Shell powder as a novel bio-filler for thermal insulation coatings☆
2019-03-20PeigenZhangJingwenTangQiangTangMinzhaoZhangLuweiShenWubianTianYameiZhangZhengmingSun
Peigen Zhang,Jingwen Tang,Qiang Tang,Minzhao Zhang,Luwei Shen,Wubian Tian,*,Yamei Zhang,Zhengming Sun,*
1Jiangsu Key Laboratory of Advanced Metallic Materials,School of Materials Science and Engineering,Southeast University,Nanjing 211189,China
2Jiangsu Key Laboratory of Construction Materials,School of Materials Science and Engineering,Southeast University,Nanjing 211189,China
Keywords:Bio-filler Fluorocarbon coating Shell powder Thermal insulation
A B S T R A C T The feasibility of employing shell powder as a novel bio-filler to prepare fluorocarbon coating is demonstrated.According to the relevant Chinese standards,the thermal and mechanical properties of the shell powder-filledfluorocarbon coating were evaluated,and compared with those filled by commercial calcium carbonate.All the shell powder-filled coatings can meet the requirements stated in the relevant standards,and with decreasing the particle sizeoftheshell powders,the performance of thethermalinsulation coating isenhanced.The coating(SC3)filled by shell powders with an average particle size of 2.81 μm possesses a better thermal insulation performance than the coating(CC)filled by commercial calcium carbonate.The coating SC3 has comparable adhesive force and washing resistance with the coating CC,and in the washing resistance test,after 2000 cycles,the coating SC3 was still able to cover totally their substrates.This work demonstrates a high value-added disposal method for the aquacultural wastes.
1.Introduction
As the aquaculture industry rapidly grows,alongthecoastsin China,an increasing amount of mollusk shells has brought tremendous pressure on theenvironment.Shell is a hard,protectiveexoskeleton created by the mollusks.The seashell primarily consists of calcium carbonate(~95%),with minor organics,such as glycoproteins,polysaccharides,chitin,and other proteins[1,2].Because the shells endure very much,they can accumulate if no appropriate disposing method is carried out.In addition to visual discomfort,when decompose,they produce unpleasant odors which are harmful to human beings.In many cultures,some shells are used asornaments,but thehuge volume of shell wastes has to be disposed in other ways.The effective utilization of mollusk shells at large scale,like other bio-wastes,such as eggshell[3,4]and ricehulls[5],issignificantinoursocietynotonlyforeconomic concerns but also for sound environment.Currently,the feasibility of utilizing shell powders as adsorbents to remove dyes and metal ions from aqueous solutions has been investigated[2,6-9],which would prove to benefit the environment.These applications primarily harness the adsorption capacity of the shell powders.In addition to being used as adsorbents due to their adsorption capacity,shell powders might be used as bio-fillers thanks to their major chemical composition,calcium carbonate.Based on the major composition of shells,to overcome the environment problem and add more value on the shell waste,several other utilizations of shell powders have been explored,such as reinforcement phase in cement mortar[10],and bio-filler for ABS and epoxy composites[1,11].In this study,we extend the scope of the utilization of shell by demonstrating a higher value-added application of shell powders as fillers in cool coatings.
In buildings,temperature rise increases the demands for air conditioning and energy consumption,leading to the increase of heat emission and further temperature rise of local microclimates within urban areas.In industry,solar radiation leads to the rise of temperature of oil storage tanks,which not only brings oil evaporation,but also causes a lot of potential problems such as explosions[12-14].In order to reduce the surface temperature of the tank,thus to reduce oil evaporation loss,a conventional method is to cool storage tanks with water spray,which leadstoawasteofenergyandwater[15-17].Athermalinsulationcoatingcanenhancethecomfort level ofoccupants by reducingthetemperature of the buildings in hot areas in summer,and cool the industrial storage tanks[18-20].The major components of a coating are the binder and filler combined with other additives[21-23].The binder provides a continuous phase(film)and determines some main characteristics of the coating,such as strength and weatherability,and thefiller is discontinuously distributed in the film,bettering the performance of the coating.The selection of different raw materials and their compositions have important influences on the final performance of the coating.

Table 1 Compositions of the coatings

Table 2 Sample codes of coatings and their fillers
In recent years,nanometer calcium carbonate is the most widely used filler for coating and has been well studied[24,25].However,the manufacturing of nanometer calcium carbonate is resourceconsuming and expensive[26,27].This study will demonstrate the feasibility of replacing CaCO3with shell powders,and it focuses mainly on the effect of different types of fillers and particle sizes on the thermal and mechanical properties of the final thermal insulation coatings.
2.Experimental
2.1.Raw materials and the formula of the coatings
Samples were prepared by employing fluorocarbon emulsion as a binder,TiO2as a pigment,hollow glass microspheres and differentsized shell powders or commercial calcium carbonate as fillers,whose formula is shown in Table 1.The coating filled by commercial calcium carbonate is for comparison purpose.The samples are denoted as SC1,SC2,SC3,and CC,(cf.Table 2).The commercial rutile TiO2,grade Ti-Pure R-902,was purchased from DuPont Chemicals Co.,Ltd.Shells were collected on the coast of Qingdao,Shandong province.The mechanical impurities were removed by washing,and the rinsed shells were dried in an oven.Finally,the shells were grinded and sieved into three powders(SP1,SP2 and SP3)with different particle sizes,which was characterized by a laser granularity analyzer.The crystal components included in the shell powders were identified by XRD(D8-Discover,Bruker).Other ingredients in the recipe of the coatings in this study were purchased from Sinopharm Chemical Reagent Co.,Ltd.

Fig.2.Size distribution of the three shell powders.
2.2.Characterization and measurements
The surface morphology of fillers(shell powders and CaCO3)and coatings was characterized using a field emission scanning electron microscope(XL 30,FEI-Philips).
The coating SC1 was prepared as follows.The fluorocarbon emulsion,TiO2pigment,dispersant,anti-foaming agent and water were firstly poured into a mixing tank and stirred at a high speed of 1000 r·min−1for 30 min,resulting in a homogeneous mixture.Then,thehollowglassspheres,shellpowders(SP1),thickener,andcoalescent agent were added to the mixture,followed by stirring at a low speed of 300 r·min−1for 30 min.Finally,the balancing water was added to adjusttheviscositytoatargetvalue.Thepreparationprocessesforcoatings SC2,SC3 and CC are virtually identical to that of SC1.The coatings wereappliedbyafilmspreadertodifferentsubstratestotesttheperformances according to the corresponding standards,which will be described in later sections.
Accordingto theChinese constructionmaterials standard JC/T 1040-2007,the solar reflectance of the shell powders,commercial calcium carbonate was measured,respectively,by using a UV/VIS/NIR spectrophotometer(Agilent Cary 5000)equipped with an integrating sphere.Inthisstandard,thesubstrateforreflectancemeasurementsisanaluminum plate(40 mm×40 mm×5 mm),and the scanning range is from 250 to 2500 nm.

Fig.1.The schematic of the equipment used for insulation performance test.

Fig.3.The XRD pattern of the shell powders.
Thermal insulation performance tests were carried out by using a lab-developed setup,which is schematically shown in Fig.1.The equipment is composed of an iodine tungsten lamp,a sealed polystyrene box covered with tinfoil,two thermal couples(TC1 and TC2),and a data recorder.The 30 cm×30 cm×30 cm box had five walls and a plate made of concrete or steel on which the coating was brushed.The thickness of all the five walls was 2 cm.The thickness of the concrete plate and steel plate was 2 cm and 2 mm,respectively.The lamp was placed 50 cm away from the coated lid.One thermal couple was placed in the body center of the box and the other one was fixed in the center of outside surface of the coated lid,to monitor the indoor and the lid surface temperatures.
The washing resistance was examined by a tester according to the Chinese National Standard GB/T 9266-2009.Specifically,a coating was formed on a stainless steel plate of 430 mm×150 mm×3 mm,and then the coated substrate was placed under a reciprocating brush.The washing resistance was evaluated by counting the cycles until the substrate was completely exposed.
The adhesion strength of coatings was examined according to Chinese National Standard(GB 1720-79)by a film adhesion tester.By this standard,a coating was formed on a stainless steel slide of 100 mm×50 mm×0.5 mm,and then the coated substrate wasfixed on a platform equipped with a needle.The coating was scratched by the needle whenthe platform reciprocally moved.Thefilm adhesion strength was assessed based on the morphology of the scratches.
3.Results and Discussion
3.1.Properties of the fillers
3.1.1.Size distribution and phase composition of the shell powders
The size distributions of the three shell powders(SP1,SP2 and SP3)usedasfillerstopreparecoatingsamplesSC1,SC2andSC3werecharacterized by a laser granularity analyzer.The results as shown in Fig.2 indicate the average sizes of the three powders are 4.10 μm,3.14 μm and 2.81 μm,respectively,as listed in Table 2.
3.1.2.Phase composition of the shell powders
The typical XRD patterns of the three powders are shown in Fig.3,indicating the major chemical component of the powders to be calcium carbonate.Generally,shells have two layers:an outer prismatic layer(consisting of calcite)and an inner nacreous layer(consisting of aragonitic calcium carbonate)[28].The polymorphs of calcium carbonate which were found to exist in the shell powders used in this study are calcite,aragonite,and a small amount of vaterite,among which calcite,belonging to a monoclinic system,is the major polymorph,as it is the most stable form.The vaterite is not thermodynamically stable,so it is the minor calcium carbonate in the shell powders.
3.1.3.SEM characterization of the fillers

Fig.4.The micrographs.(A):SP1,(B):SP2,(C):SP3,and(D):Commercial CaCO3.
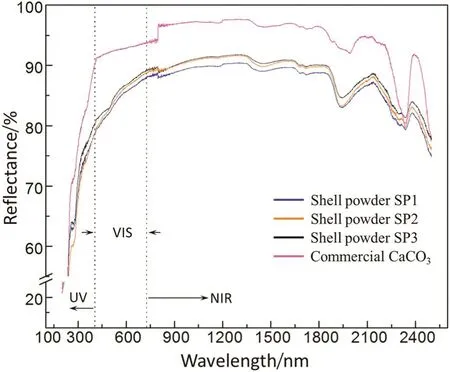
Fig.5.Solar reflectance of shell powders(SP1,SP2 and SP3)and the commercial CaCO3.
The morphology of the shell powders was characterized by SEM as shown in Fig.4(A)-(C),and compared with the commercial calcium carbonate,Fig.4(D),with respect to their shape,size distribution and dispersity.It is clear from the SEM images that the three shell powders vary in size,but they all include two major microscopic morphologies,irregular particle and platelet.The stacked platelets(less than 1 μm thick)consists of aragonitic calcium carbonate and organic materials,arrangedina“brick-and-mortar”microstructurewithanorganicmatrix interlayerthatistraditionallyconsideredasservingasgluebetweenthe single platelets[28].In addition,shell powders,Fig.4(A)-(C),have better dispersity compared with the commercial calcium carbonate shown in Fig.4(D),which might be understood that the organic matters contained in shells,after milling,improve the dispersity of the calcium carbonate powders.The better dispersity of the shell powders is an advantage to increase the thermal performance of the final coatings.
3.1.4.Solar reflectance of the fillers
The solar reflectance of the shell powders and the commercial calcium carbonate is plotted in Fig.5.The three shell powders with different sizes virtually have the same reflectance spectrum.They have high reflectanceacrossthesolarspectrum,approaching90%,andtheirreflectivityforUVlightsisnotevenlow.Acrossthewholespectrum,commercial calcium carbonate shows higher reflectivity than the shell powderfillers,which is primarily because there exist organic matters in thefillers of shell powders.According to the formula of solar reflectance[29],the reflectance of the fillers at UV/VIS/NIR segments and the average reflectance are calculated and listed in Table 3,respectively.
3.2.Properties of the coatings
The preparation of the coating samples(described in Section 2.1)and their performance testing were carried out according to the corresponding Chinese National Standards.
3.2.1.Solar reflectance of the coatings
The solar reflectance of the coatings was measured according to the Chinese National Standard(GB/T 25261-2010)by using aspectrophotometer(Lavy500UV-VIS).Thesizeofthecoatingsubstrate is 40 mm×40 mm×5 mm,and the thickness of the coating is 100 μm.

Table 3 The calculated solar reflectance of different fillers
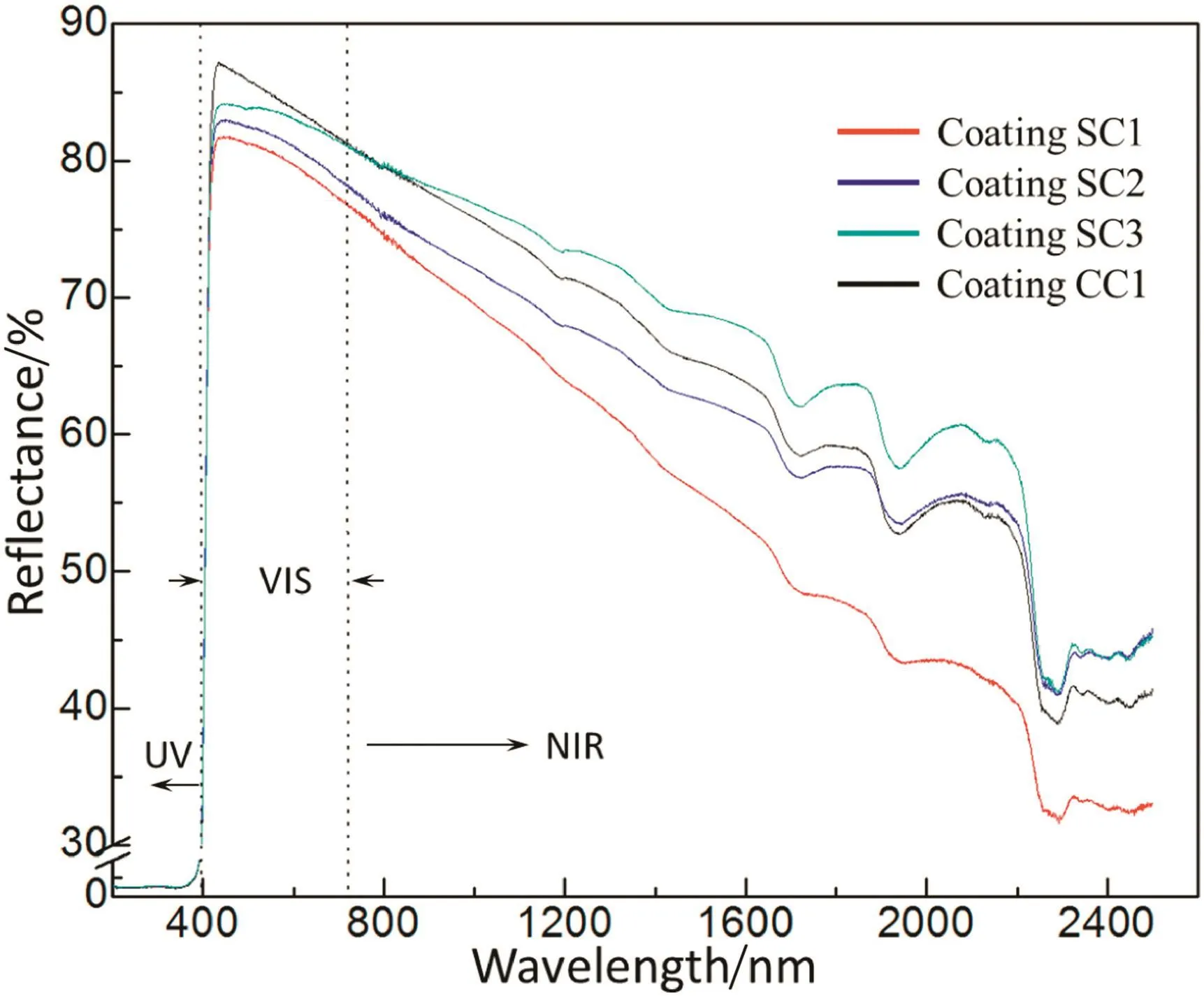
Fig.6.Solar reflectance of the coatings,SC1,SC2,SC3 and CC.
The solar reflectance of the four coatings(SC1,SC2,SC3 and CC)is shown in Fig.6.The calculated solar reflectance of the coatings according to the formula of solar reflectance[29],is listed in Table 4.Apparently read in Fig.6 and Table 4,all the four coatings strongly absorb the ultraviolet because of the existence of the TiO2(rutile phase)which is a highly efficient ultraviolet absorbent.As the wavelength shifts to visible band,the reflectance of all the coatings dramatically increases.Across the whole visible band,the coatings maintain a high refl ectance,although they reflect less lights as the wavelength goes longer.
According to the data shown in Fig.6 and Table 4,sorting the solar reflectance of the coatings in descending order gives the sequence:SC3,CC,SC2 and SC1,which have an average solar reflectance of 0.711,0.707,0.681 and 0.654,respectively.This means the size of the shell powder fillers pronouncedly affects the solar reflectance of the coatings and the solar reflectance of the coating filled by the finest shell powders(SC3)emulates that of the coating filled by commercial CaCO3(CC).Coating SC3 includes much more shell powder particles because of the small size than coatings SC1,SC2 and CC,thus there are much larger interfaces in coating SC3 than coatings SC1,SC2 and CC.The larger interface can enhance the solar reflectance of the coating SP3.
3.2.2.Surface morphology of the coatings
The morphology of the coatings was characterized by SEM,and the images are shown in Fig.7.Among these three shell powder coatings,coating SC3 has smoother surface and less cavities than the other two.Compared with the coating filled with shell powders,Fig.7(A)-(C),especially SC3,the coating CC,Fig.7(D),filled with commercial CaCO3,possesses a coarser surface,andtheagglomeration of theCaCO3particle is muchmore evident.This is explained bythe fact that the dispersity of the commercial CaCO3is worse than that of shell powders.The higherpropensity to agglomeratefor commercial CaCO3is alsoobserved in the SEM images of the fillers,shown in Fig.4.The worse dispersity of the commercialCaCO3fillersofthecoatingCCresultsinalowersolarreflectance than coating SC3.

Table 4 The calculated solar reflectance of the coatings

Fig.7.SEM images of the coatings.(A):SC1,(B):SC2,(C):SC3 and(D):CC.
3.2.3.Thermal insulation performance of the coatings
Thermal insulation effect is the most direct indicator to evaluate the thermal insulation performance of a coating.In this study,the thermal insulation effect of the coatings was examined by using the lab-made setup described in Section 2.2.The initial temperature(then room temperature)was20°C±0.5°C,andthepowerofthetungsteniodinelamp was 500 W.

Fig.8.The cooling effects of the four coatings and the reference panel on the indoor temperatures.(A):applied on concrete plates and(B):applied on steel plates.
Five identical concrete plates were prepared and four coatings were applied on the plates by a spreader,the remaining one was used as a reference without any coating on it.After the coatings were dried and ready to function,under the same conditions(radiation,environment temperature,air flow,etc.),the temperature variation inside the box covered by each of the coated plates,as well as the reference plate,were recorded,respectively.The temperature variation inside the box is shown in Fig.8(A).The steady state of temperature for some coatings was reached in 1 h.
All the four coatings,compared with the reference concrete plate,have a cooling effect,and the temperature rises(ΔT)inside the box are listed in Table 5.Coating SC3(with the lowest ΔT=9.5 °C)won out over the other shell powder coatings to cool the inside of the box,whichmeansthatthefinerthatshellpowderis,thebetterthermalinsulation performance it renders the coating.In addition,the performance of coating SC3 was also better than coating CC(ΔT=10.3 °C)filled by commercial CaCO3,which is consistent with the coatings'power of reflecting solar radiation,Fig.6.The test of the coatings was also carried out on steel plates by the same procedures,and the cooling effect of the coatings on steel plates showed the similar trends as they were applied on concrete plates,as shown in Fig.8(B).

Table 5 Inside temperature rise of the box covered by concrete/steel plate applied with different coatings

Fig.9.Washing resistance of the coatings.(A):SC1,(B):SC2,(C):SC3,(D):CC.
3.2.4.Washing resistance of the coatings
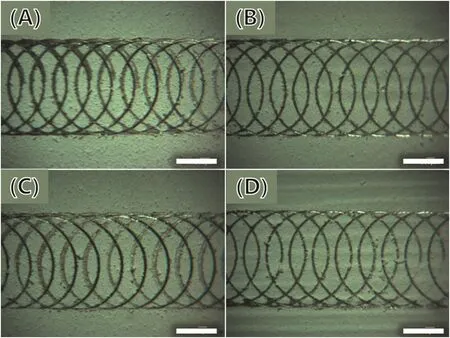
Fig.10.Results of adhesion tests of the coatings.(A):SC1,(B):SC2,(C):SC3,and(D):CC.Scale bars represent 2 mm.
Applied on exterior surfaces,the coatings must be cleaned regularly because of the pollution from their service environment.Therefore,the washing resistance of the coating directly determines its durability.Accordingto theChinese National Standard GB/T 9266-2009,thewashing resistances of the coatings were tested by recording the changes of the surface morphologies around the center of the coatings after 1000 and 2000 cycles of reciprocating brushing,respectively.The results are shown in Fig.9,indicated by arrows in each picture.Coating SC1 failed after 1000 cycles,baring its substrate,while coatings SC2,SC3 and CC could withstand these cycles.After 2000 cycles,most substrate around the center of coating SC1 was exposed,and coating SC2 obviously shed.Bycomparison,thecoatingsSC3andCCwerestillabletocovertotallytheirsubstrates,althoughthecenterofthecoatingSC3wasslightly attenuated.The results demonstrate that the washing resistance of coating SC3 is comparable with that of coating CC.But the washing resistance of coatings SC1 and SC2 filled with coarser shell powders decreased.According to GB/T 9266-2009,a coating sample is up to the standard for its washing resistance,once it passes 500 cycles.But,engineerscontinuetoenhancethewashingresistancebecausethisproperty has close relationship with the durability of coatings.The coatings filled with finer shell powders show better performance,because the finer powders have larger surfaces,and therefore,the fillers were morefirmly mounted in the coating films.
3.2.5.Adhesive force of the coatings
According to the Chinese National Standard GB/T1720-79(89),the adhesive force of the coatings was evaluated based on the morphology of the scratches on the samples after testing,as shown in Fig.10.All the four kinds of coating can satisfy the standard,and among these coatings,although the scratches on coating SC1 are severe which is caused by the larger particles of irregular shell powders.Relatively,the coating SC3 has better adhesive force than those of SC1 and SC2.
4.Conclusions
This study has revealed the benefit of the use of shells is twofold:a cheap and effective bio-filler to produce thermal insulation coating,and reducing the residue of shell wastes.The smaller shell powders render better thermal insulation performance for as-filled coatings,and the coating SC3 filled by shell powders with an average particle size of 2.81 μm possesses larger solar reflectance and better thermal insulationperformancethanthoseofcoatingsfilledbycommercialCaCO3.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Review on application of nanoparticles for EOR purposes:A critical review of the opportunities and challenges
- Drawdown mechanism of light particles in baffled stirred tank for the KR desulphurization process☆
- Optical inline analysis and monitoring of particle size and shape distributionsformultipleapplications:Scientificandindustrialrelevance
- Experimentalstudyofpowerconsumption,localcharacteristicsdistributions and homogenization energy in gas-liquid stirred tank reactors☆
- Molecular simulation of penetration separation for ethanol/water mixtures using two-dimensional nanoweb graphynes☆
- Chaotic characteristics of pseudoplastic fluid inducedby 6PBTimpellerin a stirred vessel☆
