Liquid-liquid two-phase mass transfer characteristics in a rotating helical microchannel
2019-03-22YanCaoJunLiYangJinJianhongLuoYubinWang
Yan Cao,Jun Li*,Yang Jin,Jianhong Luo,Yubin Wang
Department of Chemical Engineering,Sichuan University,Chengdu 610065,China
Keywords:Microchannels Mass transfer Extraction Immiscible fluids Multiphase flow
ABSTRACT In this work,the mass transfer characteristics of two immiscible fluids were investigated in a rotating helical microchannel with hydraulic diameter of 932 μm.Aqueous phosphoric acid solution and 80%tri-n-butyl phosphate(TBP)in kerosene were selected for the investigation of mass transfer performance in quartz glass/high density polyethylene(HDPE)microchannel.High dispersion between the two immiscible fluids can be obtained in the microchannel due to the intensifying action of centrifugal force,and the majority of the droplets with average diameter of 20-100 μm were produced in the microchannel.The flow rate and rotation speed were found to have great effects on the extraction efficiency and average residence time.The empirical correlation of average residence time based on experimental data was developed by theoretical analysis and data fitting method,and a mathematical model of the mass transfer coefficient in dispersed phase was proposed.
1.Introduction
Liquid-liquid extraction as an important unit operation in the process of chemical separation plays a significant role,such as petroleum,hydrometallurgy,and food[1,2].However,many liquid-liquid mass transfer devices including mixer-settlers,extraction column,and vibrating sieve tray tower have the shortcomings of slow extraction rate and long residence time.Consequently,a series of problems are caused,such as bulkiness and industrial scale-up difficulties of the mass transfer devices.Therefore,it is necessary to intensify the mass transfer process.
Microchemical engineering technology as a new technology has received great attention over the last two decades due to its tremendous advantages in the enhancement of heat and mass transfer[3,4].The mass transfer capability is improved by 1-3 orders of magnitude compared with most of the conventional large-scale devices,owing to the large surface-to-volume ratio and short mass transfer distance in the microstructural devices[5].As a consequence,the residence time can be shortened effectively,which allows the miniaturization of extraction devices.On the basis of the advantages mentioned above,the microstructural devices have been widely applied in chemical synthesis,extraction,preparation of nanoparticles,and biochemical analysis,etc.[6-9].
The mass transfer performance shows extreme dependency on flow regimes.There are six different flow regimes such as slug,slug-drop,deformed interface,parallel/annular,slug-dispersed,and dispersed flow observed in microchannel for liquid-liquid systems[10].Generally,parallel flow[11,12]and slug flow[13]are the most researched flow patterns for two immiscible fluids in microchannel devices.In the parallel flow,two phases conduct mass transfer through molecular diffusion.And in the slug flow,convection within the individual liquid slug and diffusion between adjacent slugs are two basic mass-transfer mechanisms.However,the interfacial areas of parallel flow and slug flow are limited by the size of microchannel,therefore,it is difficult to further improve the extraction efficiency[14].
Currently,mass transfer enhancement by dispersing one phase into another in microchannels has been paid more attention by many researchers[15,16].Dispersed flow plays a vital role in further intensification of mass transfer due to its much larger interfacial area compared with slug flow and parallel flow.Therefore,lots of researchers have designed different types of microstructured devices,such as microchannel junction[17],micro-sieve array device[18]and multifunctional micromixer[19],where microscale droplets can be generated and mass transfer can be greatly enhanced.However,those microstructured devices were either limited in processing capacity(usually from a few microliters to several milliliters per minute)or hard to manufacture due to the complexity of their structures.In addition,in order to realize the enhancement of mass transfer,a third medium was introduced into the microchannel to promote the formation of highly dispersed droplets[20-22].Su et al.[23]developed a method of intensifying liquid-liquid mass transfer by introducing an inert gas into microchannels.It was reported that high dispersion between immiscible liquid-liquid two phases can be obtained when the flow rate of the gas was high enough.Su et al.[24]also presented another method of mass transfer enhancement by packing micro-particles in a T-shaped microchannel.The diameter of droplets produced in the microchannel was close to 10 μm,which assured larger mass transfer area of two-phase immiscible fluids and consequently improved the mass transfer performance greatly.However,the introduction of a third medium in microchannel led to challenges in operation control and increased process complexity.Moreover,the packing microparticles in microchannel would bring higher specific energy dissipation.
On the basis of the idea that improving the hydrodynamics and contact of two immiscible fluids can achieve better mass transfer performance,a new rotating helical microchannel was proposed in our group.Because the interfacial tension as the dominant force in microchannels can be easily overcome by the inertia force due to the rotation of drum,highly dispersed droplets can be generated in the microchannel.Thus,it is not necessary to add a third medium into the system.Efficient extraction can be realized in the rotating helical microchannel and it is easy to operate and control.Moreover,it has good adaptability to liquid-liquid two-phase systems with high volumetric flux ratio.
It is well known that the prediction of mass transfer performance for novel microreactor is necessary for its optimization design.Zhao et al.[25]has proposed a semi-empirical correlation of the volumetric mass transfer coefficient for parallel flow which was a function of the Reynolds number of immiscible liquid-liquid two phases,the volumetric flux ratio and the hydraulic diameter of microchannel.On the basis of the dual-film theory,Bai et al.[26]presented a relatively simple correlation of the global volumetric mass transfer coefficient for dispersed flow,which was related to the Reynolds number of two-phase mixtures and the two-phase volumetric flux ratio.However,the mass transfer characteristics of the dispersed flow which is influenced by centrifugal force in microchannel have not been reported so far.Thus,the internal mechanism of microscale mass transfer needs to be further explored and a reliable mathematical model should be established and developed.
In this work,aqueous phosphoric acid solution and 80%tri-n-butyl phosphate(TBP)in kerosene were selected as research fluids.The effects of flow rate,rotation rate,average diameter of droplets and average residence time on mass transfer performance,as well as the effects of flow rate and rotation rate on average residence time were investigated in a rotating helical microchannel.Furthermore,an empirical correlation was developed to predict the average residence time,and a mathematical model of the mass transfer coefficient in dispersed phase was also established.
2.Experimental Section
2.1.Materials
TBP(purity≥98.5%)and sulfonated kerosene were provided by Sichuan Xinruiyuan Technology Development Co.,Ltd.TBP was saturated with water before use.Phosphoric acid(85%,AR grade),SUDAN III and methylene blue were obtained from Chengdu Kelong Chemical Co.Ltd.In the visualization of flow phenomena,minute amounts of Sudan III and methylene blue were dissolved in the organic phase and aqueous phase,respectively.De-ionized water(electrical resistivity≥18.5×104Ω·m)produced by Aquapro RM-220(Ever Young Enterprises Development Co.,Ltd)was used to prepare the aqueous phase solutions.
2.2.Experimental device and setup
Fig.1 shows the schematic diagram of the rotating helical microchannel device used in this work.The device had an open inlet and an open outlet.It was considered that the channel consisted of numerous unit channels,each of which was composed of a 400 μm channel(d1,distance from the outer edge of the drum to the inner edge of the smooth outer cylinder)and a micro-helical channel with 250 μm depth(d2)and 2000 μm(h1)width.The drum was made of high density polyethylene(HDPE)with hydrophobic properties and the outer cylinder was made of quartz glass with hydrophilic properties.The microhelical channel was fabricated by micromachining technology at the outer surface of the drum,and was designed for draining the mixed fluids out of the device in case of overflowing.In all experiments,the drum rotated at a given speed and the outer cylinder always remained stationary.
Fig.2 shows the schematic diagram of the experimental setup used in this work.In all experiments,the volume flux ratio of organic phase to aqueous phase was 4/1.The aqueous phase and organic phase were fed by two peristaltic pumps(BT100S/YZ15,Baoding Lead Fluid Technology Co.,Ltd.).Then,the two-phase fluids flew out of respective feed pipes and fell on the top surface of the drum.Due to the effects of centrifugal force and gravity,the two-phase fluids flew into the microchannel and conducted mass transfer in the microchannel.A centrifuge tube was used to collect samples from the outlet streams,and the collected samples were immediately transferred into a high-speed centrifuge(TG16-WS,Xiangyi Centrifuge Instrument Co.Ltd.),where the aqueous phase was completely separated from the organic phase at the centrifugal speed of 8000 r·min-1to prevent continuous mass transfer during the stay.To ensure the stability of samples,each experimental sample was obtained after the extraction had been going for at least 1 min.The flow characteristics for two-phase immiscible fluids in the microchannel were observed by a high-speed CCD camera(pco.dimax CS1,PCO).

Fig.1.Schematic diagram of the rotating helical microchannel.

Fig.2.Schematic diagram of the experimental setup:(1)aqueous tank,(2)organic tank,(3)peristaltic pump for aqueous phase,(4)peristaltic pump for organic phase,(5)feed pipe for aqueous phase,(6)feed pipe for organic phase,(7)microchannel apparatus,(8)centrifuge tube,(9)high-speed centrifuge,(10)CCD camera.
2.3.Experimental analysis
In this work,extraction experiments for investigating the mass transfer characteristics in the rotating helical microchannel were performed under ambient conditions(ca.0.1 MPa,25 °C).The viscosity was measured with an Ubbelohde viscometer,and the interfacial tension of the working system was measured with an automatic surface tensiometer(BZY-201,Shanghai Fangrui Instrument Co.,Ltd).Relative measurement errors were 1%and 3%,respectively.The physical properties of the working systems are listed in Table 1.
The droplet size can be measured by a particle size analyzer(BT-9300S,BETTERSIZE INSTRUMENTS LTD.,China)and an electron microscope(XDS-1B,Chongqing Photoelectric Instrument Co.,Ltd.,China).The droplet sizes were measured by the particle size analyzer under the condition of high rotation rate(R=300-700 r·min-1).The droplet sizes at low rotation rate(R=100-200 r·min-1)were bigger.It was found that the accuracy of the particle size analyzer in measuring the size of larger droplets was poor,because larger droplets were prone to break up in the process of dispersion.Thus,the electron microscope was used to measure the droplet sizes at low rotation rate.The measurement of average diameters of droplets with the particle size analyzer was similar to the measurement of droplet sizes employed by Zou et al.[27].A sample was taken into the sample pool which was filled with kerosene as a solvent.Under the condition of continuous measurement,the average size of droplets was determined.The measurement ofaverage diameters of droplets with the electron microscope had the similarity with the measurement of droplet sizes mentioned by Li et al.[28]and Xu et al.[29].The images were captured by the electron microscope and stored in the computer memory for further analysis.The number of droplets used for droplet sizes measurement is no less than 100.The images were analyzed using a software of ChemDemo 3.0,by which the droplets were identified manually and the droplet sizes were measured automatically.The average diameters of droplets obtained by the above two methods were the mass mean droplet diameter(d43)[27],which can be defined as a ratio of fourth and third moments of a droplet size distribution.

Table 1 Physical properties of the working systems at 25°C and atmospheric pressure

where niis the number of droplets in size interval i and diis the droplet diameter in size interval i.A particle size distribution is shown in Fig.3.
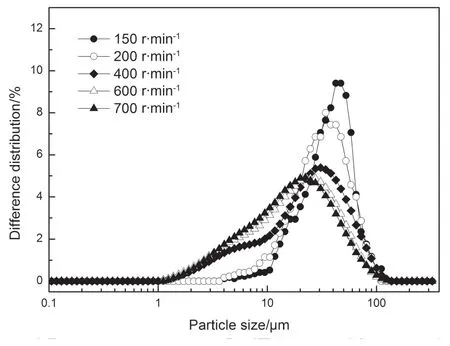
Fig.3.A particle size distribution under the conditions of Qaqu=4 ml·min-1,R=150,200,400,600,700 r·min-1.
The measurement of average residence time was performed by a stopwatch(ZS-2B,Shanghai sasey clock&watch Co.,Ltd).At first,feed pipes were filled with respective liquids in advance.Then,the peristaltic pumps and the stopwatch were started simultaneously.When the annular liquids were observed at the end of the drum,the timing was stopped.The time shown on the stopwatch was the average residence time of fluids in the microchannel.Relative measurement error of average residence time was 3%.
The concentration of phosphoric acid in the organic phase or aqueous phase was analyzed by an automatic potentiometric titration instrument(916 Ti-Touch,Metrohm Switzerland Ltd.,Herisau,Switzerland)which was designed on the basis of the principle of potential method.The concentration of phosphoric acid can be determined based on the neutralization reaction between the sodium hydroxide as a standard reagent and the phosphoric acid in the aqueous phase or phosphoric acid stripped from the organic phase.Relative measurement error was 1%.
2.4.Definitions of relevant parameters
The extraction efficiency[30](η)can reflect the performance of the extraction and it can be defined as follows:

where Corgis the concentration of phosphoric acid in the organic phase after phase separation.Corg,0is the initial concentration of phosphoric acid in the organic phase(approximately Corg,0≈0 in our system).Corg*is the equilibrium concentration of phosphoric acid in the organic phase.In this work,Corg*can be determined by our experimental measurements:the experiments were performed in a 250 ml beaker,where 40 ml of aqueous phase was mixed with 160 ml of organic phase;the two phases were stirred at a speed of 300 r·min-1for 2 h,then allowed to settle for 2 h;the aqueous phase was separated from organic phase by a separating funnel;the concentration of phosphoric acid in the organic phase can be determined by the automatic potentiometric titration instrument.
It was observed that in most cases the outlet streams from the microchannel were turbid and opaque,so it was predicted that waterin-oil droplets were presented in the microchannel.Furthermore,blue aqueous droplets in the experimental system of TBP/kerosene with SUDAN III dye/phosphoric acid with methylene blue can be observed by CCD camera,and a dispersed flow is shown in Fig.4.
For a single droplet,the amount of solute released from the droplet is equal to the amount of solute deceased in the droplet in differential time(dt)by the law of conservation of mass.Thus,a mass conservation equation[31]can be obtained as follows:

Fig.4.A dispersed flow observed in the middle segment of the microchannel by CCD camera(the image taken under the conditions:Qaqu=12 ml·min-1,Qorg=48 ml·min-1,R=400 r·min-1).

where kdis the mass transfer coefficient of dispersed phase,A is the surface area of droplet,Vdis the volume of droplet,Caquis the concentrations of phosphoric acid in the aqueous phase,and Caqu,iis the interfacial concentration of phosphoric acid in the aqueous phase.Then,Eq.(3)can be expressed as following integral equation:
where τ is the average residence time,Caqu,0is the initial concentration of phosphoric acid in the aqueous phase,andis the average diameter of droplets which is indicated as the mass mean droplet diameter(d43)in this work.The Eq.(4)can be further expressed as follows:

Based on the double film theory,the interfacial concentrations of phosphoric acid in the aqueous phase and organic phase should meet the following equilibrium relation:

where Corg,iis the interfacial concentration of phosphoric acid in the organic phase.The molecular diffusivities of phosphoric acid in the aqueous phase(Daqu)and organic phase(Dorg)which are calculated by Wilke-Chang formula[32]are 1.86×10-10m2·s-1and 9.76×10-10m2·s-1,respectively.Due to the higher diffusivity and lower viscosity in the organic phase,it can be assumed that the mass transfer resistance in the liquid film of organic phase is very small and can be neglected approximately.Then,there is the following relation:

A further assumption is that there is little change in the concentration of phosphoric acid in organic phase,which is approximately considered as a constant,then the equilibrium relationship of two phases can be approximated as follows:

Combining Eq.(8)with Eqs.(6)and(7),the following relation can be obtained

Furthermore,Eq.(5)can be expressed as follows:

On the basis of the following relations,

the kdcan be expressed as the following equation:

Based on the content of Section 2.2,it was known that the channel of the device was composed of numerous unit channels as shown in Fig.1.Therefore,the hydraulic diameter(dH)of the microchannel can be defined on the basis of one unit channel,and the formula for dHis as follows:

where d1,d2,h1,and h2are the related sides on the cross section of the unit channel as shown in Fig.1.
3.Results and Discussion
3.1.Mass transfer characteristics of microchannel extraction
3.1.1.Comparison of conventional agitation extraction and microchannel extraction
The conventional agitation extraction was performed in a 250 ml beaker,where 40 ml of aqueous phase was mixed with 160 ml of organic phase;the two phases were stirred at a speed of 200 r·min-1.At the meanwhile,the microchannel extraction was carried out with the same system for comparison.The results are shown in Fig.5.The extraction of H3PO4almost completed in 20 s in the microchannel device,while the extraction efficiency was only about 43%in conventional agitation device.Even when the residence time was 60 s,the extraction efficiency was only about 65% in the conventional agitation device.Therefore,it is obvious that the faster extraction can be achieved in the microchannel device compared with the conventional agitation device.
3.1.2.Effects of flow rate on extraction efficiency
In order to investigate the effects of flow rate on extraction efficiency,two rotation rates of 100 r·min-1and 300 r·min-1were chosen.As shown in Fig.6,the extraction efficiency decreased with the increase in flow rate,and the extraction efficiency decreased more rapidly with the increase in flow rate at the rotation rate of 100 r·min-1than that at the rotation rate of 300 r·min-1.The results indicated that extraction efficiency was affected by both average residence time and interfacial area of the two immiscible fluids.Furthermore,the average residence time was influenced by flow rate and rotation rate,and the interfacial area was directly related to the droplet sizes which were also decided by flow rate and rotation rate.

Fig.5.Comparison of conventional agitation extraction and microchannel extraction.
At the rotation rate of 100 r·min-1,the effect of centrifugal force was rather weak.As a result,the aqueous phase was difficult to be dispersed into the organic phase but to be agglomerated into a mass as shown in Fig.7a.In turn,the interfacial area of the two immiscible fluids became very small.Meanwhile,the fluids in the microchannel were simultaneously affected by gravity,which caused the fluids flow out of the microchannel vertically rather than follow the micro-helical channel.Consequently,a flow pattern of“falling-rotating-falling”was created,which resulted in a shortened average residence time.As the flow rate increased,the velocity of fluids in the microchannel increased,which led the average residence time to be much shorter.The small interfacial area and short residence time would lead to low extraction efficiency.As Fig.6 showed that the extraction efficiency was the lowest at the rotation rate of 100 r·min-1and flow rate of 120 ml·min-1.
At the rotation rate of 300 r·min-1,centrifugal force played a more dominant role than gravity.As a result,the fluids flew out of the microchannel along the micro-helical channel rather than fell vertically in the annular microchannel as shown in Fig.7b.In this way,the average residence time of fluids can be effectively guaranteed.Although the average residence time would decrease slightly with the increase of rotation rate and flow rate,the interfacial area of the two phases was greatly improved for the formation of dispersed droplets in the microchannel as shown in Fig.4.Because the positive impact of increased interfacial area greatly overcame the negative impact of decreased residence time,the high extraction efficiency of over 94%still can be ensured.
3.1.3.Effects of rotation rate on extraction efficiency
In this section,7 groups of different flow rates were chosen to investigate the effects of rotation rate on extraction efficiency as shown in Fig.8.The extraction efficiency increased obviously with the increase in rotation rates from 100 r·min-1to 300 r·min-1,and then gradually leveled off to a steady efficiency by further increasing the rotation rates from 300 r·min-1to 700 r·min-1.The result implied that the extraction efficiency was mainly affected by the interfacial area of two immiscible fluids at a given flow rate,and the interfacial area was mainly affected by the rotation rate.In the microchannel,the imbalance between the interfacial tension and the inertia force would cause the fracture of the aqueous phase and organic phase,and one phase would be dispersed into another phase in the form of droplets[33].In this work,when the inertia force resulting from the rotation of drum was greater than the interfacial tension,the aqueous phase would be torn easily and dispersed droplets would be formed in the organic phase.The higher the rotation rate was,the smaller the droplet size was.The consequence was the interfacial area of the two immiscible fluids would become larger.However,when the droplet size was reduced to a certain level,the mass transfer was basically saturated.Even if the rotation rate continued to increase,there was little effect for the enhancement of mass transfer.Therefore,the extraction efficiency basically maintained at a constant value.
3.1.4.Effects of average diameter of droplets on extraction efficiency
The droplet size is a very important parameter in the study of dispersed flow.Therefore,the average diameter of droplets under different conditions of flow rate or rotation rate is measured in this work.As shown in Fig.9,the average diameters of droplets increased with the increase in flow rate and decreased with the increase in rotation rate.It can be explained that the average residence time of fluids decreased with the increase of flow rate,which led to weaker dispersion of the aqueous phase.The inertia force increased with the increase of rotation rate,which caused the aqueous phase to be dispersed more easily.Furthermore,the interfacial area increased with the decrease of droplet size,which was contributed to the improvement of extraction efficiency.Therefore,the trend reflected in Fig.9 coincided with that in Fig.8.In order to further find the association between the average diameter of droplets and extraction efficiency,we plotted η withunder the same conditions of flow rate and rotation rate as shown in Fig.10.In general,the extraction efficiency decreased with the increase in the average diameter of droplets.However,the extraction efficiency changed little(from 94%to 99%)when the average diameters of droplets were within 100 μm.The result can be explained that the extraction efficiency was affected by the interfacial area which was decided by the droplet sizes,and smaller interface area due to bigger droplet size led to lower extraction efficiency.As the discussion in Section 3.1.3,when the droplet size was reduced to a certain level,the mass transfer was basically saturated.The further decrease in droplet size had little effect on the improvement of extraction efficiency.Therefore,it can be concluded that if the droplet size can be well controlled in the range of 50-100 μm,a high extraction efficiency can be achieved,and it was not necessary to consume extra energy to further reduce the droplet size.

Fig.6.Effects of flow rate on extraction efficiency.
3.2.Effects of average residence time on extraction efficiency
3.2.1.Effects of rotation rate and flow rate on average residence time
In general,the average residence time can be changed primarily by changing the volume of the microchannel and/or the flow rate[34,35],while it can be seen from Fig.11 that the average residence time was influenced not only by the flow rate but also by the rotation rate in the microchannel device.In this work,the microchannel of the experimental device was placed in the vertical direction.Due to the effect of gravity,the fluids in the microchannel had a vertical velocity which increased with the increase in flow rate.Therefore,the average residence time was shortened with the increase of flow rate at the same rotation rate.Meanwhile,the fluids in the microchannel were affected by centrifugal force which drove the fluids to leave the device rapidly along the micro-helical channel.The greater the centrifugal force was,the higher the tangential velocity of fluids was.Thus,the average residence time essentially became shorter with the increase of rotation rate at the same flow rate.
However,a turning point of the average residence time appeared with the increase of rotation rate as shown in Fig.11;in other words,there was a slight increase of average residence time with the increase of rotation rate.The result can be explained that the volume of the microchannel was no longer a“fixed”value but a changed and higher value due to the effect of centrifugal force which would make the falling liquid to be raised again.As a result,the average residence time would increase slightly.In addition,the turning point of average residence time shifted to the right slightly with the increase of flow rate.As shown in Fig.11,the turning point appeared at the rotation rate of 150 r·min-1when the flow rates were 40~80 ml·min-1,while the turning point moved at the rotation rate of 200 r·min-1when the flow rates were 100 ml·min-1and 120 ml·min-1.This was because the gravity of fluids increased with the increase of flow rate,so that greater centrifugal force was needed to overcome gravity to raise the falling liquids again.

Fig.7.Comparison of effects of gravity and centrifugal force on fluids:(a)rotation rate of 100 r·min-1,(b)rotation rate of 300 r·min-1.

Fig.8.Effects of rotation rate on extraction efficiency.
Furthermore,the distribution range of average residence time became narrower with the increase of rotation rate.As shown in Fig.11,the distribution range of average residence time was from 6 s to 20 s at the rotation rate of 100 r·min-1,while the distribution range of average residence time was from 4.7 s to 5.2 s at the rotation rate of 700 r·min-1.That was because the centrifugal force of fluids was much smaller when the rotation rate was lower,and the contribution of gravity to the average residence time was greater than that of the centrifugal force.As a result,a slight change of flow rate would cause dramatic changes in the average residence time.While,the centrifugal force of fluids was much greater when the rotation rate was higher and the centrifugal force gradually replaced gravity as the dominant force acting on the average residence time,so that the average residence time at the same rotation rate basically remained the same even if the flow rate changed greatly.
3.2.2.Relation map between average residence time and extraction efficiency

Fig.9.Effects of rotation rate and flow rate on average diameter of droplets.

Fig.10.Effects of average diameter of droplets on extraction efficiency.
It was reported[30]that the extraction efficiency was the same at the same average residence time.In order to investigate the relation between the average residence time and the extraction efficiency in this microchannel device,an extraction efficient zone map,which was related to average residence time,were drawn as shown in Fig.12.The enclosed area of the broken line was the extraction efficient zone,where the extraction efficiency was over 95%.It can be seen from Fig.12 that the extraction efficiency over 95%can probably be reached in the range of average residence time from 4-20 s,and the extraction efficiency may be different even when the average residence time was the same.Therefore,the results indicated that the extraction efficiency was not completely correlated with the average residence time in this microchannel device.
This extraction efficient zone map can be used to predict the extraction performance of the working system under certain operating conditions and achieve the control to the average residence time by the adjustment of operation parameters,so that the extraction of the working system can be always kept in the high efficient zone.
3.3.Correlation of average residence time
It was clear that the average residence time was influenced not only by the flow rate but also by the rotation rate on the frontal discussions in Section 3.2.1,which made the calculation of average residence time to be complicated.Because the average residence time played an important role in the construction of mass transfer model,it is crucial to establish the average residence time model.By the force analysis for the droplet,it is clear that gravity deprived from flow rate will make the droplet have a vertical velocity,and centrifugal force deprived from rotation rate will make the droplet have a tangential velocity.The average residence time can be obtained by the calculation where the length of micro-helical channel is divided by the synthetic speed of vertical velocity and tangential velocity.

Fig.11.Effects of rotation rate and flow rate on average residence time.

Fig.12.Extraction efficient zone map.
For the vertical velocity,it can be defined by the following equation:

and φ is the gravity influence factor,which can be defined as:

where h is the height of the drum,Q is the total flow rate of the aqueous phase and organic phase,V is the volume of the microchannel,ReMis the Reynolds number of the two-phase mixtures,and k1is the correction factor.In this work,because the microchannel had an open inlet and outlet,there would be a little air occupying the microchannel,so that the microchannel cannot be completely filled.The filling volume of liquids in the microchannel increased with the increase in flow rate.Thus,the filling volume of liquids was variable.However,the V in Eq.(14)is a constant,so a gravity influence factor(φ)associated with flow rate should be introduced into Eq.(14)to correct the V.Considering the effects of flow rate on the velocity of fluids,and the effects of the velocity of fluids on the motion of fluids,the ReMwhich can be used to characterize the motion of fluids has been introduced into Eq.(15).Then,the vertical velocity can be further expressed as:
For the tangential velocity,it can be normally defined as:

where k2is the correction factor,ω is the angular velocity of drum,and Dois the external diameter of drum.
The correlation for the average residence time is defined by the following equation:

where L is the length of micro-helical channel.Taking the Eqs.(16)and(17)into Eq.(18),the correlation for the average residence time can be expressed as follows:

In the rotating helical microchannel,the related structural parameters are as follows:
L=11.1 m,h=0.2 m,V=1.9×10-5m3,Do=0.053 m
Then,the fitting results by the software of 1stopt1.0 for Eq.(19)are as follows:
k1=1988.27,k2=1.1867,α=-0.546,R2=0.9550
Those values are taken into Eq.(19)and the final empirical correlation for the average residence time is obtained,as shown in Eq.(20).

The experimental and calculated values of the average residence time were compared and the result was shown in Fig.13.The relative deviations between the experimental and calculated values were within 20%.Therefore,it is reasonable to carry out theoretical calculation for the average residence time by Eq.(20).
3.4.Modelling of mass transfer
Li[36]has studied the mass transfer performance for the system of phosphoric acid and TBP in liquid-liquid phase micro extraction,and has established a mathematical model of mass transfer coefficient inside droplet.However,the model of mass transfer coefficient in continuous phase with higher viscosity,in which there was greater mass transfer resistance,was not considered.
In this work,the aqueous phase as dispersed phase has higher viscosity and lower diffusivity compared with the organic phase as continuous phase.It can be considered that the extraction process is controlled by diffusion in dispersed phase.Therefore,it is crucial to establish the model of the mass transfer coefficient in dispersed phase.Based on the modification of the classical Newman's full model for mass transfer in drops[37,38],the empirical correlation for the mass transfer coefficient in dispersed phase can be obtained,as shown in Eq.(21).

It is well known that the Reynolds number(Re=ρudH/μ)and Weber number(We=ρu2dH/σ)are the dimensionless numbers related to the inertial force,therefore,the Reynolds number and Weber number can be used to express the enhancement effect of inertial force in the mass transfer process between the two immiscible fluids.Furthermore,the mass transfer performance was closely related to the mass transfer area which was determined by the size of droplets on the frontal discussions in Section 3.1.4.Therefore,/dHcan be used to further strengthen the action of inertia force.As a consequence,a formula of correction factor fdwith Reynolds number,Weber number and/dHhas been established,as shown in Eq.(22).

The formula of correction factor fdcan be obtained by the optimization method of Levenberg-Marquardt and Universal Global Optimization for the software of 1stopt1.0 and it is shown in the following:

Based on the established mass transfer correlations Eqs.(21)and(23),the comparison between the experimental values and the calculated values of mass transfer coefficients(kd)for 56 experimental data points is shown in Fig.14.The relative deviations between the experimental and calculated values of mass transfer coefficient were basically within 15%.Therefore,it is reasonable to carry out theoretical calculation for mass transfer coefficient of the dispersed phase by the theoretical model.The establishment of the mass transfer coefficient model in dispersed phase will provide a good theoretical basis for the optimization design of the microchannel.
4.Conclusions
A new rotating helical microchannel that could intensify the mass transfer of two immiscible fluids was investigated.High dispersion between two immiscible fluids can be obtained by the intensifying action of centrifugal force.It was found that both the extraction efficiency and average residence time were influenced by flow rate and rotation rate,and low flow rate and high rotation rate can ensure high extraction efficiency(usually over 95%);a high extraction efficiency can also be achieved if the average diameter of droplets was controlled well within 100 μm;the extraction efficiency was not completely correlated with the average residence time in the rotating helical microchannel.An extraction efficient zone map associated with average residence time was obtained and it can be used to predict the extraction performance of the working system.An empirical correlation for the average residence time was developed on the basis of experimental data.Furthermore,a mathematical model of the mass transfer coefficient in dispersed phase was established.The relative deviations between the experimental and calculated values of kdwere basically within 15%by the proposed model.The results obtained are important for the optimization design and the scale-up design of microchannel.

Fig.14.Comparison of experimental and calculated values of kd.
Nomenclature
A surface area of droplet,m2
Caquconcentrations of phosphoric acid in the aqueous phase,mol·L-1
Caqu,0initial concentrations of phosphoric acid in the aqueous phase,mol·L-1
Caqu,iinterfacial concentration of phosphoric acid in the aqueous phase,mol·L-1
Corgconcentrations of phosphoric acid in the organic phase,mol·L-1
Corg,0initial concentrations of phosphoric acid in the organic phase,mol·L-1
Corg,iinterfacial concentration of phosphoric acid in the organic phase,mol·L-1
Daqumolecular diffusivity of phosphoric acid in the aqueous phase,m2·s-1
Dorgmolecular diffusivity of phosphoric acid in the organic phase,m2·s-1
Dothe external diameter of drum,m
d43mass mean droplet diameter,m
dHhydraulic diameter of microchannel,m
didroplet diameter,m
diaverage diameter of droplets,m
fdcorrection factor
h height of drum,m
kdmass transfer coefficient of the dispersed phase,m·s-1
k1,kicorrection factor
L length of micro helical channel,m
m1-5correction factor
Q total flow rate of aqueous phase and organic phase,m3·s-1
Qaquflow rate of aqueous phase,ml·min-1
R rotation rate,r·min-1
Re Reynolds number
ReaquReynolds number of aqueous phase
ReMReynolds number of two-phase mixtures
uvelocity of fluid,m·s-1
uGvertical velocity of mixed fluids,m·s-1
urtangential velocity of mixed fluids,m·s-1
V volume of the microchannel,m3
Vdvolume of droplet,m3
We Weber number
WeaquWeber number of aqueous phase
η extraction efficiency,%
μ viscosity,Pa·s
ρ density,kg·m-3
σ interfacial tension,N·m-1
τ average residence time,s
φ gravity influence factor
ω angular velocity of a drum,rad·s-1
Subscripts
aqu aqueous phase
M mixture of the immiscible liquid-liquid two phases
org organic phase
0 initial
Acknowledgments
Project supported by the National Natural Science Foundation of China(No.21776180,21776181,21306116).
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Synthesis plasmonic Bi/BiVO4photocatalysts with enhanced photocatalytic activity for degradation of tetracycline(TC)☆
- Food processing wastewater purification by microalgae cultivation associated with high value-added compounds production—A review☆
- A state-of-the-art review on single drop study in liquid-liquid extraction:Experiments and simulations☆
- Thermal cracking characteristics of n-decane in the rectangular and circular tubes
- Stochastic modeling of subgrid-scale effects on particle motion in forced isotropic turbulence☆
- Performance comparison of heat exchangers using sextant/trisection helical baffles and segmental ones☆
