimens of T.albolabris compared were colleted from Guanghzou,Guangdong Province,China.Photographed by Shengchao SHI except B by Liang ZHANG.
Snout-vent length :513 mm;Tail length :142 mm;Total length :655 mm;Tail length/Total length :21.7 %.
Ventral scale:158;Subcaudal scale:69,all paired;anal shield entire.
Dorsal scale row:23-21-15 scales,rhomboid,feebly keeled,gradually smoother towards ventrals,first row smooth.
Rostral trapezoidal in front view,base twice as broad as top and 1.6 times of height,front edge protruding and nearly rounded,visible in profile.Nasals large,sub-rectangular,undivided,partially fused with the first upper labial.Internasals trapezoidal,width twice of height,edged with four small scales,and separated from supraoculars by four small scales,in contact with each other.Three canthal scales bordering the canthus rostralis present between the internasal and corresponding supraoculars,not larger than adjacent snout scales.Scales on snout dorsal small,smooth,irregular in shape.Twelve Cephalic scales presents between supraoculars.Occipital scales rhomboid,flat,feebly keeled.Temporals rhomboid,slightly larger,mostly feebly keeled,two most outer rows smooth.
One loreal present on both sides.Two elongated preoculars above the loreal pit,the lower form upper margin of loreal pit.Two postoculars present on both lateral sides of head.Supraoculars entire,with eight/ten small scales bordering left/right supraoculars.One subocular present on both lateral sides of head,elongated and crescent-shaped,surrounding nearly half of eyes from loreal to lower postoculars,with eleven scales bordered on left.
Ten supralabials present on left.First supralabial triangular,small.Second supralabial separated from prelacunal.Third supralabial largest,wider than high.Fourth supralabial wider than high,separated from subocular by a scale as large as temporals.Fifth supralabial separated from subocular by two scales the same size.Sixth SL similar in size to the first,smaller than second to fourth.Two tiny scales present above the third SL,below subocular.Twelve infralabials on left.The first pair of infralabials contact each other in ventral view.Anterior three infralabials in contact with chin shields.
Coloration in preservation (Figure 1)
.Description based on holotype when preserved in 80% ethanol for three months.Dorsal head and body olive,body with faint black transverse bands with a width of approximately one to three dorsal scales.Lateral head olive above lower margin of eye,greenish yellow below.The color of lateral body gradually turns from olive near spine to olive-yellow on first row of dorsal scales.Ventral head yellowish white.Ventrals after head light yellowish white,gradually turning yellowish green towards vent.Dorsal tails mostly dark red,inner two rows of dorsal tail scales dark red,other dorsal scales olive.Most subcaudal scale yellowish green,posterior 30% gradually dark red.
Figure 3 Comparison in head scales and coloration between Trimeresurus guoi sp.nov.and T.albolabris. Left,doral view of head;right,lateral view of head.A,B,female of T.guoi sp.nov.;C,D,female of T.albolaris;E,F,male of T.guoi sp.nov.;G,H,male of T.albolaris.A,C,E and G:Different head shape (more elongated skull in new species) in both genders;B and D:Different iris color (firebrick-red vs.copper) in female;F and H:Different iris color (firebrick-red vs.copper) and postocular stripe (absent vs.present) in male.Specimens of T.albolabris compared were colleted from Guangzhou,Guangdong Province,China.Photographed by Shengchao SHI except D by Liang ZHANG.
Coloration in life (Figures 2-3)
.Description based on three males DL2019111701,DL 2019111702 DL2019090830.Dorsal head and body jungle green.Lateral head jungle-green above lower margin of eyes,and green-yellow below,without postocular stripes.Dark transverse bands the width of one to three dorsal scales present on body,.Lateral body mostly jungle-green,gradually lighter from spine to ventrals.First row of dorsal scales grass-green,with a narrow green-yellow longitudinal stripe,which forms a faint green-yellow ventrolateral line.Ventral body yellow-green,becoming lighter in color anteriorly.Tail with a dark red longitudinal stripe in inner two rows of dorsal scales,other dorsal scales on tail jungle-green.Most SC green-yellow,posterior one third gradually dark red.Iris firebrick-red,pupils edged with lighter color.Tone coloration maroon.Hemipenis (Figure 4 left).
Description based on male paratype DL2019111701 (SVL 454 mm).Hemipenis elongated,reaching 23/32subcaudal when unextruded/extruded;bifurcates at 6subcaudal,covered with weak sparse papillae after the bifurcation to about 11subcaudal.Skin on hemipenis after 11subcaudal feebly calyculate.Thesulcus spermaticus
shallow,divides near the base of the organ.Intraspecific morphological variation.
All ten specimens were of similar body coloration and body shape.Females without ventrolateral line (Figure 2A),outermost dorsal scales yellow green.Males with relatively longer tail and more subcaudal scales than females.Supralabials vary between 10 or 11 (N
=10);Infralabial vary from 11 to 13 (N
=10);Cephalic scales vary from 9 to 11 (N
=8) (More details in Table 1).Comparison.
The new species is morphologically and phylogenetically (see below) referred to the subgenusTrimeresurus
(Malhotra and Thorpe,2004;Davidet al.
,2009),and is morphologically similar toT.albolabris
,T.septentrionalis
,andT.caudornatus.
See main characters separating it from these three species in Table 2.
Table 2 Summary of selected characters for members of similar species.
Trimeresurus guoi
sp.nov.
is distinguishable fromT.albolabris
as follows (Figures 2-4):(1) Ventral body yellow green vs.creamy white or light yellow.(2) Ventrolateral line unicolor,indistinct,yellow-green,present only on first row of most dorsal scales of males vs.multicolor,distinct in males,basically white,upper margin light yellow,lower margin fern-green,present on first two rows of dorsal scales.(3) Postocular stripe absent vs.white postocular stripe extending from loreal pit to lateral body.(4) Iris firebrick-red vs.iris copper.(5) Stripe on dorsal tail dark red vs.stripe on dorsal tail bronze.(6) Hemipenis longer,reaching 23subcaudals when unextruded,papillae relatively small and sparse vs.hemipenis relatively shorter,reaching 18subcaudals when unextruded,papillae pointy,well developed and dense (Figure 4H).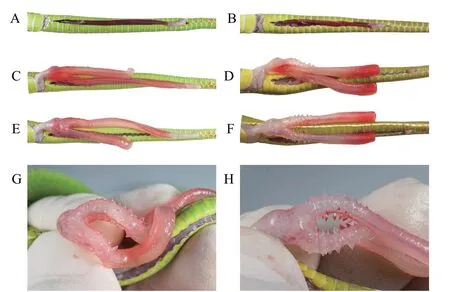
Figure 4 Comparison in hemipenis between Trimeresurus guoi sp.nov.(Left) and T.albolabris (Right).A and B:fresh unextruded hemipenis (longer in new species);C and D sulcate view of fresh extruded hemipenis (longer in new species);E and F:asulcate view of fresh extruded hemipenis (longer in new species);G and H:spines after bifurcation (papillae relatively small and sparse vs.papillae pointy,well developed and dense).Photographed by Shengchao SHI.
Trimeresurus goui
sp.nov
.is distinct fromT.septentrionalis
by:(1) Fewer ventrals,154-163 in males (N
=7),158-160 in females (N
=3) vs 164-170 in males (N=
5) and 166-171 in females (N=
13).(2) Fewer SC,58-72 in males (N=
7),52-59 in females (N=
3) vs.74-80 in males (N=
5) and 56-66 in females (N=
13).(3) Lateral head jungle-green above lower margin of eyes,and green-yellow below,without postocular stripes,iris firebrickred vs.head below the eye green,only slightly or not lighter,iris yellow.Trimeresurus guoi
sp.nov
.is distinct fromT.caudornatus
by:(1) DSR 23-21-15 vs DSR 21/22-21-15.(2) Iris firebrickred in both genders vs.iris golden yellow in both genders.(3) The orange-red stripe along the ventral part of the tail absent vs.present.(4) Hemipenis relatively shorter,reaching 32subcaudal when extruded vs.reaching 37or 38subcaudal when extruded.Trimeresurus guoi
sp.nov
.is distinct fromT.salazar
by:(1) Postocular stripe absent in both genders vs.reddish orange postocular stripe extending from loreal pit to lateral body in male.(2) Ventrolateral line yellow-green in male and absent in female vs.yellow with a faint orange in males and yellow in females.(3) Iris firebrick-red in both genders vs.iris golden yellow in both genders.Trimeresurus guoi
sp.nov
.is distinct fromT.insularis
by:(1) Dorsal scale row feebly keeled except the outermost rows vs.strongly keeled expect the first row.(2) Lateral head junglegreen above lower margin of eyes,and green-yellow below vs.head green above,blue-green,or light green (derived from photos in Vogel 2006).(3) Geographically isolated from type locality of the latter (continental,from southwestern China and adjacent area vs.islands of Soe and Timor,Indonesia).Trimeresurus guoi
sp.nov
.is distinct fromT.erythrurus
by: (1) Scales in 21 rows at mid-body vs.usually 23-25 longitudinal rows.(2) Head scales feebly keeled vs.strongly keeled.(3) Ventrolateral stripe absent in females vs. a thin white line present in most females.Trimeresurus guoi
sp.nov
.is distinct fromT.purpureomaculatus
by the following characters:(1) Scales in 21 longitudinal rows at mid-body vs.25-27 (N=
29).(2) Head scales feebly keeled vs.strongly keeled.(3) Body jungle-green vs.usually not green (except some specimens from Sumatra).(4) Lateral head jungle-green above lower margin of eyes,and green yellow below vs.upper labials usually not lighter than body but in some populations yellow.(5) Fewer Cephalic scales,10-11(N=
6) vs. 13-18 (N=
11).Trimeresurus guoi
sp.nov
.is distinct fromT.macrops
by:(1) No postocular stripe vs.prominent lateral white or light blue stripe in males.(2) Ventrolateral stripe indistinct,faint green yellow vs.prominent white lateral stripes in males.(3) Eyes relatively smaller the horizontal eye diameter/head length 13.4 % (vs.the horizontal eye diameter/head length 16.1%).(4) Head oval,gradually widening behind eyes (vs.head triangular,widens abruptly behind eyes).Trimeresurus guoi
sp.nov
.differs fromT.rubeus and T.cardamomensis
principally by the same characters as fromT.macrops
.It furthermore differs fromT.cardamomensis
by the iris color being firebrick-red rather than golden yellow.Trimeresurus guoi
sp.nov
.is different fromT.cantori
by:(1) Fewer dorsal scale row (21 vs. 25-29).(2) Fewer supralabial scale (10-11 vs.11-13,only very exceptionally 11.(3) Fewer Cephalic scales (10-11 vs.13-17).(4) Fewer ventrals (155-163 vs.170-182).Trimeresurus guoi
sp.nov
.is distinct fromT.fasciatus
by:(1) Dorsal body jungle-green with faint transverse dark bands on skin vs.brownish grey with olivaceous brown or dark brown crossbands on the back (Davidet al.
,2003).(2) Ventral body yellow-green dorsal tail dark red vs.greyish brown or brown.(3) Internasals broad trapezoidal,broadly in contact vs.separated from each other by one scale.(4)Trimeresurus fasciatus
is endemic to Tanahjampea Island.Trimeresurus guoi
sp.nov
.is distinct fromT.andersoni
by:(1) Dorsal body jungle-green with faint transverse dark bands on skin vs.color above and below variable,usually brown,buff,or blackish.(2) Fewer dorsal scale row at midbody (21 vs. 23 to 25,rarely 21).(3) Fewer ventrals (154-163 vs.171-183) (Gumprechtet al.
,2004).Besides,T.andersoni
is endemic to the Andaman and Nicobar Islands.Trimeresurus guoi
sp.nov
.is distinct fromT.labialis
,T.honsonensis
Grismer,Ngo and Grismer,2008,T.venustus
,andT.kanburiensis
by body color and pattern.The dorsal body ofT.guoi
sp.nov
. jungle-green with faint transverse dark bands on skin vs.brown inT.labialis
(Vogelet al.
,2014),green patterned with spotted brownish speckles inT.honsonensis
,T.venustus
(Vogelet al.
,1991),andT.kanburiensis
(Davidet al.
,2004).Etymology.
The specific name is in honor of Dr.Peng Guo (Sichuan,China),the first researcher on the taxonomy and systematics of the genusTrimeresurus sensu lato
through molecular analysis in China.We suggest the following common names as “Guo’s green pit viper” in English and “Diān Nán Zhú Yè Qīng (滇南竹叶青)” in Chinese.Phylogenetic analysis
Our DNA dataset contained 56 specimens with 2582 base pairs.The Bayesian Inference (BI) and Maximum likelihood analysis (ML) recovered trees bearing consistent phylogenetic structure (Figure 5).Phylogenetically,the specimens from Yunnan province form a clearly divergent lineage with samples from Thailand (AM A165 and AM A229),Vietnam (ROM 39389) and Myanmar (CAS 222595) (Figure 5,BPP 1.00/UFB 100),with mitochondrial Cytb
divergence 0.046-0.128 from all previously known species (Table S2).This divergence is clearly among inter-species level since the uncorrected p-distance are 0.031 betweenT.erythrurus
andT.cantori
and 0.041 betweenT.cantori
andT.andersoni
.Taking into consideration the morphology of those specimens from Thailand,Vietnam and Myanmar,we hypothesize that these individuals belong to the new species based on the short branch length and low pairwise distance between those from Jiangcheng County and Simao District,Yunnan Province,China (< 0.019,Table S2).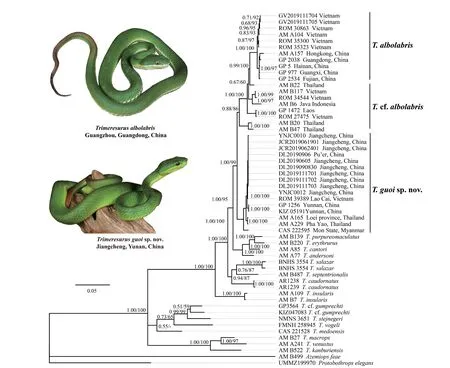
Figure 5 Bayesian Inference (BI) and maximum likelihood (ML) analyses trees of the genus Trimeresurus based on Cyt b,ND4,12S,and 16S.The major bootstrap and posterior probability values (BPP/UFB) were presented (the ones lower than 50% denoted as “-”).
Due to the lectotype (BMNH 1946.1.19.85) ofT.albolabris
being collected from Hong Kong,China (Wallachet al.
,2014),and the sequences from Hong Kong (AM A157),southern China and northern Vietnam forms a monophyletic group with strong support (Figure 5,UFB 100/ BPP 1.00),we treat those specimens asT.albolabris
.However,these specimens from Thailand (AM B22),Vietnam (ROM 27475),Laos (GP 1472),and Java (AM B6) were outlying to theT.albolabris
,the deep divergences,both intra-species (0.000-0.049,Table S2) and inter-species (>0.029,Table S2),indicated that specimens are cryptic.We therefore refer to these specimens asT.
cf.albolabris
and their taxonomic status remains unresolved.The uncorrected sequence divergence in the studied Cytb
fragment showed the new species to be divergent from the previously recognized species by interspecific genetic distances of 0.048-0.061 forT.albolabris
,0.034-0.049 forT.
cf.albolabris
,0.045-0.125 all other known species (Table S2).Distribution and habitat.
Trimeresurus guoi
sp.nov
.is known from Jiangcheng County and Simao District,Southern Yunnan Province,China,and adjacent Vietnam (Lao Cai),Thailand (Loei and Pha Yao) and Myanmar (Mon State) (Figure 6).It is found in tropical monsoon forests with clearly defined dry and rainy seasons,at elevations reaching approximately 1,400 m in Jiangcheng,Yunnan,China (Figure 7).
Figure 6 Distribution of Trimeresurus guoi sp.nov.,T.albolabris and T.cf.albolabris.

Figure 7 Habitat of T.guoi sp.nov.in Jiangcheng,Yunnan Province,China.
4.Discussion
The Asian green pit vipers are notoriously difficult to classify due to highly similar squamation morphology.Specimens of the new species were treated asT
.albolabris
for a long time.
However,multiple evidence including molecular and morphological data based on fresh and living specimens collected near or from the type locality showed thatT.guoi
sp.nov.
differs fromT.albolabris
as described above.Furthermore,several lineages in the subgenusTrimeresurus
need to be reexamined in detail and might lead to the description of other new species.The description ofT.guoi
sp.nov.
once more emphasizes the underestimation of the diversity of green pitvipers in Yunnan,where six species were recorded:T.yunnanensis
,T.gumprechti
,T.stejnegeri
,T popeiorum
,T.yingjiangensis
,andT.caudornatus
(Guoet al.
,2015;Chenet al.
,2019;Chenet al.
,2020,this paper).Yunnan Province is located in the southeastern-most corner of the Tibetan Plateau,with high mountains and deep valleys,which may cause isolation and accelerate diversification.According to Figure 6,the Red River might be a boundary of the new species andT.albolabris
.Ecological differences can drive speciation (McKelvy and Burbrink,2017).T.guoi
sp.nov
.andT
.albolabris
occupy different ecological niches.Specimens ofT
.albolabris
collected from Hong Kong and Guangdong were found in southern subtropical monsoon forests at low elevation (altitude under 500 m) with annual mean temperatures of 21.5-22.5 ℃ (Xuet al.
,2007).In contrast to this,T.guoi
sp.nov.
was found in tropical monsoon forests with clearly defined dry and rainy seasons and a higher elevation (reaching above 1,400 m),the annual mean temperature is 14.8-18.9 ℃ (Liu,2000).Further studies on how ecological differences drive speciation of these two species are recommended.Acknowledgements
This project was funded by the Biodiversity Investigation,Observation and Assessment Program of Ministry of Ecology and Environment of China (2019-2023) and the Natural Science Foundation of Jiangsu Province,China (BK20160103).We thank Patrick Campbell (BMNH),Jens Vindum and Alan Leviton (CAS),Andreas Schmitz (MHNG),Alain Dubois and Annemarie Ohler (MNHN),Silke Schweiger,Richard Gemel and Georg Gassner (NMW),Pim Arntzen and Esther Dondorp (RMNH,Leiden),Mark-Oliver Rödel and Frank Tillack (ZMB),Jakob Hallermann (ZMH,Hamburg) for data about the collections under their care.We thank Liang Zhang and Hangzhang Cai for providing photographs.We especially thank Tom Charlton for the language editing.杂志排行
Asian Herpetological Research的其它文章
- A New Leaf Litter Toad of Leptobrachella Smith,1925 (Anura,Megophryidae) from Sichuan Province,China with Supplementary Description of L.oshanensis
- A New Species of the Genus Achalinus from Huangshan,Anhui,China (Squamata:Xenodermidae)
- Phylogeography and Cryptic Species Diversity of Paramesotriton caudopunctatus Species Group (Salamandridae:Paramesotriton) in Guizhou,China
- Phylogeny and Biogeography of Common Toad (Bufo bufo) in Xinjiang,China
- Evolution of Phenotype and Mitochondrial Genome Reveals Limbless and Body-elongated Squamates may Change Their Energy Basis for Locomotion
- Male Advertisement Call of the Endangered Leptobrachella tengchongensis (Anura:Megophryidae) from Mount Gaoligongshan,Yunnan Province,China
