Molecular simulation of penetration separation for ethanol/water mixtures using two-dimensional nanoweb graphynes☆
2019-03-20WeiZhangZhijunXuXiaoningYang
Wei Zhang,Zhijun Xu,Xiaoning Yang*
State Key Laboratory of Material-Orientated Chemical Engineering,College of Chemistry and Chemical Engineering,Nanjing Tech University,Nanjing 210009,China
Keywords:Graphynes Membrane separation Ethanol/water Molecular simulation
A B S T R A C T Graphyneis expected to bea new-class ofhighly-efficientsieving membranesdue to its controllable uniformpore structure and ultrathin single-atom thickness.Herein,we computationally investigate the permeation performance of liquid ethanol-water mixtures across polyporous two-dimensional γ-graphyne sheets.It was found that,in the mixture,ethanol with larger molecular diameter permeates faster through the graphyne pores than water.The simulations demonstrate that pristine graphynes could act as highly-efficient ethanol-permselective membranes forseparation of ethanol-water mixtures,with ethanolpermeability remarkablyhigherthan conventional membranes.This separation mechanism is distinctly different from the molecular-size dependent sieving process.The stronger hydrophobic interfacial affinity between graphyne and ethanol makes ethanol molecules preferentially adsorb on graphyne surface and selectively penetrate through graphyne pores.This penetration mechanism provides new understanding of molecular transport through atomically thick two-dimensional nanoporous membranes and this work is expected to be valuable in the potential development of highly-efficient membranes for liquid-phase mixture separation.
1.Introduction
Graphyne is a new family of carbon allotrope structures formed by both sp-and sp2-hybridized carbon atoms[1-3].As one form of graphynes,γ-graphyne[4,5]is composed of phenyl rings joined together by acetylene bonds,in which the graphyne-n corresponds to nacetylenic bonds.The acetylenic linkages between two neighboring phenyl rings form uniform and repeating triangular nanowebs with well-defined nanopores in graphynes.The pore size can be adjusted by changing the number of acetylene bonds(defined as n).Currently,various monomeric and oligomeric structures of graphyne can be prepared with the advances in radialene and annulene chemistry[6-10].Especially,large-area graphdiyne sheet has been successfully prepared and fabricated on top of copper substrates[11].
Graphyne nanomaterials have a rich variety of excellent physicochemicalproperties[4,5,12-14],whichbringaboutnumerouspromising applications[15-18].Especially,pristine graphyne has been promisingly reported as a new-class of highly permeable membranes in gas separation[19-22]and water desalination[23-26]due to its one-atom thickness and natural uniform well-defined pore structure.However,to our best knowledge,there is very limited research regarding graphyne sheets as nanoporous membranes for the separation of miscible alcohol-water mixtures.In our recent study[27],it was demonstrated that hydrophobic graphyne surface exhibits strong affinity toward ethanol molecules,which suggests that polyporous graphyne surfaces can possibly act as a two-dimensional separation membrane.
The separation of ethanol-water liquid mixtures is of significant importancenotonlyinconventionalchemicalindustries,butalsointheproduction of bioethanol,one of the main biofuels,from biomass[28,29].At present,membraneseparationhasbeenproposedasthesuperioralternative approach to conventional distillation and extraction for the separation of ethanol-water mixtures due to high selectivity and low energy cost[28,30].In particular,pervaporation technique is identified as one of the feasible methods for recovering bio-ethanol,one of the main biofuels,from aqueous solution[28,29].The critical step of the pervaporation process is to develop new-typed membranes with highflux and high selectivity.Therefore,it is of great scientific interest and huge practical value to explore the potential application of single-layer graphyne membrane in liquid phase ethanol-water separation.
Herein,molecular dynamics(MD)simulations were conducted to investigate the molecular permeation of ethanol-water mixtures through the graphyne pores.The γ-graphyne-n(G-n)sheets with n=3,4 and 5 were considered.We computationally demonstrate that graphyne sheets with specific uniform nanoscale pores have strong potential as ethanol-permselective sieving membranes for the separation of ethanol-watermixtures.Ethanol molecules have larger pore penetration ability than water molecules.This penetration mode across atomically thick two-dimensional(2-D)graphynes is different from the pore-size selection mechanisms in conventional nanoporous membranes[31,32],which is due to surface affinity difference.Our result will provide new understanding of molecular transport across nanoporous 2-D membranes.
2.Model and Computational Details
In this work,MD simulations were performed using the Lammps package[33].TheLennard-Jones(L-J)12-6potentialandtheCoulombic interaction were implemented to describe the intermolecular interactions.Water was represented by the SPC/E model[34]and ethanol wasmodeledusingtheOPLS-AAforcefield,whichhasbeensuccessfully used to reproduce the ethanol properties[35-37].The SPC/E water model can capture the properties of water and it is well known for its satisfactory performance in the simulation of both interfacial and dielectric properties of water[38-41].In addition,the SPC/E model can reasonably match with the OPLS force field[42-44].The bond lengths and lattice parameters of graphynes were obtained from the optimized graphyne geometries from the reference[45].The carbon atom of graphynewasdescribedasunchargedL-Jparticle[46].Thepotentialparameters have been successfully applied to describe the graphyne membrane for investigating the water desalination[26,47],which in principle possesses highly similar characteristics to the simulation system of ethanol-water separation.Following the previous simulations of ethanol-water mixtures,the geometric mixing rules were applied for the L-J interactions between different particles[48,49].The particle meshEwaldmethodwasusedtocalculatethelong-rangedelectrostatic interactionandacutoff distanceof1.0nmwasadoptedfortheL-Jinteraction.For all simulation systems,the simulated temperature was controlled at 298.15 K by the Nose-Hoover thermostat with a relaxation time of 0.1 ps.A time step of 1 fs was used and the data were collected every 1 ps for the property analysis.
In this non-equilibrium permeation simulation,the penetrating transport through the different graphyne membranes was simulated under applied pressure with the different initial configurations of equilibrium ethanol-water mixtures in the feed side.The different conditions in the permeation side were considered.Periodic boundary conditions were applied along the x-y directions.After a preequilibrium simulation,the defined pressure was imposed to the system along the direction perpendicular to the graphyne sheet for 10 ns by applying an external force on the graphene piston.The applied force acting on an individual carbon atom of graphene piston is determined by f= ΔP ·A/n,where ΔP is the controlling pressure,which is the difference between the feed and permeate pressures.At the permeateside,weusedtheatmosphericpressureasthepermeatepressure;at thefeedside,weusedhighpressureasthefeedpressure.Aistheareaof the graphene sheet,and n is the total number of carbon atoms in graphene piston.Fig.1 illustrates the typical non-equilibrium permeation simulation system,in whichgraphynesheet wasfixed in themiddle of a simulation box.The graphyne structures(seeing Fig.S1)were frozen dueto thenegligible effect of flexible structure on thesimulation results[40,41],although the rigid structure might not represent the actual configuration.The frozen structure of nanoporous membrane also canreducethecomputationalcost[50].Inthenon-equilibriumMDsimulation,graphene-like pistons were placed on both sides of the simulation cell and external force was imposed to the pistons in order to produce hydrostatic pressure[23,25,51-56].The hydrostatic pressure,rangingfrom 20 to 500 MPa,wascreated along the negative z direction.Higher pressure condition used in the non-equilibrium simulation[51,57]can reduce the thermal noise and enhance signal/noise ratio.The detailed information for the simulation systems was listed in Tables S1 and S2.
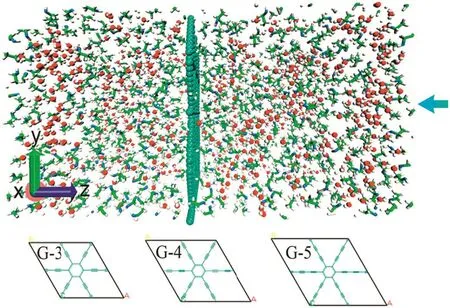
Fig.1.A snapshot ofsimulation system containingthe50mol%mixture inboth sides with a pressure applied along negative z direction.The bottom shows unit cells of graphyne-3,graphyne-4 and graphyne-5.
3.Results and Discussion
We first studied the permeation behavior of the ethanol-water mixture(50mol%)passingthroughtheG-4membrane,wherevariousexternal hydrostatic pressures were imposed(see Fig.2).Fig.2a plots thefiltered molecular numbers of ethanol and water as a function of time under various pressures.Obvious larger permeation number appears for ethanol than water,indicating that the G-4 membrane has the selective permeation for ethanol.Fig.2c presents the pressure-driven permeations for pure species,and pure water was found to permeate faster throughtheG-4membranethanpureethanol.Thecomparisonofpermeation behavior between the mixture and the pure species further highlights the competitive and selective penetration of ethanol relative to water.Foreachtime-dependentpenetrationcurve(Fig.2a),theparabolic shape in the initial time region reflects the pre-equilibrium stage of penetration flow.The linear regime in the flow curves can be used to compute the permeation fluxes for each species,which is defined as theflowing molecular number per unit time and per unit membrane area.The fluxes(Fig.2b)show roughly approximately linear increase with the pressure,consistent with previous simulation results[25,47].As expected,the flux of ethanol in the mixture is always higher than that of water.Comparatively,the linear penetration behavior of pure species through the G-4 membrane is better than the mixture,which is possibly duetothecompetitioneffectofethanolandwaterduringthepenetration course.Inaddition,weconductedthesupplementarypermeationsimulation with the 50 mol%mixture in the feed side and pure liquid ethanol in the downstream side.This simulation might represent the pressuredriven osmotic or nanofiltration process.As seen in Fig.S2,there also appears higher permeation of ethanol relative to water.Thus,all these simulation results computationally demonstrate that graphyne-4 sheet has promising potential as the membranes for the ethanol separation from ethanol-water mixtures.
In order to understand the above penetration performances,we computed the interfacial density profiles of both the mixture and the pure species on the G-4 surface in the equilibrium state.As shown in Fig.3a(upper panel),in the ethanol-water mixture there is favorable adsorption of ethanol relative to water on the G-4 surface with signi ficantadsorptionpeak(~0.4nm)forethanolmolecules.Thisisconsistent with the previous experimental[58]and theoretical results[35,37,49,59]that the amphiphilic ethanol molecules are able to predominately concentrate/adsorb on hydrophobic(or organophilic)carbon surfaces.The hydrophobic performance on graphyne surfaces has been similarly reported for other carbon-based surfaces.For example,a segregation phase separation phenomenon[60]with ethanol molecules preferably concentrated near graphene surfaces was observed for ethanol-water mixtures confined within graphene slit pores[59].In addition,it was foundthatamphiphilicethanolmoleculesareabletopredominatelyadsorb on hydrophobic carbon nanotube surfaces[57,59].All these results provide the conclusion that the stronger surface affinity of ethanol in the mixture could exclude water from the carbon-based hydrophobic surface.The specifically enhanced adsorption of ethanol molecules on graphyne carbon surface might increase ethanol crossing rate through the inherent single-atom-thick graphyne pores,possibly leading to preferential pore penetration of ethanol relative to water.

Fig.2.(a)The number of filtered water and ethanol molecules as a function of simulation time for the applied pressures ranging from 40 to 100 MPa.(b)The mass fluxes of water and ethanol as a function of the applied pressures.(c)The filtered molecular number as a function of simulation time for pure water and pure ethanol through G-4 membrane under 100 MPa.(d)The moving trajectories of the representative ethanol and water molecules in the z direction as a function of the simulation time,different colors represent different molecules.The inset is a snapshot of one typical ethanol molecule transporting through the graphyne-4 membrane.The arrow represents the movement direction.

Fig.3.(a)Density distributions along z axis for pure water and pure ethanol(left)and for the 50 mol%mixture(right)on the graphyne-4 surface.(b)Left:Potential of mean force(PMF)for one water molecule across the pore center along the direction perpendicular to the graphyne-4 sheet.The total PMF included the solution interaction and the graphyne interaction(1 cal=4.184 J).Right:Same as left but for ethanol.The reference z=0 corresponds to the G-4 position(vertical broken lines).
Fig.2c shows the moving trajectories of several representative ethanol/water molecules passing through the G-4 membrane.As displayed in the trajectories,ethanol molecules could remain stable for a longer time in the adsorption layer(z~0.4 nm)before passing through the graphyne pores.This is in accordance with the longer residence time of ethanol in the surface contact layer(Fig.S3).On the contrary,thewatermoleculesshownoticeableoscillationintheinterfacial region.The behavior signifies the surface adsorption of ethanol might benefit the ethanol permeation,and meanwhile impede the water approaching toward the pore.This is qualitatively similar with the previous studies that increased adsorption on graphene surface might improve the pore diffusion of molecules[61,62].However,as compared with porous graphene,graphyne displays more enhanced molecular penetrability owing to its high pore density.In addition,this result also suggests that the ethanol molecules hardly pass through theporesdirectly from thebulkphase,whichis differentfrom the passingmodesforgasmoleculesacrossnanoporousgraphene[63].Moreintuitively,the translocation configurations for one typical ethanol molecule through the graphyne pore under different times are shown in the snapshot(see inset of Fig.2c).We can observe that this ethanol molecule usually stays for a while in the adsorption layer and then adopts the special alignment when passing through the graphyne pore.
To further explore the underlying mechanism of the selective permeation of ethanol over water,we further simulated the PMFs(potential of mean force)for ethanol and water in the mixture(50 mol%)passing through the pore center of graphyne-4.The PMF along the direction perpendicular to the membrane as a function of the position z was calculated by,

where W(z0)is the reference value and it was chosen to be zero at the furthest target position(2 nm in this work),z is the axial coordinate of the target species from the center of the graphyne-4 pore.The constraining force f(z)was calculated from the average constraint force exerted on the target species.In the constrained MD simulation,both the G-4 sheet and the center of mass of the target species were kept fixed,while the target species can rotate around the center of mass and the other solvent molecules were completely free.After the pre-equilibrium simulation of 1 ns,we collected the following production to calculate the average constraint force.
As shown in Fig.3b(down panel),there is a free-energy well for ethanol through the G-4 pore,whereas water faces a free-energy barrier at the pore center.The free-energy profiles indicate that it is thermodynamically favorable for ethanol molecules to occupy the graphyne-4 pores.This is consistent with the significant pore occupation of ethanol observedintheprecedingdensityprofile(Fig.3a).Essentially,themagnitude of free-energy barrier(~3.8 kBT)for water passage is not very huge,which is not able to make sufficient resistance on the water passage.As a result,the reason of lower permeation flux of water in the mixture could be considered as the competitive effect.The different thermodynamic preferences between ethanol and water,when locating in the graphyne pores,might cause the selective pore penetration.
In Fig.3b,we decomposed the total PMF into the solvent contribution and the graphyne contribution.The solvent interaction is generally repulsive for molecules approaching toward the pores,while the graphynesheetimposesattractiveimpactonthemolecularpenetration.As expected,the graphyne-ethanol interaction is stronger than the graphyne-water interaction,in line with the enhanced surface adsorption of ethanol.Therefore,the molecular trapping inside the pore is driven by the enhanced hydrophobic interaction between graphyne and ethanol,and the significant difference in the surface affinity for the two species is the main molecular origin of the selective ethanol penetration.Fig.S4 shows the relevant PMF profiles in the G-3 membrane.UnliketheG-4pore,itisthermodynamicallyunfavorableforethanol to locate inside the G-3 pore.Although ethanol molecules show favorable adsorption near the G-3 surface,they bear larger repellence when approaching the G-3 pore.This behavior is reasonable by considering the small pore size in the G-3 membranes.This result agrees well with the consequent simulation result(Fig.4a)that strong external force is generally required to achieve the ethanol permeation passing through the G-3 pore.From the above results,the penetration separation mechanism for ethanol-water mixtures through the single-layer graphyne membrane can be presumably ascribed asthesurface affinity.Graphynesurfaceisfoundtopreferentiallyadsorbethanoloverwaterin themixture due to its hydrophobic feature.This enhanced surfaceaffinity might increase the possibility of molecular transport across the graphyne pores.For the graphyne-4 membrane,ethanol molecules preferentially occupy the pores and display larger penetration ability.Meanwhile,the preferential pore trapping of ethanol might block the penetrating pathway of water.When the pore size further increases in the graphyne-5 membrane,the superiority of pore occupation and penetration of ethanol molecules becomes less significant,and consequently the water penetration shows a slight increase.However,for the graphyne-3 membrane,the pore size is too small for ethanol to easily permeate.

Fig.4.(a)The mass fluxes of water and ethanol in the 50 mol%mixture as a function of the applied pressure for the three graphyne membranes.(b)The penetration snapshots of the 50 mol%mixture passing through the G-4 membrane at 100 MPa during the simulation course.Color scheme:green,ethanol;red,water;cyan,graphyne.
The separation mechanism is distinct from the pore-size sieve process[31,32],but is considered as thechemical affinity separation mechanism,already proposed in previous works[27,62,64].In the conventional nanoporous membranes,competitive diffusion and adsorption within the inside structure of pore for various species can be exploited as the separation mechanism.However,in the ultrathin graphyne membrane,the special separation mechanism is determined not only by the selective molecular trapping in the atomically thick polyporous structure,but also,more critically,by the different adsorptionaffinitiesontheplanargraphynesheet.Thejointfunctionoftheenhanced hydrophobic surface adsorption and pore trapping effect in the pristinegraphyneleadstotheuniquepreferentialchemicalaffinitywith ethanol,making single-layer graphyne sheet have high selective penetration of ethanol,just like a two-dimensional molecular sieve.In fact,the ethanol-selective mechanism of polyporous graphyne is somewhat comparable to that observed in hydrophobic or organophilic polymer membranes[63,66],which has been widely applied in the ethanol pervaporation process.However,the ultrathin graphyne membranes allow superfast molecular transport,as compared to conventional polymer membranes.
In the following section,we investigated the pervaporation separation process of ethanol from aqueous solutions,in which the pressuredriven permeation of the ethanol-water mixture passing through the graphyne membranes from solution phase to vacuum phase was simulated.Fig.4ashowsthemassfluxofeachspeciesasafunctionofapplied pressure for the ethanol-water mixture(50 mol%)through three types of graphynemembranes.The mass permeation flux,defined astheflow massperunitmembranearea andperunittime,isdeterminedfromthe time-dependence profiles of molecular penetrating number and was shown in Fig.S5.The ethanol flux is higher than water.The linear behavior of fluxagainst pressure suggests theresult couldbe extrapolated to low pressure condition.In particular,the obvious difference in the penetration fluxes between the two species suggests γ-graphynes have the potential as the pervaporation membrane for the ethanol separation.For the G-3 membrane,no molecular penetration can be observed under the pressure below 100 MPa and much higher pressure is generally required for the permeation owing to the pore size restriction.
The pervaporation simulation of the ethanol-water mixture was also conducted for a different initial condition,where the species moleculeswererandomlymixedinthefeedsidewithoutpre-equilibrium.As shown in Fig.S6,the almost identical result was obtained,with favored ethanol penetration relative to water.This further confirms that the pervaporation simulation result is rational.Moreover,this result means that the enhanced surface affinity of graphyne is able to ensure the selective pore penetration of ethanol regardless of the starting state in the feed mixture.Fig.4b shows the typical snapshots for the mixture permeation through the G-4 membrane.It is clearly observed that,during the simulation process,more ethanol molecules pass through the graphyne-4 membrane,demonstrating the enrichment of ethanol in the permeate side.The detailed dynamic pervaporation process for the ethanol-water mixture can be clearly visualized in the supplied video file.
In Fig.5,we compared the separation performance of the graphyne membranes by calculating the hydraulic permeability(in unit of kg·m−2·h−1·bar−1,1 bar=105Pa)and the molar permeation ratio.For the G-4 and G-5 membranes,ethanol demonstrates significantly higher permeability than water.The G-3 membrane has relatively lower permeability for bothethanol and water.However,the simulated permeabilities(100-1200 kg·m−2·h−1·bar−1,1 bar=105Pa)of graphyne membranes are several orders of magnitude larger than those in the current pervaporation membranes[65,67].The molar permeability ratio of ethanol to water is also shown in Fig.5.However,the molar permeation ratio of graphyne membranes is generally low.This high permeability and low permeation ratio is closely associated with the monoatomic thickness of graphyne structures.In addition,thelarge pore densityinthegraphyneplane alsoprovides thecontribution to this low selectivity.Comparatively,among the three types of graphyne membranes,the G-4 membrane possesses acceptable separation performance.

Fig.5.A comparison of the separation performance between the three graphyne membranes and the existing ethanol-permselective membranes for the separation of ethanol/water mixture(1 bar=105Pa).
We also investigated the effect of the mixture concentration in the initial feed side on the separation performance by using the G-4 membrane.Fig.S7 shows the penetration results under a series of various concentrationconditions.Although,inthecaseoflowerfeedconcentration,ethanol penetration declines due to reduction of ethanol adsorption,the permeated number of ethanol is generally larger than that of water.In particular,the penetration number of ethanol was larger than water during the first 4 ns simulation time even in the lower ethanol concentration.Fig.6 shows the variation of ethanol concentrations in thepermeation side with theinitial feed concentrations under different simulation times.It was observed that,even though the ethanol concentration in the permeate side decreases with the simulation time,the permeate concentration is always higher than the initial feed concentration.These results show the graphyne-4 membrane could maintain static permeation separation capacity for ethanol-water mixtures under wide concentration ranges.Specially,the reasonable penetration selectivity under lower ethanol concentration demonstrates the graphyne-4 membrane can serve as a highly-efficient energy-saving ethanol-permselective membrane in actual operation.
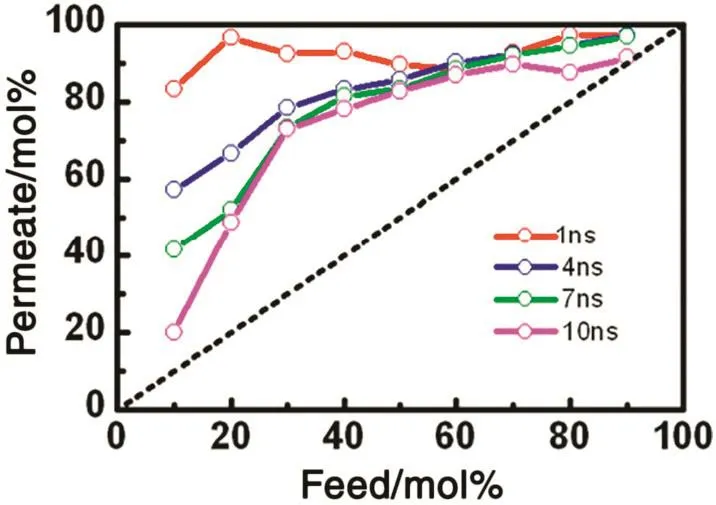
Fig.6.The variation of ethanol concentrations in the permeation side with the initial feed concentrations under different simulation times.
Accordingtotheabovesimulation,the selective penetrationis largely determined by the interfacial affinity of graphyne pores.This sieving mechanism could be generally applied to other two-dimensional nanoporouscarbonstructuresforseparationofvariousliquid-phasemixtures.However,the ethanol selectivity of the graphyne membrane is not very high compared with the conventional pervaporation membranes.This low selectivity is obviously associated with the larger pore density in the graphyne plane.The ethanol selectivity could be improved by designing specific membranes by combining multiple graphyne sheets in a serial way.For instance,it is expected that dual-graphyne membrane structure is able to significantly eliminate the water permeation and increase the ethanol selectivity.In addition,due to the high permeability,this graphyne-based ethanol-permselective pervaporation process could be integrated with other unit processes to achieve high ethanol permeation or concentration[29,30].Although the simulation was conducted in the higher pressures,this does not mean that actual operation of membrane separation must run in higher-pressure conditions.Meanwhile,the simulation result can be extended to the low-pressure condition.Moreover,previous computation has demonstrated that graphyne membraneshavestrongermechanicalstrength[23,25]andhigherchemical stability[26].These superior properties will ensure the operation of graphyne membranes.
4.Conclusions
Our simulation demonstrates that pristine graphynes can possibly act as the filtration membrane for separation or enrichment of ethanol-water liquid mixtures with remarkably high ethanol permeability and acceptable selectivity.The ethanol permeability is several orders of magnitude larger than conventional pervaporation membranes.The unusual penetration separation mechanism for the graphyne membranes,presented in this work,is attributed to the combination of hydrophobic carbon surface and unique polyporous structure with single-atomthickness,bothofwhichareabletoprovidefavoredsurface adsorption and pore trapping of ethanol molecules.This simulation result will be valuable in designing and developing new-typed nanoscale hydrophobic(ororganophilic)membranesthatcouldbeappliedtoseparateethanolfromaqueoussolution.Itshouldbeemphasizedthatthere are still huge challenges in the application of graphyne structures,such as the feasibility of synthesizing large scale graphite acetylene and the difficulty of preparing single-layer free-standing graphyne membrane with controllable structures.However,we should believe that,with rapid development in the material preparation technique,the novel graphyne materials will be applied to develop new membrane separation technique,even though the development of graphyne-based 2-D membrane is far from being realized in the present time.
Supplementary Material
Supplementary data tothis article canbefoundonlineathttps://doi.org/10.1016/j.cjche.2018.02.028.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Review on application of nanoparticles for EOR purposes:A critical review of the opportunities and challenges
- Drawdown mechanism of light particles in baffled stirred tank for the KR desulphurization process☆
- Optical inline analysis and monitoring of particle size and shape distributionsformultipleapplications:Scientificandindustrialrelevance
- Experimentalstudyofpowerconsumption,localcharacteristicsdistributions and homogenization energy in gas-liquid stirred tank reactors☆
- Chaotic characteristics of pseudoplastic fluid inducedby 6PBTimpellerin a stirred vessel☆
- Investigation of the liquid recycle in the reactor cascade of an industrial scale ebullated bed hydrocracking unit
