Solubility and mass transfer of H2,CH4,and their mixtures in vacuum gas oil:An experimental and modeling study☆
2019-03-22ZhigangLeiYifanJiangYaoLiuYichunDongGangqiangYuYanyongSunRuiliGuo
Zhigang Lei ,Yifan Jiang ,Yao Liu,Yichun Dong,Gangqiang Yu, *,Yanyong Sun,Ruili Guo
1 State Key Laboratory of Chemical Resource Engineering,Beijing Key Laboratory of Energy Environmental Catalysis,Beijing University of Chemical Technology,Beijing 100029,China
2 School of Chemistry and Chemical Engineering,Shihezi University,Shihezi 832003,China
Keywords:H2CH4Vacuum gas oil(VGO)Solubility Mass transfer COSMO-RS model
ABSTRACT In this work,the solubility data and liquid-phase mass transfer coefficients of hydrogen(H2),methane(CH4)and their mixtures in vacuum gas oil(VGO)at temperatures(353.15-453.15 K)and pressures(1-7 MPa)were measured,which are necessary for catalytic cracking process simulation and design.The solubility of H2and CH4in VGO increases with the increase of pressure,but decreases with the increase of temperature.Henry's constants of H2and CH4follow the relation of ln H=-413.05/T+5.27 and ln H=-990.67/T+5.87,respectively.The molar fractions of H2and system pressures at different equilibrium time were measured to estimate the liquid-phase mass transfer coefficients.The results showed that with the increase of pressure,the liquid-phase mass transfer coefficients increase.Furthermore,the solubility of H2and CH4in VGO was predicted by the predictive COSMO-RS model,and the predicted values agree well with experimental data.In addition,the gas-liquid equilibrium(GLE)for H2+CH4+VGO system at different feeding gas ratios in volume fraction(i.e.,H285%+CH415%and H290%+CH410%)was measured.The selectivity of H2to CH4predicted by the COSMO-RS model agrees well with experimental data.This work provides the basic thermodynamic and dynamic data for fuel oil catalytic cracking processes.
1.Introduction
After the catalytic cracking of residual oil,the produced vacuum gas oil(VGO)is dense and viscous and also contains metals,high sulfur,nitrogen,and residual carbon[1].It is a challenge for refineries to control sulfur in VGO to an ultralow level to meet the need of environmental protection because of its complex nature[2-4].Of all the processes,hydrogenation is a good way to process the VGO.It should be noted that the VGO has a relatively large molecular mass,complex composition,high impurity content,and high boiling point,and it is difficult to react with hydrogen(H2)in the hydrogenation process.Therefore,it is a very important basis of achieving the hydrogenation reaction to ensure H2dissolved in the VGO liquid phase.In this case,the solubility of H2in VGO is a key factor that affects the hydrogenating efficiency.At present,most of the literatures proposed are about the solubility of H2in organic solvents.There are relatively few studies on H2solubility data in the petroleum fractions[5],not to mention H2solubility data in VGO.Moreover,in the actual VGO hydrogenation process,H2is usually mixed with a small amount of CH4.Thus,the study on H2,CH4and even their mixture solubility in VGO is important to complement the basic data for hydrogenation process simulation and design.
Saajanlehto et al.[6]investigated the solubility of H2in heavy oil systems at the temperature range from 498 to 598 K and the pressure range from 2 to 11 MPa.The results showed that the solubility of H2can increase with increasing pressures and temperatures.Liu and Que et al.measured the H2solubility in methane,n-heptane,atmospheric gas oil and atmospheric residue,and found that H2solubility increases with increasing temperatures and pressures[7].Liaw et al.focused on the solubility of H2in the presence of CH4and ethane in coal liquids[8].At a constant temperature and H2partial pressure,the solubility of H2in tetralin first increases,but as the methane and ethane partial pressure increase,it then decreases.Zhou et al.studied the solubility of H2in pyrolysis gasoline,benzene,toluene and BTXS(the mixture of benzene,toluene,xylene and styrene)[9].The Soave-Redlich-Kwong(SRK)cubic equation was used to correlate the H2solubility,indicating that the model is suitable and the calculated results agree well with experimental data.Unfortunately,VGO is a multi-component hydrocarbon mixture,so it is difficult to obtain those necessary critical parameters.Moreover,H2and CH4solubility data in VGO are still missing so far.It is impossible to obtain the relatively reliable results for H2and CH4solubility in VGO predicted by those thermodynamic models aforementioned.Thus,it is required to find out a more appropriate predictive model to describe the VGO system.
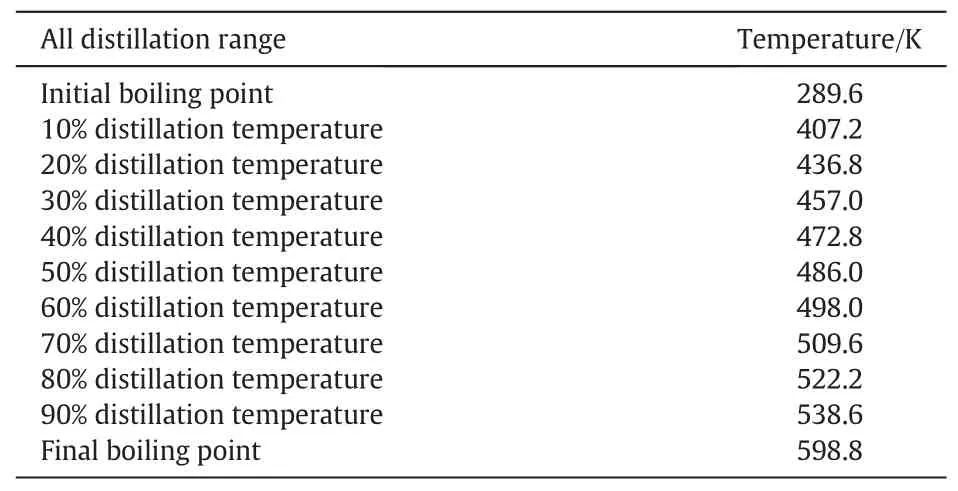
Table 1.ASTM distillation data of vacuum gas oil(VGO).
The conductor-like screening model for real solvents(COSMO-RS)was originally proposed in 1995[14],which doesn't depend on experimental data.It is based upon the unimolecular quantum chemical calculations to predict the thermodynamic properties of pure or mixed solvents[14-18].The COSMOthermX is an advanced software tool based on the COSMO-RS method to predict gas solubility,vapor pressure,activity coefficients and Florusse et al.measured the solubility of H2in heavy n-alkanes up to 30 mol% and described the GLE of these mixtures through a molecular-based equation of state based on the statistical associating fluid theory(SAFT),with the result that the calculated values agree with experimental data[10].

Fig.1.Schematic diagram of the experimental setup(1,hydrogen cylinder;2,constant temperature jacket;3,magnetic agitated view-cell reactor;4,magnetic stirring;5,thermocouple;6,vacuum pump;7,monitor of temperature and pressure).
On the other hand,Field et al.measured the solubility of CH4in methylcyclohexane and toluene from 283 to 313 K,and compared the experimental data with the results obtained through the scaled particle theory(SPT),indicating that both agree well[11].Hesse et al.measured the solubility of CH4in n-alkanes at 298.15 K[12].The results showed that the longer the carbon chain,the less the solubility of CH4.Zirrahi et al.investigated the solubility of CH4in bitumen,and used the Krichevsky-Ilinskaya(KI)equation to predict the gas solubility[13].
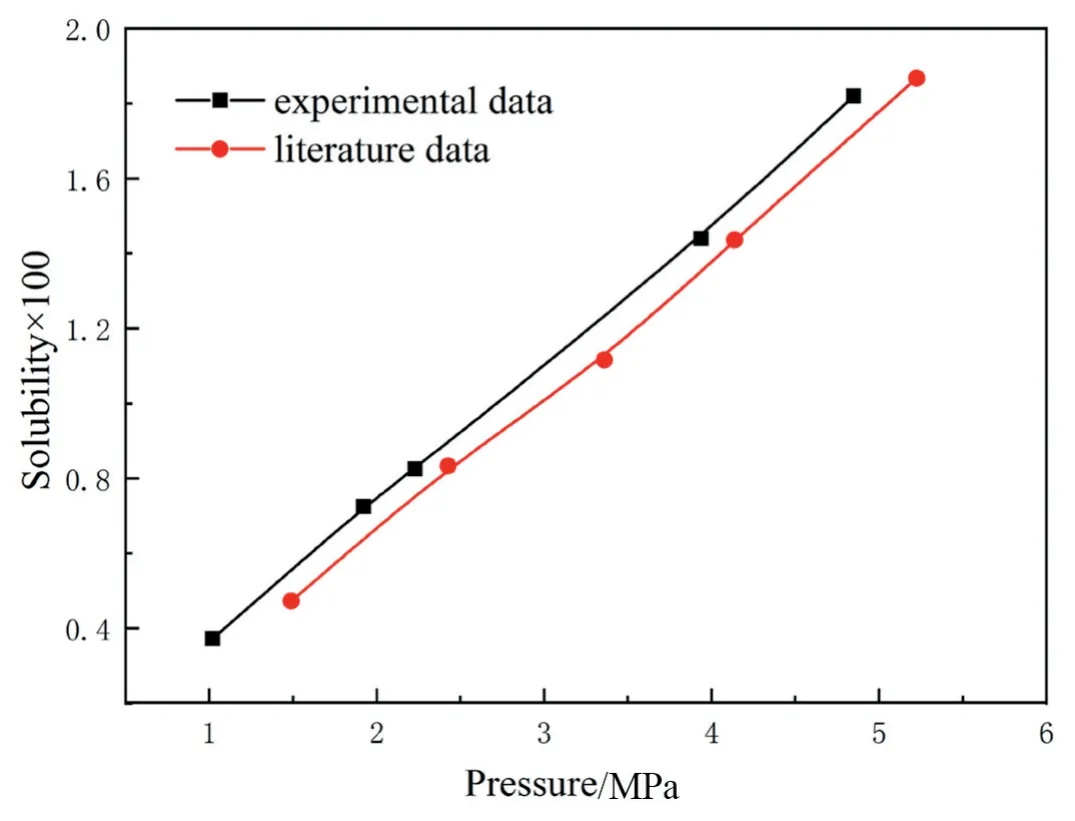
Fig.2.Comparison of H2solubility in toluene at 323.15 K between experimental data measured in this work and literature data.

Table 2.Physicochemical properties of vacuum gas oil(VGO).

Table 3.Hydrocarbon type composition of vacuum gas oil(VGO).
Overall,there are a variety of predictive molecular thermodynamic models like perturbed-chain statistical associating fluid theory(PC-SAFT),Peng-Robinson(PR)equation of state,SRK equation of state,and group contribution(GC)equation of state that can be used to predict the gas solubility.In particular,some critical parameters of compounds in the system are required when using these equations of state models.Henry constants[19].Through COSMOthermX,users can obtain the chemical potentials and sigma-profiles of individual compounds as well as thermophysical,equilibrium and transfer properties of pure compounds and mixtures[20].It is generally believed that this tool can be used to estimate the gas solubility with correct trends in most cases[21-23].Thus,in this work,we decide to apply the COSMO-RS model to predict the H2and CH4solubility in VGO.
This article consists of three parts as follows.First,the solubility of H2,CH4and the mixture of H2+CH4in VGO was measured at the temperature range from 353.15 to 453.15 K and pressure range from 1 to 7 MPa.Second,Henry's constants and gas-liquid mass transfer coefficients of pure H2and CH4in VGO were studied.Finally,the solubility of pure and mixed gas in VGO was predicted by the COSMO-RS model using the COSMOthermX software package(version C30_1301),and the predicted results were compared with the measured data.On this basis,the predicted selectivity of H2to CH4in VGO was also compared with the experimentally measured data to further check the applicability of the COSMO-RS model.

Fig.3.Optimized geometries of six virtual model compounds C34H46(a)and charge distribution on the molecular surface(b).

Table 4.Experimental solubility data of H2in vacuum gas oil and the predicted results by COSMO-RS model at different temperatures and pressures.
2.Experimental
2.1.Materials
The VGO was supplied by Sinopec Fushun Research Institute of Petroleum and Petrochemicals.The ASTM(American Society for Testing and Materials)distillation data are listed in Table 1,which were obtained using a BSY-103 type distillation analyzer according to GB/T6536.H2,CH4and mixed gas were purchased from Beijing Beiwen Special Gases Factory.The mass fractions of H2and CH4are both 99.99%(mole fraction),and they were used without further purification.Toluene was purchased from Sinopharm Group Chemical Reagent Co.,Ltd.(purity≥99.9 wt%).

Fig.4.Solubility of H2in vacuum gas oil at different temperatures and pressures(points,experimental data;solid lines,predicted values by the COSMO-RS model).
2.2.Experimental setup and procedure
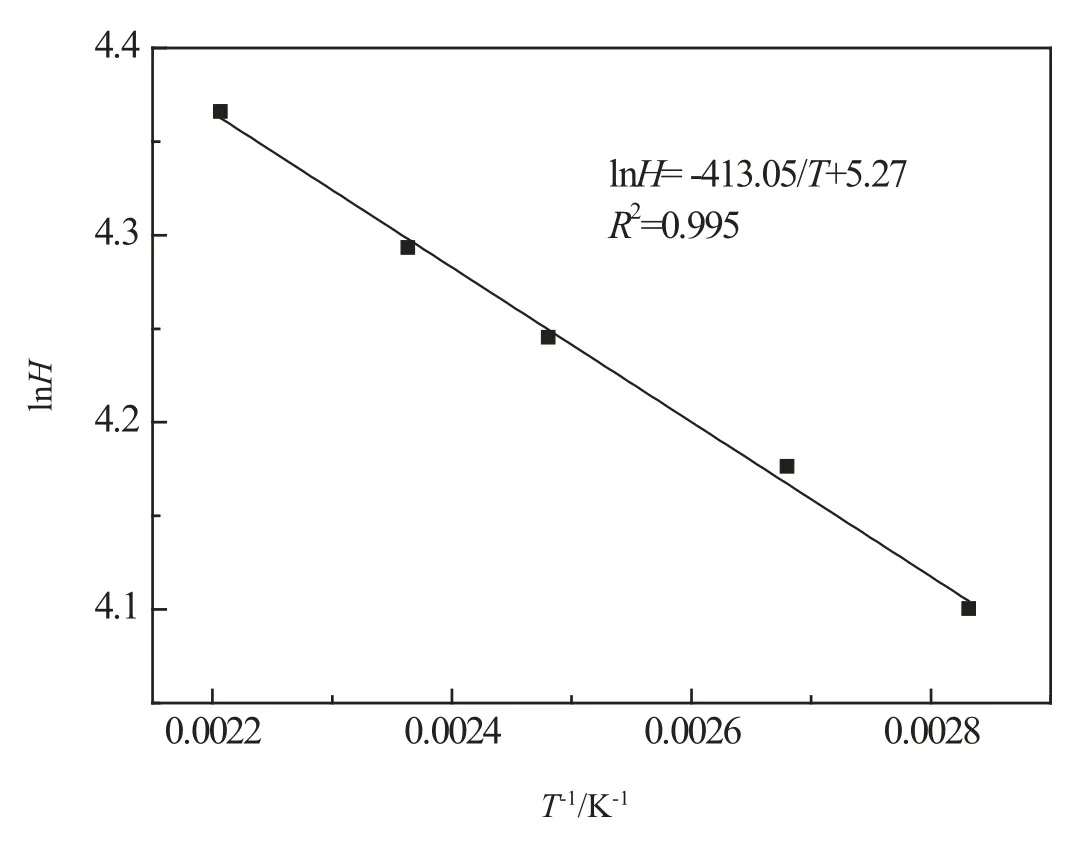
Fig.5.Henry's constant of H2in vacuum gas oil at different temperatures.

Fig.6.System pressure P as a function of equilibrium time t at 373.15 K and 2.549 MPa.
The experimental apparatus used to measure the gas solubility and mass transfer coefficients in VGO is a high-pressure and high-temperature magnetic agitated view-cell,as shown in Fig.1.In the experimental system,a pressure sensor with an accuracy of±0.001 MPa and a temperature sensor with an accuracy of±0.1 K were used to measure the system pressure and temperature,respectively.They can control the total uncertainty within 1% including the uncertainty of experimental method and apparatus.The mass of VGO was measured by an electronic balance(CPA 1003S,Sartorius),its precision being±0.001 g.The experimental method for measuring the solubility of H2and CH4in VGO adopted in this work is similar to the previous work reported by Lei et al.[24,25]and Dai et al.[26].
In experiment,the initial pressure,the varied pressure over time,and the balance pressure were recorded.The procedure is similar to the previous works[27-30]as described below.First,a certain amount(mVGO)of VGO was added into the equilibrium cell.When the system temperature reached the desired value,the equilibrium cell was evacuated with a vacuum pump until the pressure was constant(P=Pm).It must be sure that there was no air in the equilibrium cell.Next,H2(or CH4or their mixture)was rapidly loaded into the cell,and the initial pressure(t=t0and P=P0)was recorded at once.A magnetic stirrer was stirring at a constant speed in the cell to accelerate the gas dissolution.Finally,the system pressure Ptwas gradually reduced with the increase of equilibrium time,and recorded every minute until the pressure didn't change for a long time.That is,the system reached the gas-liquid equilibrium(GLE)and the equilibrium pressure should be recorded as Pe.
2.3.Solubility measurement
During the experiment,the solubility of H2(x)in VGO can be calculated by

where MVGOis the molecular weight of VGO;nH2,nH2,0and nH2,erepresent the amount of H2in the liquid phase and gas phase at initial state as well as in the gas phase at equilibrium state,respectively;Vcellis the total volume of equilibrium cell;VL,0and VL,erepresent the initial and equilibrium volumes of liquid phase in the cell,respectively;Vm,0and Vm,e,which can be obtained from NIST(National Institute of Standards and Technology)database,are the molar volumes of H2under the initial pressure P0and equilibrium pressure Pe,respectively.In addition,VL,ecan be derived from

To verify the accuracy and reliability of experimental method and apparatus,the experimental data of H2solubility in toluene at 323.15 K were compared with the data coming from the literature[32].As shown in Fig.2,the experimental data measured by this apparatus agree very well with literature values,demonstrating that the experimental method and apparatus presented in this work are reliable.In addition,the liquid-phase mass coefficient was measured by the dynamic pressure step method[33,34],and can be calculated by

where,Pm,P0,Ptand Peare called the pressure after evacuated,the initial pressure,the pressure at any time t and the equilibrium pressure in the system of equilibrium cell.Since a liner relationship exists between ln((P0-Pe)/(Pt-Pe))and equilibrium time t,kLa can be deduced from the slope of straight line directly[35-37].
3.COSMO-RS Model
In this work,the COSMO-RS model using COSMOthermX software package(version C30_1301)was used to predict the solubility of H2and CH4in VGO based on activity coefficients[38-41].The COSMO-RS model has been widely applied to the research fields in chemical engineering[42-51].For the H2-VGO and CH4-VGO systems,the gas-liquid equilibrium(GLE)can be expressed as.


Fig.7.Liquid-phase mass transfer coefficient kLa of H2in vacuum gas oil at 373.15 K.

Table 5.Experimental solubility data of CH4in vacuum gas oil and the predicted results by COSMO-RS model at different temperatures and pressures.
where xiand yirepresent the mole fractions of H2(or CH4)in the liquid and gas phases,respectively;P is the system pressure;ϕi(T,P,yi)is the gas phase fugacity coefficient,which can be obtained from the Peng-Robinson(PR)equation of state[25];represents the saturated vapor pressure of gases(H2and CH4),which can be obtained from the NIST database or Antoine equation[52];and γiis the liquid phase activity coefficient and it can be assumed asbecause of the extremely small gas solubility.Moreover,the gas phase is taken on as pure gas because of the very high boiling point of VGO,that is,yi=1.

Fig.8.Solubility of CH4in vacuum gas oil at different temperatures and pressures(points,experimental data;solid lines,predicted values by the COSMO-RS model).
The composition of VGO is extremely complex,and no definite molecular structure can be adopted for the COSMO-RS calculation.Thus,several virtual molecules were designed in accordance with the physicochemical properties and hydrocarbon composition of VGO,as listed in Tables 2 and 3.
The physicochemical properties,i.e.,hydrocarbon composition and C/H atom ratio,are corresponding to the real compounds with the designed molecular formula C34H46.All these molecules were first optimized by TURBOMOLE at the BP86/defTZVP level to generate the COSMO files,and then the files were introduced into the COSMOtherm package.The optimized geometries of these model compounds and the surface charge distributions are shown in Fig.3.

Fig.9.Henry's constant of CH4in vacuum gas oil at different temperatures.

Fig.10.System pressure P as a function of equilibrium time t at 353.15 K and 3.078 MPa.
4.Results and Discussion
4.1.Solubility of H2in VGO
The solubility of H2in VGO was first measured at the temperature range of 353.15-453.15 K and the pressure range of 1-7 MPa,and the experimental data and COMO-RS predicted values are listed in Table 4.Moreover,the average relative deviations(ARDs)defined as ARD%=are also included in Table 4,where xi,predpresents the predicted values by the COSMO-RS model.It can be seen that most of the predicted values agree with the experimental data to some degree,indicating that the COSMO-RS model can qualitatively describe the H2solubility in VGO.Especially,the predicted values obtained from the model compound no.3 are the closest to the experimental data.The P-diagram is illustrated in Fig.4,which shows that the solubility of H2in VGO increases with the increase of pressure,but decreases with the increase of temperature.
Henry's constant was calculated by the method of linear extrapolation at xi(i=H2and CH4)→0.The calculation formula can be written as follows

Fig.11.Liquid-phase mass transfer coefficient kLa of CH4in vacuum gas oil at 353.15 K.

It is interesting to find that the relationship between Henry's constant H and temperature T can be fitted by the equation ofas illustrated in Fig.5.This demonstrates that lnH has a good linear relationship with 1/T,i.e.,lnH=-
The relationship between the system pressure P and the equilibrium time t at T=373.15 K and P=2.549 MPa is shown in Fig.6.
It is clear that there is also a linear relationship between ln((P0-Pe)/(Pt-Pe))and t,which is written as ln((P0-Pe)/(Pt-Pe))=0.00463t+0.5265.From the slope of straight line,the liquid-phase mass transfer coefficient(kLa)of H2in VGO can be derived.
Furthermore,Fig.7 shows the effect of pressure on kLa at T=373.15 K.It can be seen that kLa increases with increasing the system pressure,indicating that H2dissolves more quickly at high pressure.
4.2.Solubility of CH4in VGO
In the similar way,the solubility of CH4in VGO was also measured under the same temperature and pressure range as that of H2.The experimental data,as well as the predicted values by the COSMO-RS model,are listed in Table 5.It can be seen that the predicted values agree with the experimental data to some degree,indicating the COSMO-RS model can be also used to qualitatively predict CH4solubility in VGO,and CH4is more easily dissolved in VGO than H2.Similarly,the model compound no.3 among the six COSMO-RS model compounds has the best fitting to experimental data.Fig.8 shows the effect of pressure on CH4solubility in VGO.It can be seen that CH4solubility in VGO increases with increasing pressures,but decreases with increasing temperatures,which is consistent with the H2solubility rule.Anyway,the COSMO-RS model is an effective and prior thermodynamic tool that does not depend on experimental data for predicting qualitatively the H2and CH4solubility in VGO.
Henry's constant of CH4in VGO was calculated by the same method as that of H2,and the relationship between lnH and 1/T,i.e.,lnH=is shown in Fig.9.
Fig.10 shows that when CH4gradually dissolves in VGO,the system pressure first decreases rapidly,and then decreases slowly to a constant value at T=353.15 K and P=3.078 MPa.
Furthermore,the effect of pressure on liquid-phase mass transfer coefficient at 353.15 K is illustrated in Fig.11.It can be seen that there exists a linear relationship between ln((P0-Pe)/(Pt-Pe))and t,i.e.,ln((P0-Pe)/(Pt-Pe))=0.00252t+0.11193.
4.3.Selectivity of H2to CH4in VGO
In the actual catalytic cracking processes,H2will be mixed with a small amount of CH4.So the solubility of mixed gas in VGO should be also measured at 353.15-453.15 K and 1-6 MPa.In this case,it is necessary to analyze the compositionof both gas and liquid phases when measuring the solubility data of H2+CH4in VGO.

Table 6.Experimental P(pressure)-T(temperature)-x(mole fraction in the liquid phase)data for the H2(1)+CH4(2)+VGO system at different pressures under the same inlet H2mole fraction 0.85 in the gas mixture.
The gas compositions yH2and yCH4in the gas phase,as well as the gas compositions xH2′ and xCH4′ desorbed from the liquid phase at low pressures,were detected by the gas chromatograph(GC 4000A)equipped with a thermal conductivity detector(TCD)and TDX-01 column(Φ3 mm×2 m).Thus,the solubility of H2and CH4in VGO was calculated by


Fig.12.Influence of temperature on the absorption selectivity S12of H2(1)to CH4(2)in vacuum gas oil at different pressures when the feeding gas composition is 85% H2+15%CH4(points,experimental data;solid lines,predicted values by the COSMO-RS model).

In this work,the mixed gas solubility in VGO at different feeding ratios in volume fractions(H285%+CH415% and H290%+CH410%)was measured.The detailed data are listed in Tables 6 and 7.
The real selectivity S of H2to CH4at the temperatures of 353.15-453.15 K was calculated by

The ideal selectivity S∞can be written as

where the activity coefficient at infinite dilution(γ∞)is calculated by the COSMO-RS model,as well PH2Sand PCH4S represent the saturated vapor pressures of H2and CH4,respectively.
The comparison between the real and ideal selectivities at two different feeding gas compositions is shown in Figs.12 and 13.
It can be seen that the ideal selectivity S∞predicted by the COSMO-RS model only depends on the temperature,while the real selectivity S depends on temperatures,pressures and the feeding gas composition.Moreover,both selectivities decrease with increasing temperatures,exhibiting the similar trend.In this case,the COSMO-RS model can only provide qualitative prediction.
5.Conclusions

Fig.13.Influence of temperature on the absorption selectivity S12of H2(1)to CH4(2)in vacuum gas oil at different pressures when the feeding gas composition is 90% H2+10%CH4(points,experimental data;solid lines,predicted values by the COSMO-RS model).
In this work,the solubility data and liquid-phase mass transfer coefficients of H2and CH4in VGO were first measured for providing the basic data for fuel oil catalytic cracking processes.It was found that the solubility of pure H2and CH4gases increases with the increase of pressures and the decrease of temperatures.There exists a good linear relationship between lnH and 1/T for both H2+VGO and CH4+VGO systems.Moreover,the liquid-phase mass transfer coefficients kLa increase with increasing pressures at a given temperature,due to the decreasing viscosity in the case of more gas dissolved in VGO.On the other hand,the experimental solubility data are in good agreement with the predicted values by COSMO-RS model using six virtual model compounds for pure H2and CH4.Among others,the model compound no.3 is the best one to predict the gas solubility in VGO.Besides,the real and ideal selectivities exhibit the similar trend with temperature,confirming that the COSMO-RS model can be used to qualitatively predict the gas solubility and selectivity in VGO.It is worth mentioning that the results presented in this work on the dissolution thermodynamics(e.g.,solubility and selectivity)and dynamics(e.g.,liquid-phase mass transfer coefficients)of H2,CH4and their mixtures in VGO may be directly extended to other gas-fuel oil systems.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Synthesis plasmonic Bi/BiVO4photocatalysts with enhanced photocatalytic activity for degradation of tetracycline(TC)☆
- Food processing wastewater purification by microalgae cultivation associated with high value-added compounds production—A review☆
- A state-of-the-art review on single drop study in liquid-liquid extraction:Experiments and simulations☆
- Thermal cracking characteristics of n-decane in the rectangular and circular tubes
- Stochastic modeling of subgrid-scale effects on particle motion in forced isotropic turbulence☆
- Performance comparison of heat exchangers using sextant/trisection helical baffles and segmental ones☆
